Abstract
The proto-oncogene KIT encodes the receptor tyrosine kinase KIT. Gain-of-function mutations in the juxtamembrane domain of KIT have been reported in human gastrointestinal stromal tumors. In a family with multiple gastrointestinal stromal tumors and diffuse hyperplasia of myenteric plexus layer, we have identified another mutation of KIT, a single base mutation, resulting in the substitution of Glu for Lys642 in the kinase I domain, and studied its biological effect in a cellular system. The mouse homologue of the human KIT mutant was generated by site-directed mutagenesis and stably transfected into the interleukin-3-dependent Ba/F3 murine cell line. The oncogenic potential of the mutated KIT was assessed in vitro by a proliferation assay and in vivo by transplantation into nude mice. Transfected Ba/F3 cells grew autonomously in absence of growth factors and formed tumors in nude mice. Substitution of Glu for Lys642 is an oncogenic mutation in the tyrosine kinase domain of KIT. As germline heterozygous mutation, it causes a diffuse hyperplasia of myenteric interstitial cells of Cajal during embryonic development and occurrence of multiple gastrointestinal stromal tumors at adulthood.
The KIT proto-oncogene is the cellular homologue of the oncogene v-kit present in the genome of Hardy-Zuckerman 4-feline sarcoma virus. 1 It encodes the transmembrane receptor tyrosine kinase KIT that is member of the same subfamily as the receptors for platelet-derived growth factor and for colony-stimulating factor 1. 2,3 KIT consists of an extracellular domain with five immunoglobulin-like repeats, a transmembrane domain, a juxtamembrane domain, and a kinase domain separated into two parts (I and II) by an insert. 2-4 The ligand for KIT is stem cell factor (SCF). 5 The SCF-KIT system plays crucial roles for the development of melanocytes, erythrocytes, germ cells, mast cells, 4 and interstitial cells of Cajal (ICCs). 6 ICCs are mesenchymal cells, closely associated with nerves and smooth muscle cells of the gastrointestinal (GI) tract. 7 Absence of the SCF-KIT signaling pathway causes the lack of ICCs surrounding the myenteric plexus (ICCs-MP) and the lack of pacemaker component (electrical slow waves) in stomach and intestine. 8,9
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the human GI tract. A majority of GISTs are KIT-immunoreactive 10,11 and several mutations in the juxtamembrane domain of KIT have been reported in GISTs. These mutations cause a constitutive, ligand-independent, activation of KIT responsible for their oncogenic potential. 10,12 Furthermore, a germline mutation in the juxtamembrane domain of KIT gene has been identified in familial and multiple GISTs. 13 We have studied a family with multiple GISTs and diffuse hyperplasia of ICCs-MP in the gut unaffected by GISTs. No mutation was found in the juxtamembrane domain, but a novel mutation in the kinase domain I was identified, and we further investigated the functional aspects of this mutant.
Materials and Methods
Patients
A French woman and her son (67 and 40 years old, respectively) presented with multiple (20 and 13 tumors, respectively) macroscopic GISTs, measuring from 1 to 8 cm, in duodenum and jejunum. All tumors examined were of low malignancy grade (ie, mitotic index <1 mitosis per 10 high-power fields) and in both cases there was no metastasis. There was no familial history of von Recklinghausen’s complex, multiple endocrine neoplasia, or intestinal ganglioneuromatosis. A detailed report of the cases will appear elsewhere. Tissues from GISTs, intestinal mucosa unaffected by GISTs, and normal pancreatic tissues were obtained from both patients as surgical waste at the time of pancreato-duodenectomy performed to relieve intestinal obstruction.
Preparation of Complementary DNA, Genomic DNA, and Sequencing
RNA was extracted from frozen tissues by a RNA extraction kit (RNeasy Mini Kit, Qiagen, Valencia, CA). Single-strand complementary DNA (cDNA) was synthesized by AMV reverse transcriptase (Boehringer Mannheim, Mannheim, Germany). The double-strand cDNA was then amplified by polymerase chain reaction (PCR) and sequenced, directly or after subcloning into Bluescript I KS(−), as described. 10 Genomic DNA was isolated from frozen GISTs by the use of NaOH boiling preps. The genomic DNA was amplified by PCR using the forward primer (GTCGCTGTAAAGATGCTCAAG) in the exon 12 and the reverse primer (TAGCAAGAGAGAACAACAGT) in the intron 13 of human KIT DNA, and was sequenced after subcloning into Bluescript I KS(−).
