Abstract
Interleukin-6 (IL-6), a major cytokine with diverse effects on cells mainly of the immune and hematopoietic systems, has been linked to several neurological disorders such as acquired immune deficiency syndrome dementia, multiple sclerosis, and Alzheimer’s disease. Central nervous system (CNS)-specific expression of IL-6 caused neurodegeneration, massive gliosis, and vascular proliferation in transgenic mice. However, the effects of systemically circulating IL-6 and its receptor IL-6Rα on the CNS are unknown. IL-6Rα is the specific component of the IL-6 receptor system and hence an important co-factor of IL-6. IL-6Rα is bioactive in a membrane-bound and in a soluble (s) form. We investigated the effects of systemically elevated levels of either human IL-6 or human sIL-6Rα or both on the CNS of transgenic mice. Although IL-6 and sIL-6Rα single transgenic mice were free of neurological disease, IL-6/sIL-6Rα double-transgenic mice showed neurological signs, such as tremor, gait abnormalities, and paresis. However, these mice also frequently showed prominent general weakness probably because of the systemic effects of IL-6/IL-6Rα such as liver damage and plasmacytomas. IL-6/sIL-6Rα transgenic mice exhibited massive reactive gliosis. Lack of signs of neuronal breakdown versus ample astrogliosis suggested that astrocytes were selectively affected in these mice. There was neither vascular proliferation nor inflammatory infiltration. Ultrastructural analysis revealed blood-brain barrier (BBB) changes manifested by hydropic astrocytic end-feet. However, albumin immunohistochemistry did not reveal major BBB leakage. Our results indicate that increased and constitutive systemic expression of IL-6 together with its soluble receptor sIL-6Rα is less harmful to the brain than to other organs. The BBB remains primarily intact. IL-6/IL-6Rα, however, might be directly responsible for the selective activation of astrocytes.
Interleukin-6 (IL-6) was initially described as a central mediator of the immune system, hemopoiesis, and acute phase reaction. 1 IL-6 is a member of the neurocytokine family, which also includes ciliary neurotrophic factor, leukemia inhibitory factor, oncostatin M, and cardiotrophin-1. These molecules have overlapping biological activities; they possess a similar secondary structure and exert their activities through related multisubunit receptors. 2 The binding of IL-6 to its receptor IL-6Rα triggers the association of the IL-6Rα subunit with a second membrane glycoprotein, gp130, which transduces the IL-6 signal. 2,3 Neurons and glial cells can produce IL-6. 4-8 IL-6 has pleiotropic effects within the nervous system 9,10 including neurotrophic 7,8 and neuronal differentiation promoting activities. 11 In addition, IL-6 promotes peripheral nerve fiber outgrowth in vivo. 12
Elevated IL-6 blood levels are found in diseases such as plasmacytoma/myeloma, osteoporosis, autoimmune diseases, and acquired immune deficiency syndrome (AIDS). 1 IL-6 has also been implicated as an important mediator of pathophysiological processes in the central nervous system (CNS), such as demyelination, 13,14 neurodegeneration, 15 and neoplastic transformation of glial cells. 16-18 In two human disorders involving the nervous system, Castleman’s disease and polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes (POEMS) syndrome, highly elevated systemic IL-6 levels have been detected, suggesting that systemically increased IL-6 might be harmful to the nervous system. 19-23 Mice overexpressing IL-6 in bone marrow cells develop a syndrome resembling Castleman’s disease. 24 In both conditions, polyneuropathy can be a prominent symptom; additionally, chordoid meningeal tumors with prominent lymphocytic infiltration have been reported in Castleman’s disease. 19,22,25
Transgenic mouse models have advanced the understanding of the CNS pathobiology of IL-6 in vivo. 26 These studies highlighted the central role of astrocytes. For example, expression of murine IL-6 from the prenatally active astrocyte-specific glial fibrillar acidic protein (GFAP) promoter 27 induced a strong and lifelong reactive gliosis as well as microglia activation, vascular proliferation, and neuronal damage. 27,28 The central role of IL-6 in regulating astrocyte responses is emphasized by the reciprocal mouse model deficient in IL-6 showing impairment of neuroglial activation after injury. 29 In addition, a ciliary neurotrophic factor transgene under the control of GFAP promoter sequences also induced prominent reactive gliosis in mice. 30 Wild-type mice that had been injected with ciliary neurotrophic factor into uninjured brain showed the same glial response. 30 When IL-6 was targeted to neurons by the rat neuron-specific enolase promoter, neuron-specific enolase-IL-6 transgenic mice also developed reactive astrocytosis and an increase in ramified microglial cells, but no apparent neuronal damage. 31 In GFAP-IL-6 mice, the blood-brain barrier (BBB) never developed completely. 32
While the role of IL-6 expressed in astrocytes and neurons has been extensively investigated, the effects of systemically circulating IL-6 and sIL-6Rα in the CNS have not been addressed so far. Because IL-6Rα is bioactive in a membrane-bound and in a soluble form, slL-6Rα is an important cofactor of IL-6. In fact, the IL-6/sIL-6Rα complex rather than IL-6 is believed to be the active form in vivo. 33 Interestingly, mice transgenic for IL-6 and a membrane-anchored IL-6Rα developed hypertrophy of ventricular myocardium. 34 Involvement of the CNS has not been described in this model, even though mice overexpressing IL-6 locally in the brain display marked CNS alterations and at least two human conditions associated with high IL-6 levels, Castleman’s disease and polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes syndrome, show nervous system involvement (see above). Therefore, we investigated the effects of high levels of circulating IL-6 and its soluble α receptor on the CNS in a transgenic mouse model, focusing on astroglial and BBB alterations.
