Abstract
Increasing evidence suggests that tachykinins are involved in the control of pathophysiological states, such as inflammation. The precise localization of tachykinin receptors is of paramount importance in the search for their possible physiological and pathological role; in this study, therefore, we attempted to define cellular sites of substance P (NK-1R) and neurokinin A (NK-2R) receptor expression in the healthy and the inflamed human intestine by in situ hybridization and immunohistochemistry. In the normal ileum and colon, NK-1R and NK-2R were localized to smooth muscle cells of the muscularis mucosae and propria and a few inflammatory cells of the lamina propria; NK-1R expression was also found in the muscular wall of submucosal blood vessels, enteric neurons and, to a lesser degree, in surface epithelial cells. Patients with Crohn’s disease and ulcerative colitis showed a dramatic increase in NK-1R density relative to controls, in both the inflamed and the uninvolved mucosa. Up-regulation of NK-1R was particularly evident on epithelial cells lining the mucosal surface and crypts, as well as on endothelial cells of capillaries and venules. Also, a marked increase in NK-2R expression was found in both groups of patients on inflammatory cells of the lamina propria, especially eosinophils. Our findings demonstrate that in the normal human intestine NK-1R and NK-2R are expressed in multiple cell types, which are endowed with different physiological functions; in addition, they demonstrate that both NK-1R and NK-2R are up-regulated in patients with Crohn’s disease and ulcerative colitis. Taken together, these observations may have important physiological and pathophysiological implications, and provide the rationale for the use of NK-1R and NK-2R antagonists in the treatment of inflammatory bowel disease.
Substance P (SP) and neurokinin A (NKA), the two most thoroughly characterized members of the tachykinin family of neuropeptides, are putative neurotransmitters that exert important physiological functions in both the central nervous system and peripheral tissues. 1
SP and NKA abound in the small and large intestine of a variety of mammalian species, including humans, where they are mainly expressed by intrinsic enteric neurons and extrinsic primary afferent nerve fibers originating from dorsal root ganglia and vagal sensory neurons. 2,3 Additional sources of these two neuropeptides are provided by enterochromaffin cells within the gastrointestinal epithelium 4 and blood-derived or resident immune cells of the lamina propria. 5,6
In keeping with their co-localization on secretory vesicles, 7 SP and NKA are co-released on application of depolarizing stimuli and when intestinal motility is reflexly activated. 8 Once released, SP and NKA exert their biological effects on target cells by interacting with specific receptors, which have been cloned, characterized, and found to have seven transmembrane spanning sequences and to be coupled to G-proteins and the phosphoinositide-signaling pathway. 9-13 To date, three distinct receptors have been identified, termed neurokinin-1 receptor (NK-1R), neurokinin-2 receptor (NK-2R), and neurokinin-3 receptor (NK-3R). SP preferentially activates the NK-1R, NKA the NK-2R, and neurokinin B the NK-3R; however, at high ligand concentrations each tachykinin can activate each of the tachykinin receptors. 13-15
Within the gastrointestinal tract, SP and NKA are involved in the physiological control of several digestive functions, including motility, fluid and electrolyte secretion, blood flow, and tissue homeostasis. 1,3,16 In addition, there is mounting evidence that tachykinins play a pivotal role in the regulation of immunoinflammatory responses, and that bi-directional communication exists between the enteric nervous and mucosal immune systems. 17-19
Given the broad spectrum of SP and NKA actions, it has been hypothesized that an unbalanced function of the tachykinin system may profoundly influence the pathophysiology of acute and chronic intestinal inflammation, contributing to the motor, secretory, and immunological disturbances which characterize human inflammatory bowel disease (IBD). 16,20 Consistent with this hypothesis, a massive increase in SP receptor binding sites has been reported by Mantyh and co-workers 21 in small blood vessels, lymphoid aggregates, and enteric neurons of the small and large bowel of patients with Crohn’s disease and ulcerative colitis. In a subsequent study, the same group of authors showed that whereas the ectopic expression of NK-1R in ulcerative colitis is confined to active, pathologically positive specimens of the colon, up-regulation of NK-1R in Crohn’s disease is evident in both pathologically positive and negative samples of the small and large bowel. 22 However, it is not clear to what extent radioligand binding sites represent specific SP receptors, and accurate localization of NK-1R and NK-2R in the human gastrointestinal tract requires further investigations. The spatial resolution of autoradiographic studies with 125I-labeled Bolton-Hunter SP is in fact inadequate to properly identify cells expressing tachykinin receptors. Moreover, SP is rapidly degraded by neutral endopeptidase, 23 and radiolabeled SP can bind both NK-1R and NK-2R. Finally, to our knowledge, a detailed description of NK-2R distribution in the human intestine is still lacking. The aim of the present study was twofold: 1) to precisely define cellular sites of NK-1R and NK-2R expression in the normal human small and large bowel, and 2) to evaluate whether a difference exists in the pattern of distribution of NK-1R and NK-2R between control patients and patients with IBD. To this purpose, in addition to immunohistochemical techniques, we have used in situ hybridization to the specific mRNAs, which remains the method of choice to study gene expression in intact tissue sections.
Materials and Methods
Patients and Tissues
Tissue samples of normal and diseased human small and large bowel were obtained at surgery. The study protocol was approved by the local Ethical Committee and informed consent for participation was obtained from each patient before surgery.
A total of 20 IBD patients was studied. Twelve patients had Crohn’s disease (7 females/5 males; age range, 18 to 59 years, median 34 years) and eight ulcerative colitis (5 females/3 males; age range, 27 to 69 years, median 38 years). In the Crohn’s disease group, five patients had ileocolic disease and two had Crohn’s colitis, whereas in five patients the disease was limited to the small bowel. Indications for surgery were chronic stenosis with recurrent episodes of obstruction or enteric fistulas, or both, in Crohn’s disease, and failure of medical treatment or longstanding pancolitis in ulcerative colitis. Tissue samples were collected from both the center of inflammation and macroscopically uninvolved areas, at a distance of 4 to 6 cm from the inflamed area. To study the expression of NK-1R and NK-2R at varying distances from the main lesion within the same patient, up to six samples per specimen were collected from the uninvolved and severely affected areas as well as from the site of obstruction. Noninflamed control tissues were taken from hemicolectomy specimens resected for colon carcinoma (6 females/4 males; age range, 46 to 74 years, median 63 years), at least 8 cm away from the edge of the neoplasm.
Surgically resected specimens were obtained within 5 minutes of removal, immediately snap-frozen, and stored in liquid nitrogen until cryostat sectioning. Control specimens were used only after normal morphology had been demonstrated by routine histological examination. Moreover, in all patients with IBD, diagnosis was confirmed by an experienced gastrointestinal pathologist without knowledge of tachykinin receptor expression.