Construction and Transfection of Murine Mutant-Type KIT cDNA
Generation of the murine counterpart of the human KIT mutant cDNA was carried out by site-directed mutagenesis as described. 14 Stable transfection into the interleukin-3 (IL-3)-dependent murine lymphoid Ba/F3 cell line and selection of transfectant clones by the method of limiting dilution were performed as described. 10 Mouse wild-type KIT cDNA and two mutated KIT cDNAs encoding well-characterized constitutively activated KIT mutants, an in-frame deletion of 6 bp encoding Val-559-Val-560 (KITdel559–560) in the juxtamembrane domain and a substitution of Asp-816 to Val (KIT816Val) in the kinase domain II respectively, were also expressed in Ba/F3 cells as controls.
Immunoprecipitation and Immunoblotting
Ba/F3 clones expressing the various forms of KIT were cultivated with or without SCF (0.1 μg/ml) for 10 minutes. Cell lysates were immunoprecipitated with rat monoclonal antibody raised against mouse KIT (clone 2B8, Pharmingen, San Diego, CA). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotting was performed with anti-phosphotyrosine mouse monoclonal antibody (4G10, Transduction Laboratories, Lexington, KY) as described. 14
In Vitro Proliferation Assay
[3-(4,5-Dimethylthiazol-2-yl)−2, 5-diphenyl tetrazolium bromide] (MTT; Sigma, St. Louis, MO) colorimetric assay was performed as described. 15 Briefly, cells were cultured with various concentrations of recombinant mouse (rm) IL-3 or rmSCF for 48 hours. Cells were further cultured for 4 hours in the presence of MTT (5 mg/ml). Reaction was stopped by adjunction of acid isopropanol and the optical density at a wavelength of 540 nm was measured on a Titertek Multiskan MCC/340 (ICN Biomedicals, Costa Mesa, CA).
Tumorigenicity Assay in Vivo
Tumorigenicity assay was performed as described 15 after approval by the Veterinary Committee of the Faculty of Medicine, Université Libre de Bruxelles (Brussels, Belgium). The various transfectants and original Ba/F3 cells were injected subcutaneously into the posterior flank of nude mice (Iffa-Credo, Brussels, Belgium), 5 animals in each group. The size of tumors was measured with the vernier caliper every 4 days. Tumor volume was calculated with the formula: tumor volume = 0.5 × a × b2, where a and b are the length and width in millimeters, respectively, of the tumoral mass. Animals were euthanized between 3 and 5 weeks after injection, depending on tumor growth rate and animal health.
Results
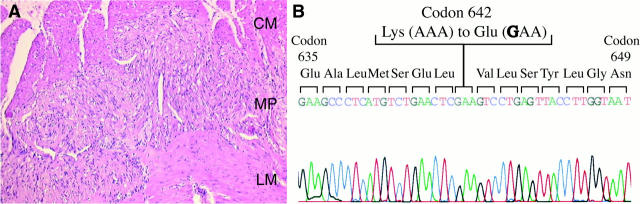
Mother and son both showed multiple GISTs but also a diffuse hyperplasia of spindle-shaped cells in the MP region along the GI tract unaffected by GISTs (Figure 1A) ▶ . GISTs, as well as ICCs in the unaffected gut, were KIT-immunoreactive (data not shown).
Figure 1.
Human familial and multiple GISTs. A: Paraffin-embedded section (stained with hematoxylin and eosin) of intestine unaffected by GISTs, showing diffuse hyperplasia of spindle-shaped cells in MP region. Original magnification, ×200. CM, circular muscle layer; MP, myenteric plexus; LM, longitudinal muscle layer. B: Mutation identified in this family: KIT642Glu, a substitution-type mutation at codon 642 (Lys to Glu) in the kinase domain I of KIT.