Materials and Methods
Generation of IL-6/IL-6Rα Double-Transgenic Mice
Heterozygous double-transgenic mice were obtained by cross-breeding metallothionine-I/IL-6 mice 35 with phosphoenolpyruvate carboxy kinase/hsIL-6Rα mice. 36 These double-transgenic mice expressed the transgenic proteins systemically under the control of the metallothionein-I promoter 37 and the neonatal active phosphoenolpyruvate carboxy kinase promoter, 38 respectively.
Detection of IL-6 and IL-6Rα Gene Products
For transgenic RNA analysis, total RNA from different organs was isolated by the guanidine isothiocyanate method. 39 Expression of IL-6 and sIL-6Rα mRNAs was analyzed previously by Northern blots probed with either a 32P-labeled cDNA of IL-6 35 or with a PstI-XhoI fragment of the IL-6Rα cDNA. 36 Transgenic protein expression was investigated by enzyme-linked immunosorbent assay and immunoprecipitation as reported earlier. 36 Biological activity of IL-6 was determined by either using the 7TD1 proliferation assay 40 or the IL-6-dependent cell line B9. 41
Histology and Immunohistochemistry
Most major organs (brain, spinal cord, heart, lung, thymus, liver, kidney, spleen, ovary/testis, intestine, eye, skeletal muscle) were fixed in formaldehyde and embedded in paraffin. Hematoxylin and eosin staining was performed on 4-μm sections. For GFAP immunohistochemistry of brain sections, the indirect peroxidase-staining method was applied using a rabbit anti-cow antiserum (DAKO, Glostrup, Denmark) and a peroxidase-conjugated goat anti-rabbit IgG (Dianova, Hamburg, Germany) secondary antibody. For the detection of BBB leakage, albumin immunohistochemistry was performed by use of a rabbit anti-human antiserum (DAKO) and a biotinylated swine anti-rabbit secondary antibody. The reaction was visualized using an avidin biotin alkaline phosphatase system and the chromogen new-fuchsin (DAKO).
The degree of gliosis was scored as follows: 0, no GFAP immunoreactive astrocytes; +, less than three GFAP immunoreactive astrocytes/high-power field (with ×40 lens); ++, less than 10 GFAP immunoreactive astrocytes/high-power field; +++, more than 10 GFAP immunoreactive astrocytes/high-power field. Liver infiltration by plasmacytoma cells and hematopoetic cells was scored as follows: 0, no infiltration; +, minor increase in mononuclear cell content; ++, moderate mononuclear cell infiltration and moderate widening of few portal canals, minor liver cell necrosis; +++, massive infiltration of numerous portal canals with major widening as well as lobular infiltration and major liver cell necrosis. The infiltration of lungs and kidneys by plasmacytoma cells was scored as follows: 0, no infiltration; +, one to three small infiltrates on cross sections of the whole organs; ++, one large or more than three small infiltrates; +++, large, confluent infiltrates. The scoring was performed by two independent observers (AGB, JW) blinded to the identity (wild type, single, or double transgenic) of the mice.
Ultrastructural Examination
For electron microscopy, brain samples of wild-type, IL-6, sIL-6Rα, and IL-6/sIL-6Rα transgenic mice were fixed in 3.9% phosphate-buffered glutaraldehyde and embedded in epoxy resin. Semithin sections were stained with p-phenyldiamine and toluidine blue. Ultrathin sections were contrast enhanced with lead citrate and uranyl acetate, and examined using a Philips EM 300 as described elsewhere. 42
Statistical Analysis
To determine whether gliosis is interrelated with age and/or mononuclear cell infiltration in lung, liver, and kidney we used Pearson correlation coefficients. 43 The Kruskal-Wallis test was used to assess statistical differences in the distribution of various parameters from the wild-type and the two transgenic mouse lines. Where differences were found a pairwise comparison with Bonferroni correction was applied to compare the different lines. 44
Results
Control Animals
Nontransgenic littermates of various ages stayed healthy and symptom-free throughout the whole duration of the study (11 months). Autopsy of all three wild-type control animals and subsequent histological examination revealed no pathological changes in any organ examined except for minor gliosis of unknown cause in one wild-type animal. The sIL-6Rα single-transgenic mice were also considered to be negative controls, because mouse IL-6 does not bind to human sIL-6 receptor α. 45 Accordingly, the three human sIL-6Rα transgenic mice were completely inconspicuous and also served as controls.
Analysis of Transgene Expression
Hemizygous IL-6/sIL-6Rα double-transgenic mice co-expressing both transgenes were used to determine the effect of the IL-6/sIL-6Rα complex on the CNS in vivo. In IL-6 mice transgene expression is driven by the metallothionein-I promoter. 35 Even though it has been described that this promoter is preferentially active in fibrous and protoplasmatic astrocytes, 46 Northern blot analysis of total RNA from several organs, including brain, had revealed that the liver is the major organ for hIL-6 mRNA expression. 35 Metallothionine-I/IL-6 transgenic mice express IL-6 constitutively in the liver and secrete the cytokine into the blood. 35 Serum concentrations ranged between 10 and 20 ng/ml. 47
Expression of the sIL-6Rα transgene was controlled by the neonatally active promoter of the phosphoenolpyruvate carboxy kinase gene that drives expression in the liver, kidney, and adipose tissue. 38,48 As shown previously, Northern blot analysis from different tissues showed transcripts of the hsIL-6Rα in the liver and kidney, but not in the heart, skeletal muscle, or brain. 45 In the brain, we could detect mRNAs of both transgenes only by use of the reverse transcriptase-polymerase chain reaction (not shown). Serum levels of sIL-6Rα were quantified by Western blot analysis and ranged between 4 and 8 μg/ml. 45 The IL-6/hsIL-6Rα complex could be immunoprecipitated from the serum. 45 IL-6/IL-6Rα double-transgenic mice had elevated IL-6 plasma levels, and the plasma half-life of IL-6 was significantly prolonged. 45
Clinical Phenotype of Double-Transgenic Animals
IL-6/sIL-6Rα double-transgenic mice have already been shown previously to differ from sIL-6Rα and IL-6 single transgenic mice mainly in three aspects: 1) plasmacytoma development was significantly accelerated and aggravated; 2) extramedullary hematopoiesis was strongly activated first in the spleen and later in the liver, and subsequently all peripheral blood cell counts were highly increased; and 3) significant hepatocellular hyperplasia and secondary liver pathology, including pellosis and areactive necroses were induced. 45,49,50
Here, we investigated the neuropathological consequences of either circulating IL-6 and sIL-6Rα or both in transgenic mouse brains. In single transgenic mouse lines neurological symptoms were absent. In contrast, all IL-6/sIL-6Rα double-transgenic mice developed neurological symptoms, such as tremor, gait abnormalities, and paresis after 2 to 6 months of life. However, these symptoms were part of a larger complex of effects of IL-6/IL-6Rα including weight loss; fur abnormalities; liver, kidney, and lung damage; and plasmacytomas and can therefore at least in part be attributed to the general weakness.