In Situ Hybridization
Frozen sections (7 μm thick) were collected onto gelatin/chrome alum-coated slides, dried briefly on a hot plate at 80°C, and fixed in 4% paraformaldehyde/phosphate buffered saline (PBS), pH 7.4, for 20 minutes. After three washes in PBS and short air drying, sections were immediately used for in situ hybridization.
For the preparation of RNA probes, the human NK-1R cDNA containing the entire 1221-bp coding sequence (kindly provided by Dr. S. Nakanishi, Kyoto University, Japan), was subcloned into the PstI site of the pBluescript SK+ vector. 11 After linearization of the plasmid with either HindIII or BamHI restriction endonuclease, T3 or T7 RNA polymerase was used to obtain run-off transcripts of the anti-sense (complementary to mRNA) or sense (anti-complementary, negative control) 35S-labeled strands, respectively. The 1,498-bp PstI fragment of the rat NK-2R cDNA, 9 containing the entire 1,172-bp coding sequence (a gift from Dr. S. Nakanishi), was subcloned into the plasmid pGEM1 (Promega Biotech, Heidelberg, Germany), at the appropriate restriction sites. After linearization of the construct with either HindIII or SalI, T7 or SP6 RNA polymerase was used to obtain anti-sense and sense RNA probes, respectively. Transcription and labeling of RNA probes were performed as described, 24 using 60 μCi of [35S]-uridine-5′-(α-thio)-triphosphate (1,250 Ci/mmol; New England Nuclear, Dreieich, Germany). The specific activity routinely obtained was 1.2 to 1.4 × 10 9 cpm/μg.
Prehybridization, hybridization, removal of nonspecifically bound probe by RNase A digestion, and further washing procedures, as well as autoradiography, were performed for both negative and positive strand RNA probes as described elsewhere. 25 After exposure for 4 to 6 weeks at 4°C, slides were developed using Kodak D19 developer (Kodak-Pathé, Chalon-S-Saône, France) for 3 minutes, rinsed in 1% acetic acid, and fixed in Kodak Unifix. After extensive washing, sections were counterstained with hematoxylin and eosin and mounted in Corbitt balsam. Sections from inflamed and noninflamed tissues were always processed in parallel, using the same batches of probes and reagents.
Immunohistochemistry
For immunohistochemistry, serial frozen sections (7 μm) were collected onto clean slides and dried overnight at room temperature. After fixation in 4% paraformaldehyde/PBS, pH 7.4, for 20 minutes, sections were incubated in 1% H2O2/methanol to block endogenous peroxidase activity.
Immunolocalization of NK-1R was performed using the polyclonal antibody L114 (a gift from Dr. S. M. Moussaoui, Rhone Poulenc Rorer, Vitry sur Seine, France) generated against the peptide sequence 19 to 32 of the rat brain NK-1R, 26 at a final dilution of 1:300 in PBS. This antibody is very specific to the NK-1R, as confirmed by solid phase enzyme-linked immunosorbent assay and immunoblotting experiments, and cross-reacts with the human NK-1R receptor (S. M. Moussaoui, personal communication). For the detection of the NK-2R, we used a polyclonal antibody directed to the C-terminal 38 amino acid residues of the rat NK-2R (K7; kindly donated by Dr. R. Shigemoto, Kyoto University, Japan), diluted 1:200 in PBS.
Immobilized antibodies were detected by the peroxidase anti-peroxidase (PAP) method, using a monoclonal PAP immune complex (DAKO, Glostrup, Denmark) diluted 1:50 in PBS and 3,3′-diaminobenzidine tetrahydrochloride (Sigma, Munich, Germany) as chromogen, as described. 25 Negative controls were performed by omitting the primary antibodies (to control the detection system), and using nonimmune rabbit serum as first layer.
Image Analysis and Statistics
Quantitative evaluation of NK-1R and NK-2R mRNA expression was performed by two independent observers who did not know the diagnosis of the tissues, with the aid of a computerized video image analysis system (Quantimet Q500MC, Leica Cambridge Ltd., Cambridge, England). Six visual fields within the mucosa and muscularis propria were chosen randomly from each section and analyzed under a dark-field microscope equipped with a ×20 lens. The autoradiographic signal corresponding to the specific hybridization was acquired by a video camera connected to the microscope, converted to digital and transformed into pixel units. The threshold of specific detection was automatically calibrated on control sections hybridized with the corresponding sense probes. The percentage of the total area occupied by the NK-1R and NK-2R autoradiographic signal in the mucosa and muscularis propria of control patients and patients with IBD was analyzed by analysis of variance, and multiple comparisons of means were made using Dunnett’s method. All data are expressed as means ± SEM, and a P value of <0.05 was accepted to denote statistical significance.
Results
Expression of NK-1R in the Healthy Human Intestine
NK-1R was found to be distributed in all layers of the morphologically normal human ileum and colon by both in situ hybridization and immunohistochemistry: specific mRNA and protein expression was localized to smooth muscle cells of the muscularis mucosae and propria (both in the longitudinal and circular muscle), neurons of the myenteric plexus, the muscular wall of submucosal blood vessels, and a few inflammatory cells of the lamina propria (Figure 1) ▶ . NK-1R autoradiographic labeling and protein immunostaining was especially evident in the inner portion of the circular muscle (Figure 1, e and f) ▶ , whereas expression on mucosal epithelial cells was scanty (Figure 1, c and g) ▶ .
Figure 1.