The whole coding region of KIT cDNAs was obtained from GISTs of both patients. Ten independent KIT cDNA clones were sequenced for each patient. A point mutation (AAA → GAA), resulting in a Lys-to-Glu substitution at codon 642 of the kinase domain I of KIT (KIT642Glu) was identified (Figure 1B) ▶ . The same mutation was also present in genomic DNAs extracted from GISTs of both patients. No other mutation in the KIT sequence was detected. Approximately half of the cDNA and genomic DNA clones from the GISTs showed the KIT642Glu mutation, whereas the remaining clones showed the normal KIT sequence, indicating that only one allele of the KIT gene carried the mutation. This was confirmed by direct sequencing of reverse-transcription PCR products (data not shown). Furthermore, the KIT642Glu mutation, together with the wild-type KIT form, was also present in DNAs extracted from normal pancreas tissues from both patients, indicating the germline and heterozygous nature of the KIT642Glu mutant present in this family.
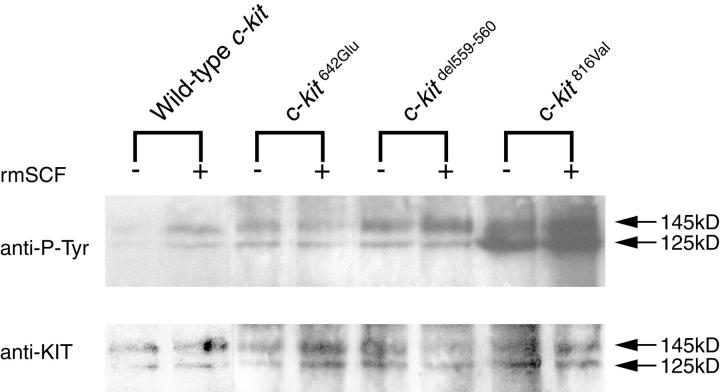
Activation of the KIT signaling pathway was evaluated by KIT autophosphorylation after immunoprecipitation and immunoblotting (Figure 2) ▶ . In Ba/F3 cells expressing wild-type KIT, strong tyrosine phosphorylation of KIT occurred only after stimulation by rmSCF. In contrast, tyrosine residues of products of KITdel559–560 and KIT816Val were phosphorylated even without stimulation by rmSCF, indicating the constitutive (ligand-independent) activation of these mutants. The magnitude of constitutive tyrosine phosphorylation was greater in Ba/F3 cells expressing KIT816Val than in cells expressing KITdel559–560. In Ba/F3 cells expressing KIT642Glu, tyrosine residues of KIT were also phosphorylated independently of rmSCF, at a level similar to that conferred by KITdel559–560.
Figure 2.
Constitutive tyrosine phosphorylation of KIT642Glu. In Ba/F3 cells expressing wild-type KIT, strong tyrosine phosphorylation of KIT occurred only after stimulation by rmSCF. In contrast, KITdel559–560 and KIT816Val were phosphorylated even without stimulation by rmSCF, indicating the ligand-independent (constitutive) activation of these mutants. The constitutive tyrosine phosphorylation of KIT816Val was greater than that of KITdel559–560. Tyrosine residues of KIT642Glu were also phosphorylated independently of rmSCF, at a level similar to that of wild-type KIT after stimulation by rmSCF and of KITdel559–560.
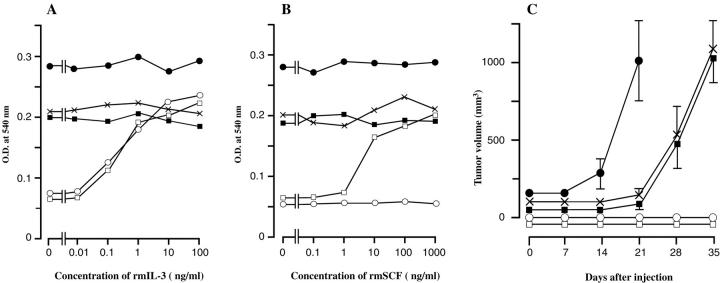
Cell proliferation was evaluated by MTT colorimetric assay (Figure 3, A and B) ▶ . Native Ba/F3 cells, which do not express KIT, proliferated in the presence of rmIL-3 but not with rmSCF. Ba/F3 cells transfected with the wild-type KIT grew in presence of either rmIL-3 or rmSCF. Ba/F3 cells expressing the constitutively activated mutants KITdel559–560 or KIT816Val grew autonomously (although at different rates), even in the absence of rmIL-3 or rmSCF. Ba/F3 cells transfected with KIT642Glu also grew autonomously without rmIL-3 and rmSCF, at a rate comparable with that of Ba/F3 cells transfected with KITdel559–560.