Histopathological Findings
At the light microscopic level, wild-type as well as sIL-6Rα transgenics were virtually free of reactive gliosis (not shown). In addition, these mice did not show impairments or defects in other organs, such as liver, lung, kidney, or muscle (not shown). A small number of reactive astrocytes could be detected in the brains of two IL-6 single transgenic animals by GFAP immunohistochemistry (Figure 1a) ▶ . In kinetical terms, gliosis in IL-6 transgenic mice did not increase during a follow-up time of 11 months. The low level of gliosis in these mice was not significantly different from the levels found in the sIL-6Rα and wild-type control mice (P = 0.074). Neurons of these mice appeared unaltered in shape and number when compared to normal control animals (Figure 1) ▶ . Compared to the brain, which showed minor to moderate gliosis in only two out of 10 animals, the lungs and kidneys of the IL-6 transgenic mice were considerably more affected; they showed infiltration by lymphoid cells in six out of 10 animals each.
Figure 1.
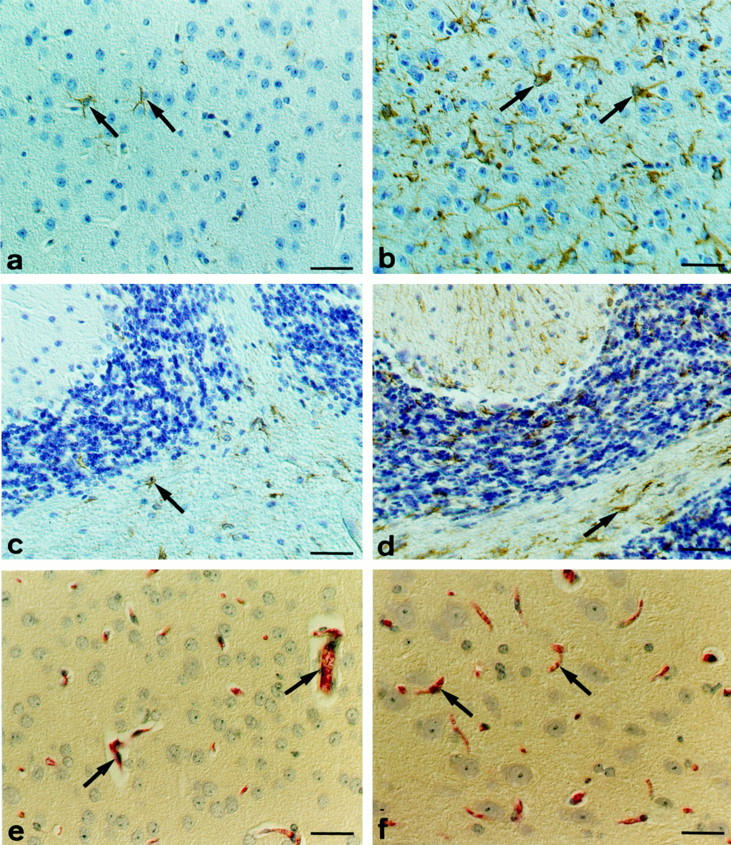
a, c: IL-6 transgenic mice with high levels of circulating human IL-6 showed minor astrogliosis reflected by a low number of GFAP immunoreactive astrocytes (arrows). In contrast, IL-6/IL-6Rα transgenics exhibited massive reactive gliosis with numerous GFAP immunoreactive astrocytes in all parts of the CNS including the cerebral cortex (b) and the cerebellum (d). Scale bars, 30 μm (a–d). However, no impairment of BBB function was detected by albumin immunohistochemistry even in the IL-6/IL-6Rα mice. There was a prominent intravasal staining in all brain sections analyzed (arrows); extravasal immunoreactivity was absent (e, normal control mouse; f, double-transgenic mouse). Scale bars, 30 μm (c), 20 μm (f).