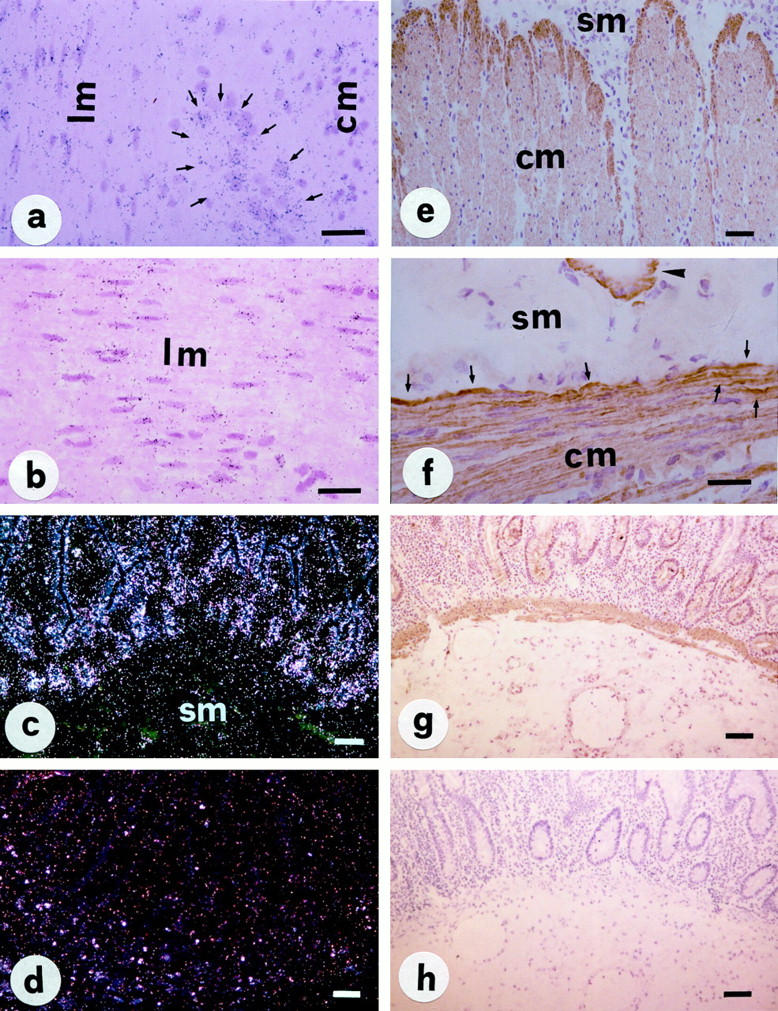
In situ hybridization with 35S-labeled NK-1R anti-sense and sense mRNA probes (a–d) and immunohistochemistry (PAP method, e–h) for NK1-R protein in normal human intestine. Expression of NK-1R mRNA and protein is localized to the muscularis mucosae (g) and propria (a, b, e, and f), especially in the inner portion of the circular muscle (e and f, arrows); moderate expression is also evident on enteric neurons (a, arrows), the muscular wall of a submucosal blood vessel (f, arrowhead) and mucosal epithelial cells (c and g). A control section hybridized with NK-1R sense probe shows only nonspecific background signal (d). No specific immunoreaction is also noted in a sections processed with nonimmune rabbit serum (h). Abbreviations: lm and cm, longitudinal and circular muscle; sm = submucosa. Scale bars, 50 μm in a, b, e, and f; 100 μm in c, g, d, and h.
Expression of NK-1R in Crohn’s Disease and Ulcerative Colitis
In surgical specimens from patients with Crohn’s disease and ulcerative colitis, in situ hybridization showed a massive increase in the NK-1R gene expression (Figure 2, a–d) ▶ . Enhanced autoradiographic labeling was particularly evident in the mucosal epithelial layer, not only in inflamed but also in macroscopically uninvolved tissues, as well as in lamina propria and submucosal inflammatory cells which, on the basis of their size, shape, and dye affinity, were tentatively identified as lymphocytes, macrophages, and eosinophilic granulocytes. NK1-R gene expression on epithelial cells was not uniform, being more evident in the lower third of the mucosa, especially in crypts. Despite the different degree of expression, the autoradiographic signal on epithelial cells was so intense that, when examined under dark-field microscope, it completely delineated the luminal aspect of mucosal glands and crypts. By immunohistochemistry, the distribution of NK-1R protein closely paralleled that of NK-1R mRNA, thus underlying the specificity of the results obtained by in situ hybridization (Figure 2, e–h) ▶ . The staining pattern was usually membranous, but immunoreactive material was constantly seen in the cytoplasm of positive cells.
Figure 2.
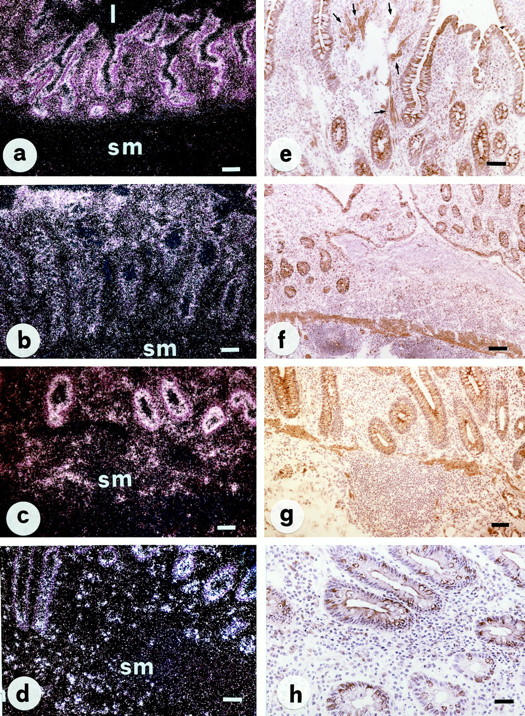
In situ hybridization (a–d) and immunohistochemistry (PAP method, e–h) for NK-1R in representative sections of Crohn’s ileitis (a, b, e, and f) and ulcerative colitis (c, d, g, and h). Increased NK-1R expression is observed in both Crohn’s disease and ulcerative colitis, not only in inflamed (b, d, f, and h) but also in macroscopically uninvolved (a, c, e, and g) tissues. Autoradiographic labeling is particularly evident on epithelial cells and completely decorates the luminal aspect of the mucosal surface and crypts. Strong NK-1R immunoreactivity is present on mucosal epithelial cells, inflammatory cells, and fibroblasts of the lamina propria (e, arrows). Abbreviations: l, lumen; sm, submucosa. Scale bars, 50 μm in h; 100 μm in a, b, c, d, e, and g; 200 μm in f.
Specific NK-1R gene and protein expression was also observed in neurons of the myenteric and submucous plexuses, the muscular wall of submucosal blood vessels, lymphoid aggregates, white thrombi within submucosal and subserosal venules, and endothelial cells of capillaries and venules (Figure 3) ▶ . Endothelial cells of the granulation tissue, probably involved in the process of new vessel generation (angiogenesis), usually expressed the highest amounts of NK-1R transcripts (Figure 3d) ▶ .
Figure 3.
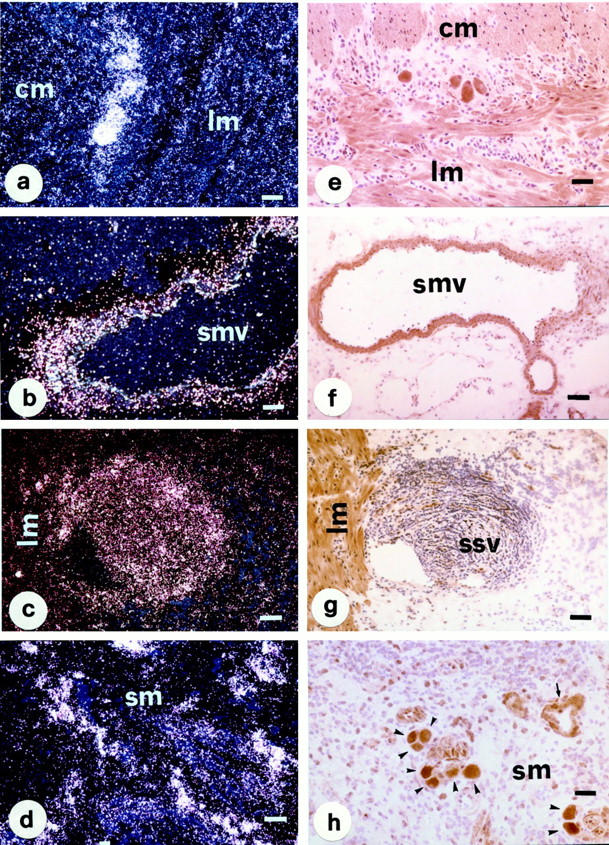
In situ hybridization (a–d) and immunohistochemistry (PAP method, e–h) for NK-1R in tissue sections obtained from a patient with Crohn’s ileocolitis at site of active inflammation. NK-1R mRNA (left) and protein (right) expression is evident in neurons of the myenteric (a and e) and submucous (h, arrowheads) plexuses, the muscular wall of a submucosal vessel (b and f), a white thrombus within a subserosal venule (c and g), inflammatory cells and endothelial cells of capillaries (d) and venules (h, arrows). Specific NK-1R mRNA (a and c) and protein (e and g) expression is also evident in the longitudinal and circular muscle layers of the muscularis propria. Abbreviations: lm and cm, longitudinal and circular muscle; sm, submucosa; smv, submucosal blood vessel; ssv, subserosal venule. Scale bars, 50 μm in a, b, d, and h; 100 μm in c, e, f, and g.