Figure 3.
Autonomous proliferation of Ba/F3 cells expressing KIT642Glu in vitro (A and B) and in nude mice (C). A: MTT colorimetric assay in the presence or absence of rmIL-3. B: MTT colorimetric assay in presence or absence of rmSCF. Optical density at 540 nm expressed as means of four wells. C: Size of tumors developed in nude mice (n = 5) after injection of Ba/F3 clones expressing various forms of murine KIT. Tumor volume is stated in mm 3 ± SE. Ba/F3 cells expressing KIT642Glu showed factor-independent growth and tumorigenicity comparable to Ba/F3 cells expressing KITdel559–560, an activating mutation of the juxtamembrane domain found in GISTs. Ba/F3 cells expressing KIT816Val, a mutant found in mast cell leukemia, showed a higher proliferation rate. Native Ba/F3 cells and Ba/F3 cells expressing wild-type KIT thrived only in presence of growth factors and did not form tumors in nude mice. Native Ba/F3 cells (○), Ba/F3 cells expressing wild-type KIT (□), Ba/F3 cells expressing KIT816Val (•, Ba/F3 cells expressing KITdel559–560 (▪), Ba/F3 cells expressing KIT642Glu (×).
The autonomous growth conferred by expression of KIT642Glu in Ba/F3 cells was confirmed by the tumorigenicity assay in nude mice (Figure 3 ▶ C). Original Ba/F3 cells and Ba/F3 cells expressing wild-type KIT did not form tumors. Ba/F3 cells expressing KITdel559–560 or KIT816Val mutants caused formation of tumors in nude mice. Ba/F3 cells expressing KIT642Glu also resulted in tumor formation, at a rate comparable with that of Ba/F3 cells expressing KITdel559–560 .
Discussion
We have identified a mutation in the tyrosine kinase domain I of the KIT gene, KIT642Glu, in members of a family with multiple GISTs and demonstrated that the resulting single amino acid substitution confers a constitutive (ie, ligand-independent) activity and oncogenic properties to KIT. Very recently, KIT642Glu has been reported in two sporadic cases of GISTs. 16 KIT642Glu caused constitutive tyrosine phosphorylation of KIT, but biological effects were not further investigated. As the mutation was encountered only in tumors (ie, as a somatic mutation) and in homozygous form, it was postulated that the constitutive activity of KIT caused by KIT642Glu would be milder than that of mutations in the juxtamembrane domain and that loss of the wild-type allele would be required for tumor formation. 16 Conversely, our results indicate that the level of constitutive activation of KIT conferred by KIT642Glu in the Ba/F3 model is similar to that of activating mutations in the juxtamembrane domain of KIT previously identified in GISTs. Furthermore, the presence of a germline KIT642Glu of only one allele of KIT gene is sufficient to induce the occurrence of multiples GISTs in the proximal gut at adulthood. Germline-activating mutations in the juxtamembrane domain of KIT reported in other familial multiple GISTs were also heterozygous. 13,17
The relationship between GISTs and ICCs has been postulated since KIT immunoreactivity was identified in a majority of GISTs. 10,18-20 The presence of KIT is an essential characteristics of ICCs throughout life. 7,21 ICCs-MP in stomach and intestine are dependant of the SCF-KIT signaling for their development and function. 7 Furthermore, differentiation and survival of embryonic ICCs in vitro also requires SCF. 22 Marked hyperplasia of KIT-positive cells in the MP layer of the GI tract unaffected by GISTs is a striking feature of our patients. This is reminiscent of another family with multiple GISTs, diffuse hyperplasia of KIT-positive spindle-shaped cells in the MP region 23 and a germline mutation in juxtamembrane domain of KIT. 17 It is noteworthy that in both families, hyperplasia of KIT-positive spindle-shaped cells was observed only in MP layer. KIT-positive ICCs in the deep muscular plexus layer (ICCs-DMP) appeared unaffected. In mouse, ICCs-DMP do not dependent on SCF-KIT signaling for their development. 7 Therefore, the presence of germline-activating mutations of KIT in both families indicates that constitutive activation of the KIT signaling pathway during development lead to both hyperplastic development of ICCs-MP and occurrence of GISTs in adulthood. A similar mechanism has been demonstrated for germline-activating mutations of another receptor tyrosine kinase, the proto-oncogene c-ret, which is involved in hyperplasia of the C cells of the thyroid gland and development of multiple endocrine neoplasia (MEN) syndromes. 24,25 Whether the hyperplastic cells observed in the GI tract of patients with germline-activating mutations of KIT gene fulfill the ultrastructural criteria of mature ICCs or of immature precursors remains to be determined but taken together, these observations favor the hypothesis of a possible common lineage for ICCs-MP and GISTs.