In general, the brains of the IL-6/sIL-6Rα double-transgenic mice were also considerably less affected compared to the extent of lesions in lungs, livers, and kidneys, which showed prominent lymphoid infiltration in most cases (Table 1) ▶ . However, numerous astrocytes in the gray and white matter of the IL-6/IL-6Rα mice displayed the typical hallmarks of reactive astrogliosis, ie, hypertrophy and strong GFAP expression (Figure 1, b and c) ▶ . Pairwise Kruskal-Wallis analysis with Bonferroni correction revealed that the levels of gliosis in the IL-6/IL-6Rα double-transgenic animals differed significantly from the degree of gliosis found in the IL-6 and IL-6Rα single transgenic as well as in the wild-type mice (P < 0.05). Histological examination of brain sections at different time points throughout a period of 2.5 to 8 months demonstrated that the degree of gliosis in IL-6/sIL-6Rα mice was stable and not time-dependent. The reactively transformed astrocytes were distributed all throughout the brain and not at preferred sites. The number of neurons in IL-6/sIL-6Rα mice appeared neither reduced nor increased when compared to the wild-type, sIL-6Rα, and IL-6 mice. There were no histopathological signs of neuronal damage such as chromatolysis or shrinkage/eosinophilia. Cerebral vascular proliferation, necrosis, inflammatory cellular infiltrates, or accumulations of macrophages or activated microglial cells were absent in the histological sections of any group examined. Importantly, no leptomeningeal infiltrates or tumors were found.
Table 1.
Histological Findings in IL-6 and IL-6/sIL-6Rα Animals
| Animal no. | Age (mo) | Liver | Lung | Kidney | Gliosis |
|---|---|---|---|---|---|
| IL-6-transgenics | |||||
| JR4 | 5.5 | + | + | + | + |
| JR5 | 7 | + | + | ++ | ++ |
| JR12 | 4 | − | ++ | ++ | − |
| JR13 | 2.5 | − | + | − | − |
| JR17 | 3 | − | − | − | − |
| JR18 | 3 | − | − | − | − |
| JR23 | 11 | − | ++ | ++ | − |
| JR24 | 11 | − | − | + | − |
| JR25 | 11 | + | ++ | ++ | − |
| JR28 | 3 | − | − | − | − |
| IL-6/sIL-6Rα transgenics | |||||
| JR2 | 5.5 | − | ++ | ++ | +++ |
| JR6 | 7 | + | + | + | +++ |
| JR8 | 2.5 | + | + | + | +++ |
| JR9 | 2.5 | + | ++ | − | ++ |
| JR11 | 4 | ++ | ++ | ++ | +++ |
| JR19 | 3.5 | − | ++ | − | ++ |
| JR20 | 3.5 | − | ++ | − | ++ |
| JR21 | 8 | ++ | +++ | + | ++ |
| JR22 | 8 | +++ | +++ | − | + |
| JR26 | 2 | + | ++ | − | ++ |
| JR27 | 2 | − | + | − | ++ |
| JR29 | 6 | ++ | +++ | +++ | +++ |
| JR30 | 6 | + | +++ | + | + |
| JR31 | 6 | +++ | +++ | ++ | +++ |
The scores were given as described in Material and Methods.
Notably, the prominent systemic effects such as liver damage and expansion of lymphoid or hematogenic progenitor cells typical for IL-6/IL-6Rα double-transgenic mice 45,47,49 did not parallel the development of gliosis in the brains of the IL-6/sIL-6Rα double-transgenic mice. For example, the 6-month-old mouse that suffered from splenomegaly and major intestinal infiltration of hematogenic precursor cells (JR22) had only minor astrogliosis. On the other hand, another IL-6/sIL-6Rα mouse 7 months of age (JR6) diagnosed with only minor liver damage was assigned strongly gliotic (Table 1) ▶ . Statistical analysis showed that there was no correlation between the degrees of infiltration of liver, lungs, and kidneys on the one hand and gliosis on the other hand in the group of IL-6/sIL-6Rα double-transgenic mice. Pearson’s coefficient analysis, Kruskal-Wallis one-way analysis of variance, and pairwise Kruskal-Wallis analysis did not show any significant correlation for all comparisons (gliosis versus liver, kidney, and lung damage).
Albumin immunohistochemistry of the brains of all mice examined in the present study did not reveal any leakage of albumin into the brain parenchyma, indicating that the BBB was primarily intact even in the IL-6/sIL-6Rα double-transgenic mice (Figure 1, e and f) ▶ .
Ultrastructural Alterations of the CNS
In the present study we focused on glial and BBB abnormalities in the CNS of IL-6 and IL-6/sIL-6Rα transgenic mice. Wild-type and sIL-6Rα transgenic mice served as controls. The capillary walls of these normal control mice (Figure 2a) ▶ , as well as of IL-6 (Figure 2b) ▶ and IL-6/sIL-6Rα (Figure 2, c and d) ▶ mice displayed no signs of endothelial damage such as fenestration or nuclear or cytoplasmic irregularities. There were no detectable alterations of endothelial tight junctions and no thickening or any other alteration of the vascular basement membranes in any mouse line. The glial sheath in the control mice looked normal with only minor focal hydropic changes (Figure 2a) ▶ . In IL-6 mice, swelling of astrocyte end-feet was moderate, if present at all. In contrast, perivascular astrocytes of IL-6/sIL-6Rα transgenics were swollen considerably (Figure 2, c and d) ▶ . These hydropic astrocytic processes were a very frequent finding. The degree of vascular luminal narrowing increased with the degree of vacuolization (Figure 2, c and d) ▶ .
Figure 2.

Electron micrographs of brain sections from different transgenic mouse lines. a: Brain capillary of wild-type control animal with normal endothelial cell, tight junctions, and basal lamina and only minor focal swelling of astroglial ensheathment (asterisk). b: Moderately hydropic astrocyte end-feet covering an endothelial cell in an IL-6 mouse. The astrocytic end-feet are moderately swollen (asterisk). c and d: Swollen astrocytic end-feet (asterisks) covering indented brain capillaries in IL-6/IL-6Rα double-transgenic mice. There are no major endothelial cell or basal lamina abnormalities. Scale bar, 0.5 μm (a–d).