Concerning the expression of NK-1R in enteric neurons and lymphoid aggregates, there was no evident difference between patients with Crohn’s disease and ulcerative colitis. Similarly, no difference in NK-1R mRNA abundance was found between patients with Crohn’s ileitis and either Crohn’s colitis or ileocolitis.
Expression of NK-2R in the Healthy Human Intestine
In the normal ileum and colon, NK-2R was predominantly localized to smooth muscle cells of the muscularis mucosae and propria (Figure 4, a and d) ▶ . Low but still detectable levels of NK-2R mRNA and protein were also observed in a very few inflammatory cells sparsely distributed in the lamina propria (Figure 4, c and f) ▶ . Of note, in contrast to the NK-1R, no specific NK-2R expression was observed on enteric neurons and mucosal epithelial cells.
Figure 4.
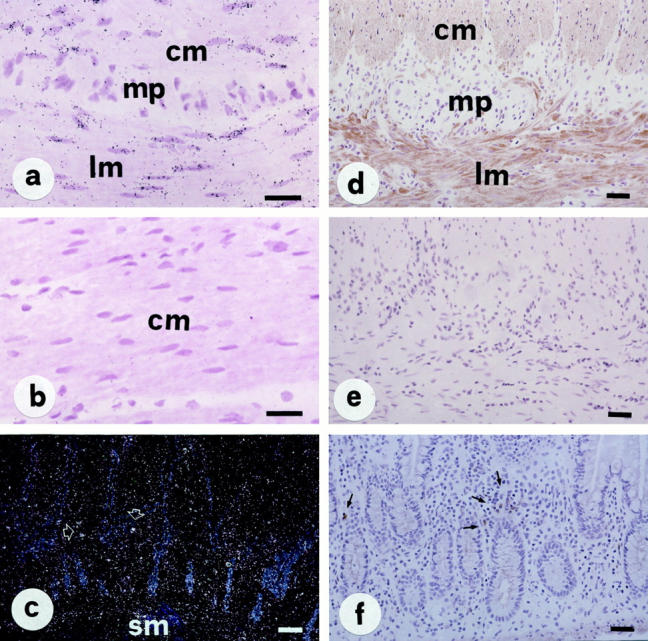
In situ detection of NK-2R mRNA (a–c) and immunolocalization of NK-2R protein (PAP method, d–f) in normal intestine. NK-2R mRNA and protein is localized to both longitudinal and circular muscle layers (a and d) and a few inflammatory cells of the lamina propria (c and f). Note that, in contrast to the NK-1R, no specific NK-2R expression is present on enteric neurons (a and d) and mucosal epithelial cells (c and f). Control sections hybridized with an NK-2R sense probe (b) or processed with nonimmune serum (e) show completely negative results. Abbreviations: lm and cm, longitudinal and circular muscle; mp, myenteric plexus; sm, submucosa. Scale bars, 50 μm in a, b, d, e, and f; 100 μm in c.
Expression of NK-2R in Crohn’s Disease and Ulcerative Colitis
With regard to the NK-2R, patients with IBD showed only a few, although substantial changes as compared with controls (Figure 5) ▶ . Particularly, an increased number of inflammatory cells expressing the NK-2R was found in the lamina propria of both Crohn’s disease and ulcerative colitis patients, whereas mucosal epithelial cells were constantly negative. Clusters of activated eosinophils showing a high number of NK-2R RNA transcripts were frequently observed around mucosal crypts, as well as within bundles of smooth muscle cells emanating from the muscularis mucosae, especially in patients with ulcerative colitis at site of active mucosal inflammation (Figure 5, d–f) ▶ .
Figure 5.
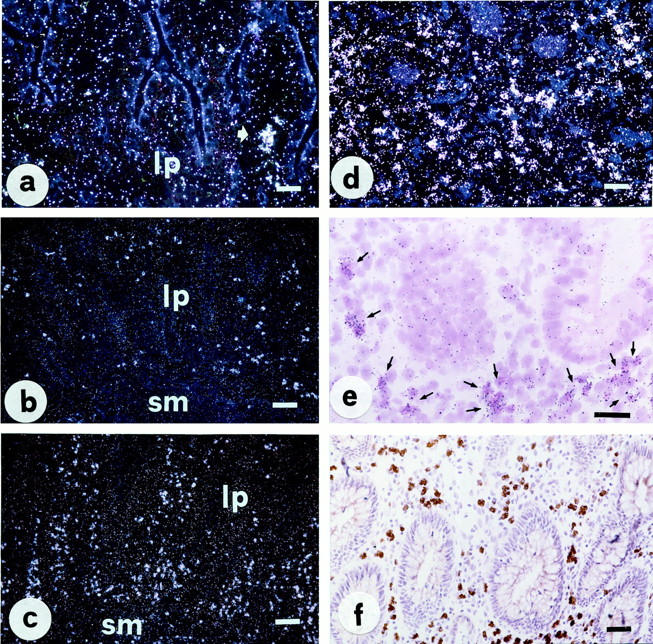
In situ hybridization (a–e) and immunohistochemistry (PAP method, f) for NK-2R in normal human intestine (a) and representative sections of ulcerative colitis (b–f). A marked increase in the number of lamina propria inflammatory cells expressing NK2-R is evident in both active (c and d) and macroscopically uninvolved (b) sections of ulcerative colitis relative to control tissue. A bright-field photomicrograph at greater magnification (e) clearly shows that most of NK-2R-positive cells are indeed eosinophils. Scale bars, 50 μm in a, d, e, and f; 100 μm in b and c.
Negative Controls
Tissue sections hybridized with sense (anti-complementary, negative control) RNA probes showed only nonspecific labeling, which cannot be distinguished from the background autoradiographic signal (Figures 1d and 4b) ▶ ▶ . Similarly, no specific immunoreaction was invariably observed in control sections processed with nonimmune rabbit serum or by omission of the primary antibodies (Figures 1h and 4e) ▶ ▶ .