Intriguingly, germline-activating mutations of KIT apparently have little, if any, influence on melanocytes, which, like ICCs-MP, are dependent on the SCF-KIT pathway for their development. 26 Some hyperpigmentation of the perineum has been reported in familial GISTs with gain-of-function mutation in the juxtamembrane domain of KIT. This feature was attributed without investigation to hyperplasia of melanocytes. 13 Our patients did not show any abnormal pigmentation. These results suggest that germline-activating mutations in the juxtamembrane domain and in the kinase domain I of KIT may have different transduction pathways in various cell lineages.
Activating mutations in the juxtamembrane domain of KIT have been previously identified in a number of KIT-positive GISTs. 10 Activating mutations in the juxtamembrane domain of KIT have been previously identified in a number of KIT-positive GISTs. 10 The recent identification of mutations in the extracellular domain and tyrosine kinase domain of KIT 16 suggests that the prevalence of KIT mutations in GISTs is very high and emphasizes the pivotal role of the proto-oncogene KIT in the ontogeny of GISTs. Mutations of KIT may be of prognosis value in GISTs 27-29 but, so far, only mutations in the juxtamembrane domain have been investigated. Reappraisal should now take into account mutations occurring in the whole coding sequence of KIT and their somatic or germinal as well as homozygous or heterozygous nature, as these factors may differentially influence the behavior of GISTs.
Acknowledgments
We thank Jean-Louis Conreur, Karine Gillard, and Huy Nguyen Tran for skillful technical assistance.
Footnotes
Address reprint requests to Jean-Marie Vanderwinden, M.D., Ph.D., Laboratoire de Neurophysiologie, Faculté de Médecine, Campus Erasme, CP 601, Université Libre de Bruxelles, 808 route de Lennik, B-1070 Brussels, Belgium. E-mail: jmvdwin@ulb.ac.be.
Supported by grants from Fonds de la Recherche Scientifique Médicale, Belgium (3.4551.98 and 3.4618.99), the Fondation Médicale Reine Elisabeth, Belgium (Neurobiology 1999–2001), and the Ministery of Education, Science, Culture and Sports of Japan. K. I. is a Research Fellow of the Japan Society for the Promotion of Science. J. M. V. is a Research Associate of the National Fund for Scientific Research (Belgium).