Electron microscopically, no signs of neuronal damage such as chromatolysis, shrinkage, or apoptotic bodies in the gray matter were found in any group. There was no apparent activation of perivascular monocytic cells or microglial cells detectable on the electron microscopic level.
Discussion
Many neurological disorders are paralleled by changes in cytokine expression. 9 Mounting evidence implies that elevated IL-6 expression is rather a cause than a result of glial as well as neuronal responses. 51 We used single-transgenic mice overexpressing human IL-6 and sIL-6Rα and double-transgenic mice overexpressing IL-6/sIL-6Rα systemically to clarify the contribution of the different components of this ligand/receptor system. In IL-6 and sIL-6Rα single-transgenic mice, neurological symptoms were absent. As predicted, sIL-6Rα mice were normal, because mouse IL-6 does not crosstalk with human sIL-6Rα. We found only few GFAP-positive reactive astrocytes in IL-6 mice even though systemic effects of IL-6 overexpression including plasmacytoma development were prominent in these mice. In contrast, IL-6/sIL-6Rα double-transgenic mice had marked neurological symptoms and showed massive reactive gliosis in the context of severe general weakness. Because neuronal density was apparently not reduced on the histological level, a selective effect on astrocytes seems to be operative in these mice.
Typically, reactive gliosis was manifested by hypertrophy and hyperplasia of astrocytes similar to the GFAP-IL-6 mice reported previously. 27 GFAP-IL-6 mice suffered from tremor, ataxia, and seizures. Life expectancy of GFAP-IL-6 mice was approximately 6 months for high expressors and 12 months for low expressors, respectively. 27 Our IL-6/sIL-6Rα mice lived for 2 to 7 months until the onset of severe symptoms which were, however, caused predominantly by extracerebral alterations such as plasmacytomas and liver cell necrosis.
Nevertheless, the GFAP-IL-6 mice are inappropriate for studying the effects of systemically circulating IL-6 on astrocytes. The profound difference between the two models lies in the source of IL-6 production. In GFAP-IL-6 mice, IL-6 expression is tissue-selective, and ensuing damage is confined locally to the CNS. In our model, the distribution of IL-6 and sIL-6Rα is systemic. Transgene expression of cytokine and ligand takes place in different organs and outside the CNS. In this way the individual mediators can float freely and interact unbiased with each other. It is interesting to speculate that circulating IL-6 and sIL-6Rα have to be complexed first extracerebrally before passing the BBB to induce gliosis. The stronger phenotype in IL-6/sIL-6Rα double-transgenic mice might be because of potentiated IL-6 signaling. 45 In this way, IL-6 is capable of inducing a vigorous response in primarily less responsive cell populations including neuronal and glial cells. 33 This point is strengthened by the fact that sIL-6Rα mice had elevated IL-6 plasma levels and a prolonged plasma half-life of IL-6 when treated with exogenous IL-6. 45
The central role of astrocytes in inducing BBB properties has been illustrated by several studies in vivo 52 and in vitro. 53 Astrocytes and their conditioned media can induce BBB properties of the endothelium in vitro, and it was suggested that this effect is mediated by astroglia-secreted IL-6. 54 However, IL-6 is also capable of inducing disruption of the BBB in vitro 55 and in vivo. 32 In this light, the perturbing effects in GFAP-IL-6 mice on BBB formation as well as the induction of other vascular abnormalities such as neoangiogenesis 27 are not surprising. In contrast to normal mice where the BBB develops between day 7 and 14, formation of normal BBB was precluded in the GFAP-IL-6 transgenic mice. 32 To obtain an animal model in which BBB formation per se is not precluded, we took advantage of cross-breeding single-transgenic mouse strains to elucidate the individual contributions of the different mediators IL-6, sIL-6Rα, and IL-6/IL-6Rα on the morphological integrity of the BBB.
In our model the edematous swelling of perivascular astrocytes in IL-6/sIL-6Rα mice presumably represents a transformational step indicating damage, but not necessarily disruption of the BBB. Opening of the BBB can generally be ascribed to three possible mechanisms: 1) separation of the interendothelial tight junctions, 2) increased vesicular transport and the formation of transendothelial channels, and 3) biochemical and structural alteration of the endothelial cell plasma membrane resulting in increased permeability. 56 We found no endothelial fenestrations, no alterations in brain endothelial tight junctions, and no ultrastructural evidence for increased vesicular transport through cerebral capillaries in the IL-6/sIL-6Rα mice. Moreover, albumin immunohistochemistry, an established method to evaluate BBB integrity, 57,58 gave no indication of a vasogenic edema with altered BBB permeability and leakage of plasma constituents into the brain. 59 Thus, even high systemic levels of IL-6 and sIL-6Rα did not cause BBB breakdown in our mouse model. This result does not support the hypothesis that systemically elevated IL-6 is a major cause of BBB breakdown. 55,60 In line with our observations, IL-6 injected intravitreally did not cause a measurable increase in blood retinal barrier permeability, whereas tumor necrosis factor-α induced increased permeability. 61 In this context, it is also interesting to note that in mice overexpressing IL-6 in neurons because of an IL-6 transgene regulated by the neuron-specific enolase promoter, no vascular changes were detected in the brain. 31 Similar to our IL-6/IL-6Rα double-transgenic mice, astrogliosis was the major finding in the neuron-specific enolase-IL-6 mice. 31 These results suggest that systemic and even local cerebral overexpression of IL-6 does not cause BBB disruption. Apparently, IL-6 overexpression has to be directly targeted to astrocytes by use of a GFAP promoter, which leads to early and sustained cerebral IL-6 overexpression from development through adulthood preventing BBB formation. 32
The following hypothesis is proposed to explain the effects of systemically increased IL-6 and IL-6Rα on astrocytes. Blood-borne cytokines have been shown to cross the BBB by saturable transport systems. 62,63 Hence, it is conceivable that IL-6, akin to ciliary neurotrophic factor and tumor necrosis factor, 64 crossed the BBB by this mechanism. Consistent with our findings, granulocyte macrophage colony-stimulating factor crossed the BBB and blood-spinal cord barrier significantly faster than the control substance, albumin. 65 Thus, IL-6 and IL-6Rα conceivably penetrated the BBB in the double-transgenic mice even though signs of major BBB disruption were absent. In addition, examination of the role of IL-6 and sIL-6Rα in IL-6 induction in vitro showed that, while treatment of astrocytes with IL-6 and sIL-6Rα resulted in modest increases in IL-6 mRNA expression, co-treatment with either tumor necrosis factor-α or IL-1β plus IL-6/IL-6Rα lead to synergistic increases in IL-6 gene expression, 66 again highlighting the pivotal role of sIL-6Rα in determining the effects of IL-6 in astrocytes.