Quantitative Image Analysis of NK-1R and NK-2R
Quantitative evaluation of NK-1R mRNA expression by computerized image analysis demonstrated a dramatic increase in the mucosa of IBD patients relative to controls (Figure 6 ▶ , upper panel). The percentage of the total area occupied by the autoradiographic signal was 2.8 ± 1.2 and 3.1 ± 1.5 in the normal ileum and colon; these values were increased by more than 1,000% in Crohn’s disease and ulcerative colitis, both in active and macroscopically uninvolved tissues (all P < 0.0001 versus the mucosa of normal controls). Similar differences, albeit less pronounced, were found when quantitative expression of NK-2R mRNA in the mucosa of patients with IBD was compared with that of controls (Figure 6 ▶ , lower panel). The percentage of total area occupied by cells expressing NK-2R mRNA in the normal ileum and colon was 2.3 ± 0.6 and 2.0 ± 0.4, respectively, and these values were increased by ∼300% in active Crohn’s disease and ulcerative colitis (P < 0.01) and by at least 100% in macroscopically uninvolved tissues from both diseases (P < 0.05 versus control mucosa). In contrast, no significant difference was found concerning the expression of either NK-1R and NK-2R in the muscularis propria of control patients and patients with IBD (data not shown).
Figure 6.
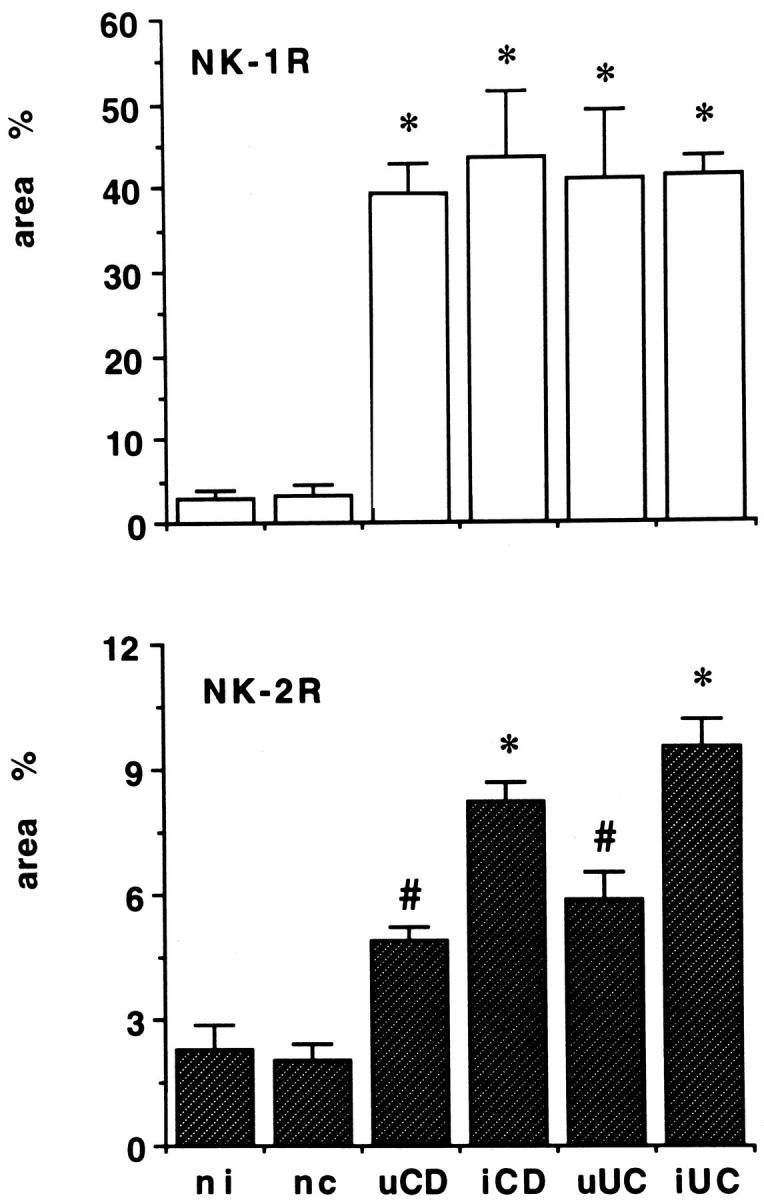
Expression of NK-1R and NK-2R mRNAs in the normal and inflamed human intestine. Quantitative evaluation was performed by computerized video image analysis on six visual fields chosen randomly from each section and analyzed under a dark-field microscope. Data, expressed as means ± SEM, represent the percentage of the total area occupied by the NK-1R and NK-2R autoradiographic signal in the mucosa of control patients and patients with Crohn’s disease and ulcerative colitis. *, P < 0.0001 (top) and P < 0.01 (bottom), #, P < 0.05 versus the mucosa of both normal ileum (ni) and colon (nc). Abbreviations: uCD, uninvolved Crohn’s disease; iCD, active Crohn’s disease; uUC, uninvolved ulcerative colitis; iUC, active ulcerative colitis.
Discussion
In the present study we have used in situ hybridization and immunohistochemistry to precisely define cellular sites of NK-1R and NK-2R expression in the normal human small and large bowel, and to evaluate whether the pattern of distribution of these receptors is significantly altered in the ileum and colon of patients with IBD. To our knowledge, this is the first study simultaneously evaluating the expression of NK-1R and NK-2R at both the gene and protein level in normal and diseased human gut. Our findings extend previous observations on the expression of SP binding sites in patients with Crohn’s disease and ulcerative colitis, 21,22 adding new insights which may be relevant to the comprehension of the role played by tachykinins in gastrointestinal physiology and pathophysiology.
Firstly, we have shown that in the normal human ileum and colon NK-1R and NK-2R are distributed more widely than previously recognized. Particularly, NK-1R mRNA and protein expression were localized to enteric neurons, the muscular wall of submucosal blood vessels, and, albeit to a lesser extent, to surface epithelial cells. In addition, both NK-1R and NK-2R were found to be expressed in smooth muscle cells of the muscularis mucosae and propria, as well as in inflammatory cells of the lamina propria. These findings provide the anatomical basis for the documented actions of SP and NKA in the gastrointestinal tract, 1,3,16 and corroborate the hypothesis that tachykinins are also involved in the regulation of mucosal immunoinflammatory response. 16-20
It is worth noting that both receptors were shown in the muscularis externa; in addition, NK-1R expression was documented in neurons of the myenteric plexus. Our results are in agreement with previous autoradiographic and functional studies, 27-29 and support the assumption that tachykinins enhance intestinal motor activity not only by direct activation of the muscle but also via acetylcholine release from enteric motor neurons. 3,30 Regarding the peculiar distribution of NK-1R in the muscularis externa, recent studies in the guinea-pig and rat gastrointestinal tract have suggested that nerve-independent facilitation of intestinal motor activity can be brought about by NK-1Rs expressed on interstitial cells of Cajal. 31,32 In agreement with Sternini et al, 31 we also localized NK-1R in the inner portion of the circular muscle layer, a region which is particularly rich in interstitial cells of Cajal; this does not prove, however, that human interstitial cells of Cajal, like those of other animal species, do express NK-1Rs. Future experiments, using in situ hybridization in combination with immunohistology for a specific cell marker 24 (eg, c-Kit) will help to clarify this point.