References
- 1.Besmer P, Lader E, George PC, Bergold PJ, Qiu FH, Zuckerman EE, Hardy WD: A new acute transforming feline retrovirus with fms homology specifies a C-terminally truncated version of the c-fms protein that is different from SM-feline sarcoma virus v-fms protein. J Virol 1986, 60:194-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarden Y, Kuang WJ, Yang FT, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A: Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 1987, 6:3341-3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu FH, Ray P, Brown K, Barker PE, Jhanwar S, Ruddle FH, Besmer P: Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family: oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J 1988, 7:1003-1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geissler EN, Ryan MA, Housman DE: The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell 1988, 55:185-192 [DOI] [PubMed] [Google Scholar]
- 5.Williams DE, Eisenman J, Baird A, Rauch C, Van Ness K, March CJ, Park LS, Martin U, Mochizuki DY, Boswell HS: Identification of a ligand for the c-kit proto-oncogene. Cell 1990, 63:167-174 [DOI] [PubMed] [Google Scholar]
- 6.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K: Requirement of c-kit for development of intestinal pacemaker system. Development 1992, 116:369-375 [DOI] [PubMed] [Google Scholar]
- 7.Sanders KM, Ordög T, Koh SD, Torihashi S, Ward SM: Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil 1999, 11:311-338 [DOI] [PubMed] [Google Scholar]
- 8.Ward SM, Burns AJ, Torihashi S, Sanders KM: Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol (Lond) 1994, 480:91-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A: W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995, 373:347-349 [DOI] [PubMed] [Google Scholar]
- 10.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad-Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y: Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279:577-580 [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Sarlomo-Rikala M, Lasota J: Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999, 30:1213-1220 [DOI] [PubMed] [Google Scholar]
- 12.Nakahara M, Isozaki K, Hirota S, Miyagawa JI, Hase-Sawada N, Taniguchi M, Nishida T, Kanayama S, Kitamura Y, Shinomura Y, Matsuzawa Y: A novel gain-of-function mutation of c-kit gene in gastrointestinal stromal tumors. Gastroenterology 1998, 115:1090-1095 [DOI] [PubMed] [Google Scholar]
- 13.Nishida T, Hirota S, Taniguchi M, Hashimoto K, Isozaki K, Nakamura H, Kanakura Y, Tanaka T, Takabayashi A, Matsuda H, Kitamura Y: Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet 1998, 19:323-324 [DOI] [PubMed] [Google Scholar]
- 14.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, Sugahara H, Butterfield JH, Ashman LK, Kanayama Y: Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest 1993, 92:1736-1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujimura T, Morimoto M, Hashimoto K, Moriyama Y, Kitayama H, Matsuzawa Y, Kitamura Y, Kanakura Y: Constitutive activation of c-kit in FMA3 murine mastocytoma cells caused by deletion of seven amino acids at the juxtamembrane domain. Blood 1996, 87:273-283 [PubMed] [Google Scholar]
- 16.Lux ML, Rubin BP, Biase TL, Chen CJ, Maclure T, Demetri G, Xiao S, Singer S, Fletcher CD, Fletcher JA: KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 2000, 156:791-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirota S, Okazaki T, Kitamura Y, O’Brien P, Kapusta L, Dardick I: Cause of familial and multiple gastrointestinal autonomic nerve tumors with hyperplasia of interstitial cells of Cajal is germline mutation of the c-kit gene (letter). Am J Surg Pathol 2000, 24:326-327 [DOI] [PubMed] [Google Scholar]
- 18.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM: Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998, 152:1259-1269 [PMC free article] [PubMed] [Google Scholar]
- 19.Sakurai S, Fukasawa T, Chong JM, Tanaka A, Fukayama M: C-kit gene abnormalities in gastrointestinal stromal tumors (tumors of interstitial cells of Cajal. Jpn J Cancer Res 1999, 90:1321-1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH: Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol 1999, 23:377-389 [DOI] [PubMed] [Google Scholar]
- 21.Vanderwinden JM, Rumessen JJ: Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech 1999, 47:344-360 [DOI] [PubMed] [Google Scholar]
- 22.Wu JJ, Rothman TP, Gershon MD: Development of the interstitial cell of Cajal: origin, Kit dependence and neuronal and nonneuronal sources of Kit ligand. J Neurosci Res 2000, 59:384-401 [DOI] [PubMed] [Google Scholar]
- 23.O’Brien P, Kapusta L, Dardick I, Axler J, Gnidec A: Multiple familial gastrointestinal autonomic nerve tumors and small intestinal neuronal dysplasia. Am J Surg Pathol 1999, 23:198-204 [DOI] [PubMed] [Google Scholar]
- 24.Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L: Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993, 363:458-460 [DOI] [PubMed] [Google Scholar]
- 25.Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, Pasini B, Hoppener JW, van Amstel HK, Romeo G: A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma (see comments). Nature 1994, 367:375-376 [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S, Kunisada T, Era T, Sakakura T: In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit dependency during melanocyte development. EMBO J 1991, 10:2111-2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst SI, Hubbs AE, Przygodzki RM, Emory TS, Sobin LH, O’Leary TJ: KIT mutation portends poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest 1998, 78:1633-1636 [PubMed] [Google Scholar]
- 28.Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M: Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol 1999, 154:53-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y: Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res 1999, 59:4297-4300 [PubMed] [Google Scholar]