In the present study, systemic overexpression of IL-6 and sIL-6Rα induced liver and kidney damage as already reported previously. 35,45,47,49,50,67 Still, several findings indicate that the vigorous astrocytic response was caused by the systemically expressed and complexed IL-6/IL-6Rα transgene product and was not a consequence of the systemic diseases of the double-transgenic animals: the intensity of gliosis was not age-dependent, whereas the systemic consequences of IL-6/IL-6Rα overexpression increase with age. 50 In addition, there was no correlation between the extent of infiltration of liver, lung, and kidneys on the one hand and astrogliosis on the other hand. Moreover, IL-6 single-transgenic mice that also showed considerable plasmacytoma development, liver damage, and other extracerebral changes 50 displayed only minor, insignificant gliosis. Finally, no histologically or electron microscopically detectable neuronal damage was found in any animal, which should be present in the case of prominent metabolic or other prominent systemic damage affecting the brain. It is, however, important to note that we did not perform detailed stereological measurements to quantify neuronal numbers.
Surprisingly, the brains of the double-transgenic mice also seemed to be protected against plasmacytoma infiltration, because there was no necrosis and no infiltration of the brain by plasmacytoma infiltrates or precursor lesions, even though other organs (ie, liver, kidneys, lung) were heavily infiltrated in many double-transgenic animals. Leptomeningeal lymphocytic infiltrates or meningeal tumors were also absent in both IL-6 and IL-6/IL-6Rα overexpressing mice, suggesting that the elevated IL-6 levels found in patients with Castleman’s disease are at least not the sole cause of these meningeal alterations.
Acknowledgments
We thank Mrs. Astrid Knischewski and Mrs. H. Mader, Aachen, for technical assistance, and Dr. Dario Janigro, University of Washington, Seattle, WA, as well as Prof. R. Janzer, Division of Neuropathology, University of Lausanne, for helpful comments.
Footnotes
Address reprint requests to Prof. Dr. J. Weis, Abtlg. Neuropathologie, Pathologisches Institut der Universität, Murtenstr. 31, CH-3012 Bern, Switzerland. E-mail: weisj@patho.unibe.ch.
Supported by the Bernese Cancer League, the Dr. med. h. c. E. Braun Foundation, Basel, Switzerland, and the Interdisziplinäres Zentrum “CNS,” Rheinisch-Westfälische Technische Hochschule Aachen, Germany.
References
- 1.Akira S, Taga T, Kishimoto T: Interleukin-6 in biology and medicine. Adv Immunol 1993, 54:1-78 [DOI] [PubMed] [Google Scholar]
- 2.Rose-John S, Heinrich PC: Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J 1994, 300:281-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Hirano T, Taga T, Kishimoto T: Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J 1990, 4:2860-2867 [PubMed] [Google Scholar]
- 4.Yasukawa K, Hirano T, Watanabe Y, Muratani K, Matsuda T, Nakai S, Kishimoto T: Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J 1987, 6:2939-2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawada M, Suzumura A, Marunouchi T: Cytokine network in the central nervous system and its roles in growth and differentiation of glial and neuronal cells. Int J Dev Neurosci 1995, 13:253-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadient RA, Otten U: Identification of interleukin-6 (IL-6)-expressing neurons in the cerebellum and hippocampus of normal adult rats. Neurosci Lett 1994, 182:243-246 [DOI] [PubMed] [Google Scholar]
- 7.Thier M, Marz P, Otten U, Weis J, Rose-John S: Interleukin-6 (IL-6) and its soluble receptor support survival of sensory neurons. J Neurosci Res 1999, 55:411-422 [DOI] [PubMed] [Google Scholar]
- 8.Marz P, Cheng JG, Gadient RA, Patterson PH, Stoyan T, Otten U, Rose-John S: Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci USA 1998, 95:3251-3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benveniste EN: Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol 1992, 263:C1-C16 [DOI] [PubMed] [Google Scholar]
- 10.Gadient RA, Otten UH: Interleukin-6 (IL-6)—a molecule with both beneficial and destructive potentials. Prog Neurobiol 1997, 52:379-390 [DOI] [PubMed] [Google Scholar]
- 11.Marz P, Herget T, Lang E, Otten U, Rose-John S: Activation of gp130 by IL-6/soluble IL-6 receptor induces neuronal differentiation. Eur J Neurosci 1997, 9:2765-2773 [DOI] [PubMed] [Google Scholar]
- 12.Hirota H, Kiyama H, Kishimoto T, Taga T: Accelerated nerve regeneration in mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J Exp Med 1996, 183:2627-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodroofe MN, Cuzner ML: Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine 1993, 5:583-588 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez M, Pavelko KD, McKinney CW, Leibowitz JL: Recombinant human IL-6 suppresses demyelination in a viral model of multiple sclerosis. J Immunol 1994, 153:3811-3821 [PubMed] [Google Scholar]
- 15.Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P: Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 1995, 202:17-20 [DOI] [PubMed] [Google Scholar]