NK-1R expression was also found in the epithelium lining the mucosal surface by both in situ hybridization and immunohistochemistry. This localization is not surprising because a wealth of evidence indicates that tachykinins have considerable influence on transmucosal water and electrolyte transport in both the small and large bowel. 16 The secretory effect of tachykinins is mediated by an enteric reflex involving both cholinergic and noncholinergic secretomotor neurons, 33 and in the normal human colon seems to be processed and amplified via cross-talk between enteric nerves, mast cells, lamina propria immune cells, and fibroblasts. 34 However, a direct action of SP on mucosal epithelial cells is well established. Evidence for the presence of SP receptors on intestinal epithelial cells has been provided by Keast et al, 35 and direct effect of SP on canine colonocytes has been reported by Rangachari et al. 36 Moreover, the presence of NK-1R on isolated colonocytes from guinea pigs has been demonstrated by Cooke et al 37 using in situ hybridization and reverse transcriptase-polymerase chain reaction techniques. In line with a recent report on normal human colonic tissue, 38 we now provide direct evidence for the localization of NK-1R mRNA and protein on epithelial cells of the normal small and large intestine. On the contrary, in agreement with previous animal data, 39 we did not find convincing evidence of NK-2R expression in the human intestinal epithelium.
Secondly, we have shown that NK-1R is dramatically increased in both Crohn’s disease and ulcerative colitis patients relative to controls. This finding is not completely new: a massive increase in SP receptor binding sites has been reported previously in patients with IBD by Mantyh et al, 21 using quantitative receptor autoradiography. Subsequently, the same group of authors reported that SP receptors are differentially expressed in patients with Crohn’s disease and ulcerative colitis; whereas in Crohn’s disease SP binding sites are ectopically expressed in lymphoid aggregates, small blood vessels, and enteric neurons of both pathologically positive and negative surgical specimens, up-regulation of NK-1R in ulcerative colitis is confined to blood vessels and lymphoid aggregates of active, pathologically positive specimens of the colon. 22 However, there are several major differences between our results and those reported by Mantyh and colleagues. 21 First, in addition to lymphoid aggregates, enteric neurons, and small blood vessels, we provide direct evidence of NK-1R up-regulation on inflammatory cells of the lamina propria, as well as on epithelial cells lining the mucosal surface and crypts. Second, we did not find any difference in the extent of up-regulation of NK-1R between patients with Crohn’s disease and ulcerative colitis. Thus, the proposal that in the 5 to 15% of IBD patients with indeterminate colitis the up-regulation of NK-1R on the enteric neurons might be used as a distinctive marker of Crohn’s disease, 22 is not substantiated by our present findings. Third, in both diseases we observed enhanced expression of NK-1R not only in surgical specimens collected from the center of inflammation but also in samples obtained from macroscopically uninvolved areas. This, we believe, is the major finding of our study. Up-regulation of NK-1R in the mucosa of macroscopically uninvolved tissues, in fact, may help to explain the high risk for recurrence after surgical resection of Crohn’s disease, as well as the well-known propensity of Crohn’s disease and ulcerative colitis to relapse after drug-induced remission. 40,41
Up-regulated expression of NK-1R may have important implications in the pathophysiology of IBD. NK-1R expression on lamina propria inflammatory cells and the endothelium of submucosal venules may participate in the margination and extravascular migration of granulocytes, lymphocytes, and monocytes into inflamed tissues, 42,43 whereas expression on endothelial cells of the granulation tissue may be involved in the process of angiogenesis, via stimulation of endothelial cell proliferation, migration, and differentiation into capillary-like structures. 44,45 Moreover, up-regulation of NK-1R on lamina propria mononuclear cells may play a central role in mucosal immunomodulation, by facilitating lymphocyte proliferation in response to mitogens and by stimulating macrophage production of inflammatory cytokines. 16-20,38,46,47 From a clinical point of view, overexpression of NK-1R on surface epithelial cells may be implicated in the pathogenesis of diarrhea which characterizes IBD. An increasing body of evidence seems to support this conclusion. Pretreatment of rats with either capsaicin, which selectively targets primary sensory neurons, or specific NK-1R antagonists dramatically reduced fluid secretion, mucosal permeability, and intestinal inflammation in animal models of acute and chronic inflammation. 6,48-51 The importance of SP and its receptor in the pathogenesis of inflammatory diarrhea is underscored by the demonstration that NK-1R mRNA expression is increased in the rat intestinal epithelium shortly after exposure to Clostridium difficile toxin A. 52 Moreover, mice genetically deficient in the NK-1R are protected from the secretory and inflammatory changes induced by C. difficile toxin A, demonstrating a major requirement for SP receptors in the pathogenesis of inflammatory diarrhea. 53
Finally, we provided new insights on the expression of the NK-2R in the human intestine. Although previous studies have examined the expression of NK-1R mRNA and SP binding sites in acute and chronic intestinal inflammation, direct evidence for the NK-2R mRNA localization and protein expression in the normal and inflamed human intestine is lacking. Data presented here show that not only NK-1R but also NK-2R expression is significantly increased in patients with IBD. In this context, the up-regulation of NK-2R in eosinophils immediately adjacent to the surface epithelium of both Crohn’s disease and ulcerative colitis patients seems of particular interest. To our knowledge, this is the first demonstration of NK-2R receptor expression in human eosinophils. A significant role for SP in the activation and migratory function of human eosinophils has indeed been reported, but these effects seem to be NK-1R-dependent. 54,55 Therefore, further studies are necessary to confirm the expression of NK-2R on human eosinophils, and to ascertain the possible role of NKA in the regulation of eosinophil functions.
In conclusion, we have shown that in the normal human ileum and colon NK-1R and NK-2R are expressed in a variety of cell types, which are endowed with different physiological functions; in addition, we have demonstrated that both the NK-1R and the NK-2R are up-regulated in Crohn’s disease and ulcerative colitis. Although the physiological and pathological implications of these findings remain purely speculative, overexpression of SP and NKA receptors in cells involved in immunoinflammatory response, as well as in enteric neurons, endothelial cells, and mucosal epithelial cells, suggest an important role for tachykinins in the pathophysiology of IBD. In a therapeutic perspective, the present results are timely, because a variety of high-affinity and selective antagonists are currently available for clinical studies. Based on present findings, these drugs may prove useful in the therapy of IBD, especially in treating diarrhea, pain, and mucosal inflammation.