- 16.Lichtor T, Dohrmann GJ, Gurney ME: Cytokine gene expression by human gliomas. Neurosurgery 1990, 26:788-792 [DOI] [PubMed] [Google Scholar]
- 17.Giometto B, Bozza F, Faresin F, Alessio L, Mingrino S, Tavolato B: Immune infiltrates and cytokines in gliomas. Acta Neurochir Wien 1996, 138:50-56 [DOI] [PubMed] [Google Scholar]
- 18.Candi E, Knight RA, Spinedi A, Guerrieri P, Melino G: A possible growth factor role of IL-6 in neuroectodermal tumours. J Neurooncol 1997, 31:115-122 [DOI] [PubMed] [Google Scholar]
- 19.Severson GS, Harrington DS, Weisenburger DD, McComb RD, Casey JH, Gelber BR, Varet B, Abelanet R, Rappaport HH: Castleman’s disease of the leptomeninges. Report of three cases. J Neurosurg 1988, 69:283-286 [DOI] [PubMed] [Google Scholar]
- 20.Donaghy M, Hall P, Gawler J, Gregson NA, Leibowitz S, Jitpimolmard S, King RH, Thomas PK: Peripheral neuropathy associated with Castleman’s disease. J Neurol Sci 1989, 89:253-267 [DOI] [PubMed] [Google Scholar]
- 21.Gherardi RK, Belec L, Fromont G, Divine M, Malapert D, Gaulard P, Degos JD: Elevated levels of interleukin-1 beta (IL-1 beta) and IL-6 in serum and increased production of IL-1 beta mRNA in lymph nodes of patients with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome. Blood 1994, 83:2587-2593 [PubMed] [Google Scholar]
- 22.Hashimoto H, Iida J, Hironaka Y, Sakaki T: Intracranial Castleman’s disease of solitary form. Case report. J Neurosurg 1999, 90:563-566 [DOI] [PubMed] [Google Scholar]
- 23.Hitoshi S, Suzuki K, Sakuta M: Elevated serum interleukin-6 in POEMS syndrome reflects the activity of the disease. Intern Med 1994, 33:583-587 [DOI] [PubMed] [Google Scholar]
- 24.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW: Dysregulated interleukin 6 expression produces a syndrome resembling Castleman’s disease in mice. J Clin Invest 1990, 86:592-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kepes JJ, Chen WY, Connors MH, Vogel FS: “Chordoid” meningeal tumors in young individuals with peritumoral lymphoplasmacellular infiltrates causing systemic manifestations of the Castleman syndrome. A report of seven cases. Cancer 1988, 62:391-406 [DOI] [PubMed] [Google Scholar]
- 26.Campbell IL: Structural and functional impact of the transgenic expression of cytokines in the CNS. Ann NY Acad Sci 1998, 840:83-96 [DOI] [PubMed] [Google Scholar]
- 27.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L: Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA 1993, 90:10061-10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang CS, Stalder A, Samimi A, Campbell IL: Reactive gliosis as a consequence of interleukin-6 expression in the brain: studies in transgenic mice. Dev Neurosci 1994, 16:212-221 [DOI] [PubMed] [Google Scholar]
- 29.Klein MA, Moller JC, Jones LL, Bluethmann H, Kreutzberg GW, Raivich G: Impaired neuroglial activation in interleukin-6 deficient mice. Glia 1997, 19:227-233 [DOI] [PubMed] [Google Scholar]
- 30.Winter CG, Saotome Y, Levison SW, Hirsh D: A role for ciliary neurotrophic factor as an inducer of reactive gliosis, the glial response to central nervous system injury. Proc Natl Acad Sci USA 1995, 92:5865-5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fattori E, Lazzaro D, Musiani P, Modesti A, Alonzi T, Ciliberto G: IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur J Neurosc 1995, 7:2441-2449 [DOI] [PubMed] [Google Scholar]
- 32.Brett FM, Mizisin AP, Powell HC, Campbell IL: Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J Neuropathol Exp Neurol 1995, 54:766-775 [DOI] [PubMed] [Google Scholar]
- 33.Marz P, Otten U, Rose-John S: Neuronal activities of IL-6-type cytokines often depend on soluble cytokine receptors. Eur J Neurosci 1999, 11:2995-3004 [DOI] [PubMed] [Google Scholar]
- 34.Hirota H, Yoshida K, Kishimoto T, Taga T: Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA 1995, 92:4862-4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fattori E, Della Rocca C, Costa P, Giorgio M, Dente B, Pozzi L, Ciliberto G: Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood 1994, 83:2570-2579 [PubMed] [Google Scholar]
- 36.Peters M, Meyer zum Buschenfelde KH, Rose-John S: The function of the soluble IL-6 receptor in vivo. Immunol Lett 1996, 54:177-184 [DOI] [PubMed] [Google Scholar]
- 37.Brinster RL, Allen JM, Behringer RR, Gelinas RE, Palmiter RD: Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA 1988, 85:836-840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGrane MM, de Vente J, Yun J, Bloom J, Park E, Wynshaw-Boris A, Wagner T, Rottman FM, Hanson RW: Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem 1988, 263:11443-11451 [PubMed] [Google Scholar]
- 39.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 40.Van Snick J, Cayphas S, Vink A, Uyttenhove C, Coulie PG, Rubira MR, Simpson RJ: Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci USA 1986, 83:9679-9683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aarden LA, De Groot ER, Schaap OL, Lansdorp PM: Production of hybridoma growth factor by human monocytes. Eur J Immunol 1987, 17:1411-1416 [DOI] [PubMed] [Google Scholar]
- 42.Schroder JM, Mayer M, Weis J: Mitochondrial abnormalities and intrafamilial variability of sural nerve biopsy findings in adrenomyeloneuropathy. Acta Neuropathol (Berl) 1996, 92:64-69 [DOI] [PubMed] [Google Scholar]
- 43.Fisher RA, Yates F: Statistical Tables for Biological, Agricultural and Medical Research, ed 6 1982, Longman, Harlow
- 44.Conover WJ: Practical Nonparametric Statistics, ed 2 1980, John Wiley, New York
- 45.Peters M, Jacobs S, Ehlers M, Vollmer P, Mullberg J, Wolf E, Brem G, Meyer zum Buschenfelde KH, Rose-John S: The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med 1996, 183:1399-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aschner M: The functional significance of brain metallothioneins. FASEB J 1996, 10:1129-1136 [DOI] [PubMed] [Google Scholar]
- 47.Peters M, Odenthal M, Schirmacher P, Blessing M, Fattori E, Ciliberto G, Meyer zum Buschenfelde KH, Rose-John S: Soluble IL-6 receptor leads to a paracrine modulation of the IL-6-induced hepatic acute phase response in double transgenic mice. J Immunol 1997, 159:1474-1481 [PubMed] [Google Scholar]
- 48.Hanson RW, Reshef L: Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 1997, 66:581-611 [DOI] [PubMed] [Google Scholar]
- 49.Peters M, Schirmacher P, Goldschmitt J, Odenthal M, Peschel C, Fattori E, Ciliberto G, Dienes HP, Meyer zum Buschenfelde KH, Rose-John S: Extramedullary expansion of hematopoietic progenitor cells in interleukin (IL)-6-sIL-6R double transgenic mice. J Exp Med 1997, 185:755-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schirmacher P, Peters M, Ciliberto G, Blessing M, Lotz J, Meyer zum Buschenfelde KH, Rose-John S: Hepatocellular hyperplasia, plasmacytoma formation, and extramedullary hematopoiesis in interleukin (IL)-6/soluble IL-6 receptor double-transgenic mice. Am J Pathol 1998, 153:639-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gruol DL, Nelson TE: Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol 1997, 15:307-339 [DOI] [PubMed] [Google Scholar]
- 52.Janzer RC, Raff MC: Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 1987, 325:253-257 [DOI] [PubMed] [Google Scholar]
- 53.Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H: Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia 1997, 19:13-26 [PubMed] [Google Scholar]
- 54.Persidsky Y, Gendelman HE: Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J Leukoc Biol 1997, 62:100-106 [DOI] [PubMed] [Google Scholar]
- 55.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J: The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol 1996, 64:37-43 [DOI] [PubMed] [Google Scholar]
- 56.Miller JD, Ironside JW: Raised intracranial pressure, oedema and hydrocephalus. Greenfield’s Neuropathology, volume 1, ed 7. Edited by DI Graham, PL Lantos PL. London, Arnold, 1997, pp 157–196
- 57.Sokrab TE, Kalimo H, Johansson BB: Parenchymal changes related to plasma protein extravasation in experimental seizures. Epilepsia 1990, 31:1-8 [DOI] [PubMed] [Google Scholar]
- 58.Sokrab TE, Johansson BB, Kalimo H, Olsson Y: A transient hypertensive opening of the blood-brain barrier can lead to brain damage. Extravasation of serum proteins and cellular changes in rats subjected to aortic compression. Acta Neuropathol (Berl) 1988, 75:557-565 [DOI] [PubMed] [Google Scholar]
- 59.Milhorat TH: Classification of the cerebral edemas with reference to hydrocephalus and pseudotumor cerebri. Childs Nerv Syst 1992, 8:301-306 [DOI] [PubMed] [Google Scholar]
- 60.Duchini A: The role of central nervous system endothelial cell activation in the pathogenesis of hepatic encephalopathy. Med Hypotheses 1996, 46:239-244 [DOI] [PubMed] [Google Scholar]
- 61.Bamforth SD, Lightman S, Greenwood J: The effect of TNF-alpha and IL-6 on the permeability of the rat blood-retinal barrier in vivo. Acta Neuropathol (Berl) 1996, 91:624-632 [DOI] [PubMed] [Google Scholar]
- 62.Banks WA, Kastin AJ, Gutierrez EG: Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett 1994, 179:53-56 [DOI] [PubMed] [Google Scholar]
- 63.Banks WA, Kastin AJ, Broadwell RD: Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2:241-248 [DOI] [PubMed] [Google Scholar]
- 64.Pan W, Kastin AJ, Maness LM, Brennan JM: Saturable entry of ciliary neurotrophic factor into brain. Neurosci Lett 1999, 263:69-71 [DOI] [PubMed] [Google Scholar]
- 65.McLay RN, Banks WA, Kastin AJ: Granulocyte macrophage-colony stimulating factor crosses the blood-testis barrier in mice. Biol Reprod 1997, 57:822-826 [DOI] [PubMed] [Google Scholar]
- 66.Van Wagoner NJ, Oh JW, Repovic P, Benveniste EN: Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J Neurosci 1999, 19:5236-5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maione D, Di Carlo E, Li W, Musiani P, Modesti A, Peters M, Rose-John S, Della Rocca C, Tripodi M, Lazzaro D, Taub R, Savino R, Ciliberto G: Coexpression of IL-6 and soluble IL-6R causes nodular regenerative hyperplasia and adenomas of the liver. EMBO J 1998, 17:5588-5597 [DOI] [PMC free article] [PubMed] [Google Scholar]