Acknowledgments
We thank Prof. S. Nakanishi for providing the NK-1R and NK-2R cDNAs, Dr. S. M. Moussaoui for the anti-NK-1R, Prof. R. Shigemoto for the anti-NK-2R, and Ms. N. C. Hargreaves for reviewing the English.
Footnotes
Address reprint requests to Antonio Calabrò, M.D., Gastroenterology Unit, Department of Clinical Pathophysiology, University of Florence, viale Pieraccini 6, I-50139 Florence, Italy. E-mail: a.calabro@dfc.unifi.it.
Supported by the Italian “Ministero della Ricerca Scientifica e Tecnologica” (grants .n° 0401–664 and 0401–610).
Portions of this work were presented at the 100th Annual Meeting of the American Gastroenterological Association and published in abstract form (Gastroenterology 1999,116:A676).
References
- 1.Otsuka M, Yoshioka K: Neurotransmitter functions of mammalians tachykinins. Physiol Rev 1993, 73:229-308 [DOI] [PubMed] [Google Scholar]
- 2.Furness JB, Costa M: The Enteric Nervous System. 1987. Churchill-Livingstone, Edinburgh
- 3.Holzer P, Holzer-Petsche U: Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther 1997, 73:173-217 [DOI] [PubMed] [Google Scholar]
- 4.Heitz P, Polak JM, Timson DM, Pearse AG: Enterochromaffin cells as the endocrine source of gastrointestinal substance P. Histochemistry 1976, 49:343-347 [DOI] [PubMed] [Google Scholar]
- 5.Metwali A, Blum AM, Ferraris L, Klein JS, Fiocchi C, Weinstock JV: Eosinophils within the healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J Neuroimmunol 1994, 52:69-78 [DOI] [PubMed] [Google Scholar]
- 6.Castagliuolo I, Keates AC, Qiu B, Kelly CP, Nikulasson S, Leeman SE, Pothoulakis C: Substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci USA 1997, 94:4788-4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deacon CF, Agoston DV, Nau R, Conlon JM: Conversion of neuropeptide K to neurokinin A and vesicular colocalization of neurokinin A and substance P in neurons of the guinea-pig small intestine. J Neurochem 1987, 48:141-146 [DOI] [PubMed] [Google Scholar]
- 8.Donnerer J, Bartho L, Holzer P, Lembeck F: Intestinal peristalsis associated with release of substance P. Neuroscience 1984, 11:913-918 [DOI] [PubMed] [Google Scholar]
- 9.Sasai Y, Nakanishi S: Molecular characterization of rat substance K receptor and its mRNAs. Biochem Biophys Res Commun 1989, 165:695-702 [DOI] [PubMed] [Google Scholar]
- 10.Hershey AD, Krause JE: Molecular characterization of a functional cDNA encoding the rat substance P receptor. Science 1990, 247:958-962 [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Tanaka A, Hara M, Nakanishi S: The primary structure and gene organization of human substance P and neuromedin K receptors. Eur J Biochem 1992, 204:1025-1033 [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi S: Mammalian tachykinin receptors. Annu Rev Neurosci 1991, 14:123-136 [DOI] [PubMed] [Google Scholar]
- 13.Guard S, Watson SP: Tachykinin receptor types: classification and membrane signalling mechanisms. Neurochem Int 1991, 18:149-165 [DOI] [PubMed] [Google Scholar]
- 14.Regoli D, Boudon A, Fauchère JL: Receptors and antagonists for substance P and related peptides. Pharmacol Rev 1994, 46:551-599 [PubMed] [Google Scholar]
- 15.Maggi CA: The mammalian tachykinin receptors. Gen Pharmacol 1995, 26:911-944 [DOI] [PubMed] [Google Scholar]
- 16.Holzer P, Holzer-Petsche U: Tachykinins in the gut. Part II. Roles in neural excitation, secretion and inflammation. Pharmacol Ther 1997, 73:219-263 [DOI] [PubMed] [Google Scholar]
- 17.McGillis JP, Mitsuhashi M, Payan DG: Immunomodulation by tachykinin neuropeptides. Ann NY Acad Sci 1990, 594:85-94 [DOI] [PubMed] [Google Scholar]
- 18.Sharkey KA: Substance P and calcitonin gene-related peptide (CGRP) in gastrointestinal inflammation. Ann NY Acad Sci 1992, 664:425-442 [DOI] [PubMed] [Google Scholar]
- 19.Maggi CA: The effects of tachykinins on inflammatory and immune cells. Regul Pept 1997, 70:75-90 [DOI] [PubMed] [Google Scholar]
- 20.Holzer P: Implications of tachykinins and calcitonin gene-related peptide in inflammatory bowel disease. Digestion 1998, 59:269-283 [DOI] [PubMed] [Google Scholar]
- 21.Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP, Jr, Vigna SR, Maggio JE, Kruger L, Mantyh PW: Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci USA 1988, 85:3235-3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantyh CR, Vigna SR, Bollinger RR, Mantyh PW, Maggio JE, Pappas TN: Differential expression of substance P receptors in patients with Crohn’s disease and ulcerative colitis. Gastroenterology 1995, 109:850-860 [DOI] [PubMed] [Google Scholar]
- 23.Okamoto A, Lovett M, Payan DG, Bunnett N: Interactions between neutral endopeptidase (EC 3.4.24.11) and the substance P (NK1) receptor expressed in mammalian cells. Biochem J 1994, 299:683-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milani S, Hermann H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabrò A, Ciancio G, Stefanini F, Burroughs AK, Surrenti C: Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol 1994, 144:528-537 [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrò A, Orsini B, Renzi D, Papi L, Surrenti E, Amorosi A, Herbst H, Milani S, Surrenti C: Expression of epidermal growth factor, transforming growth factor-α and their receptor in the human oesophagus. Histochem J 1997, 29:745-758 [DOI] [PubMed] [Google Scholar]
- 26.Moussaoui SM, Hermans E, Mathieu AM, Bonici B, Clerc F, Guinet F, Garret C, Laduron PM: Polyclonal antibodies against the rat NK1 receptor: characterization and localization in the spinal cord. Neuroreport 1992, 3:1073-1076 [DOI] [PubMed] [Google Scholar]
- 27.Mantyh PW, Mantyh CR, Gates T, Vigna SR, Maggio JE: Receptor binding sites for substance P and substance K in the canine gastrointestinal tract and their possible role in inflammatory bowel disease. Neuroscience 1988, 25:817-837 [DOI] [PubMed] [Google Scholar]
- 28.Gates TS, Zimmerman RP, Mantyh CR, Vigna SR, Maggio JE, Welton ML, Passaro EPJ, Mantyh PW: Substance P and substance K receptor binding sites in the human gastrointestinal tract: localization by autoradiography. Peptides 1988, 9:1207-1219 [DOI] [PubMed] [Google Scholar]
- 29.Korman LY, Sayadi H, Bass B, Moody TW, Harmon JW: Distribution of vasoactive intestinal polypeptide and substance P receptors in human colon and small intestine. Dig Dis Sci 1989, 34:1100-1108 [DOI] [PubMed] [Google Scholar]
- 30.Maggi CA, Catalioto RM, Criscuoli M, Cucchi P, Giuliani S, Lecci A, Lippi A, Meini S, Patacchini R, Renzetti AR, Santicioli P, Tramontana M, Zagorodnyuk V, Giachetti A: Tachykinin receptors and intestinal motility. Can J Physiol Pharmacol 1997, 75:696-703 [PubMed] [Google Scholar]
- 31.Sternini C, Su D, Gamp PD, Bunnett NW: Cellular sites of expression of the neurokinin-1 receptor in the rat gastrointestinal tract. J Comp Neurol 1995, 358:531-540 [DOI] [PubMed] [Google Scholar]
- 32.Portbury AL, Furness JB, Young HM, Southwell BR, Vigna SR: Localisation of NK1 receptor immunoreactivity to neurons and interstitial cells of the guinea-pig gastrointestinal tract. J Comp Neurol 1996, 367:342-351 [DOI] [PubMed] [Google Scholar]
- 33.Brown DR, Parsons AN, O’Grady SM: Substance P produces sodium and bicarbonate secretion in porcine jejunal mucosa through an action on enteric neurons. J Pharmacol Exp Ther 1992, 261:1206-1212 [PubMed] [Google Scholar]
- 34.Riegler M, Castagliuolo I, So PTC, Lotz M, Wang C, Wlk M, Sogukoglu T, Cosentini E, Bischof G, Hamilton G, Teleky B, Wenzl E, Matthews JB, Pothoulakis C: Effects of substance P on human colonic mucosa in vitro. Am J Physiol 1999, 276:G1473-G1483 [DOI] [PubMed] [Google Scholar]
- 35.Keast JR, Furness JB, Costa M: Different substance P receptors are found on mucosal epithelial cells and submucous neurons of the guinea-pig small intestine. Naunyn Schmiedebergs Arch Pharmacol 1985, 329:382-387 [DOI] [PubMed] [Google Scholar]
- 36.Rangachari PK, Prior T, McWade D: Epithelial and mucosal preparation from canine colon: responses to substance P. J Pharmacol Exp Ther 1990, 254:1076-1083 [PubMed] [Google Scholar]
- 37.Cooke HJ, Sighu M, Fox P, Wang Y-Z, Zimmermann EM: Substance P as a mediator of colonic secretory reflexes. Am J Physiol 1997, 272:G238-G245 [DOI] [PubMed] [Google Scholar]
- 38.Goode T, O’Connell J, Sternini C, Anton P, Wong H, O’Sullivan GC, Collins JK, Shanahan F: Substance P (Neurokinin-1) receptor is a marker of human mucosal but not peripheral mononuclear cells: molecular quantitation and localization. J Immunol 1998, 161:2232-2240 [PubMed] [Google Scholar]
- 39.Crowther R, Jukic D, Regoli D, Rangachari PK: Functional subtyping of neurokinin receptors on canine proximal colonic mucosa. J Pharmacol Exp Ther 1994, 268:1374-1380 [PubMed] [Google Scholar]
- 40.Sutherland LR: Clinical course and complications of ulcerative colitis and ulcerative proctitis. Targan SR Shanahan F eds. Inflammatory Bowel Disease: From Bench to Bedside. 1994, :pp 279-295 William & Wilkins, Baltimore [Google Scholar]
- 41.Levine DS: Clinical features and complications of Crohn’s disease. Targan SR Shanahan F eds. Inflammatory Bowel Disease: From Bench to Bedside. 1994, :pp 296-316 William & Wilkins, Baltimore [Google Scholar]
- 42.Nakagawa N, Sano H, Iwamoto I: Substance P induces the expression of intercellular adhesion molecule-1 on vascular endothelial cells and enhances neutrophil transendothelial migration. Peptides 1994, 16:721-725 [DOI] [PubMed] [Google Scholar]
- 43.Schratzberger P, Reinisch N, Prodinger WM, Kähler CM, Sitte BA, Bellmann R, Fischer-Colbrie R, Vinkler H, Wiedermann CJ: Differential chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. J Immunol 1997, 158:3895-3901 [PubMed] [Google Scholar]
- 44.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F: Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 1994, 94:2036-2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiedermann CJ, Auer B, Sitte B, Reinisch N, Schratzberger P, Kähler CM: Induction of endothelial cell differentiation into capillary-like structures by substance P. Eur J Pharmacol 1996, 298:335-338 [DOI] [PubMed] [Google Scholar]
- 46.Lotz M, Vaughan JH, Carson DA: Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988, 241:1218-1221 [DOI] [PubMed] [Google Scholar]
- 47.Lieb K, Fiebich BL, Busse-Grawitz M, Hull M, Berger M, Bauer J: Effects of substance P and selected other neuropeptides on the synthesis of interleukin 1b and interleukin-6 in human monocytes: a re-examination. J Neuroimmunol 1996, 67:77-81 [DOI] [PubMed] [Google Scholar]
- 48.Castagliuolo I, Lamont JT, Kelly CP, Jaffer A, O’Keane JC, Pothoulakis C: Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin. Gastroenterology 1994, 107:657-665 [DOI] [PubMed] [Google Scholar]
- 49.McCafferty DM, Sharkey KA, Wallace JL: Beneficial effects of local or systemic lidocaine in experimental colitis. Am J Physiol 1994, 266:G560-G567 [DOI] [PubMed] [Google Scholar]
- 50.Mantyh CR, Pappas TN, Lapp JA, Washington MK, Neville LM, Ghilardi JR, Rogers SD, Mantyh PW, Vigna SR: Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology 1996, 111:1272-1280 [DOI] [PubMed] [Google Scholar]
- 51.Pothoulakis C, Castagliuolo I, Lamont JT, Jaffer A, O’Keane JC, Snider M, Leeman SE: CP-96345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA 1994, 91:947-951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pothoulakis C, Castagliuolo I, Leeman SE, Wang CC, Li H, Hoffman BJ, Mezey E: Substance P receptor expression in intestinal epithelium in Clostridium difficile toxin A enteritis in rats. Am J Physiol 1998, 275:G68-G75 [DOI] [PubMed] [Google Scholar]
- 53.Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard NP, Pothoulakis C: Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest 1998, 101:1547-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Shazly AE, Masuyama K, Eura M, Ishikawa T: Immunoregulatory effect of substance P in human eosinophil migratory function. Immunol Invest 1996, 25:191-201 [DOI] [PubMed] [Google Scholar]
- 55.El-Shazly AE, Masuyama K, Ishikawa T: Mechanisms involved in activation of human eosinophils exocytosis by substance P: an in vitro model of sensory neuroimmunomodulation. Immunol Invest 1997, 26:615-629 [DOI] [PubMed] [Google Scholar]


