Abstract
Determination of HER-2/neu oncogene amplification has become necessary for selection of breast cancer patients for trastuzumab (Herceptin) therapy. Fluorescence in situ hybridization (FISH) is currently regarded as a gold standard method for detecting HER-2/neu amplification, but it is not very practical for routine histopathological laboratories. We evaluated a new modification of in situ hybridization, the chromogenic in situ hybridization (CISH), which enables detection of HER-2/neu gene copies with conventional peroxidase reaction. Archival formalin-fixed paraffin-embedded tumor tissue sections were pretreated (by heating in a microwave oven and using enzyme digestion) and hybridized with a digoxigenin-labeled DNA probe. The probe was detected with anti-digoxigenin fluorescein, anti-fluorescein peroxidase, and diaminobenzidine. Gene copies visualized by CISH could be easily distinguished with a ×40 objective in hematoxylin-stained tissue sections. HER-2/neu amplification typically appeared as large peroxidase-positive intranuclear gene copy clusters. CISH and FISH (according to Vysis, made from frozen pulverized tumor samples) correlated well in a series of 157 breast cancers (kappa coefficient, 0.81). The few different classifications were mostly because of low-level amplifications by FISH that were negative by CISH and immunohistochemistry with monoclonal antibody CB-11. We conclude that CISH, using conventional bright-field microscopy in evaluation, is a useful alternative for determination of HER-2/neu amplification in paraffin-embedded tumor samples, especially for confirming the immunohistochemical staining results.
HER-2 oncogene amplification and its concomitant protein overexpression is currently implicated as an important prognostic biomarker in breast carcinoma. 1 Recent studies suggest that HER-2 may also be a useful determinant of response to hormonal or cytotoxic chemotherapy. 1 Clinical importance of HER-2 diagnostics has risen even more with the increasing use of the new anti-cancer drug trastuzumab (Herceptin, Roche Ltd, Basel, Switzerland), which is a humanized monoclonal antibody against the extracellular part of the HER-2 protein product. 2 Trastuzumab therapy is effective only in patients whose tumors contain amplification and/or overexpression of HER-2. 2 Thus, HER-2 assays are now becoming an integral part of breast cancer diagnostics, in parallel with assays of hormone receptors and tumor proliferation rate. 2
The earliest studies of HER-2 used Southern and Western blotting for detection of HER-2 gene amplification and protein overexpression. These methods are not well suited for routine diagnostics and have been replaced by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). A vast majority of HER-2 studies has been done using IHC, which detects the HER-2 protein overexpression on the cell membrane. Without HER-2 oncogene amplification, the protein expression is low and undetectable by IHC. However, IHC is subject to a number of technical artifacts and sensitivity differences between different antibodies and tissue pretreatments. 3 Standardized reagent kits have recently been introduced (such as HercepTest), but mixed results have been reported from their methodological comparisons. 4-8
FISH quantifies the number of gene copies in the cancer cell nucleus. Since initial applications to detect HER-2 amplification by FISH, 9,10 a number of reports have verified its accuracy both in freshly frozen and paraffin-embedded tumor material. 6,11 FISH is done either using single-color (HER-2 probe only, DAKO, Copenhagen, Denmark) or as a dual-color hybridization (using HER-2 and chromosome 17 centromere probes simultaneously), the latter making it easier to distinguish true HER-2 amplification from chromosomal aneuploidy. FISH from entire cells (cultured cells, pulverized tissue, or imprint touch specimens from tumors) is considered straightforward, but the use of tissue sections complicates the quantitative nature of FISH because of nuclear truncation (slicing).
The main difficulty for adopting FISH in clinical diagnostics is the need to use fluorescence microscopy, which is not done in most routine diagnostic laboratories. Evaluation of FISH requires a modern epifluorescence microscope equipped with high-quality ×60 and ×100 oil immersion objectives and multi-bandpass fluorescence filters. Moreover, because the fluorescence signals fade within a few weeks, the hybridization results must be recorded with expensive digital cameras.
To overcome these practical limitations, we introduce here chromogenic in situ hybridization (CISH), in which the DNA probe is detected using a simple IHC-like peroxidase reaction. The method was compared with FISH made from frozen tumor samples and IHC (using mAb CB11 on adjacent paraffin-embedded sections).
Materials and Methods
Tumors
One hundred fifty-seven tumors were prospectively collected at the Jules Bordet Institute, Brussels, Belgium. Histopathological classification and grading was done on hematoxylin and eosin (H&E)-stained slides according to standard histopathological practice. 12
FISH
FISH was done at the Jules Bordet Institute, and the data comes from a previously published study. 12 In brief, a fresh tumor sample of 0.5 cm 3 or a freshly made imprint touch preparation were obtained immediately after surgery. Cells from tumor pieces were mechanically disintegrated, centrifuged, and treated with 0.075 mol/L KCl for 1 hour at 37°C. After washing in methanol:acetic acid (3:1) the cells were spread onto microscope slides. The slides were denatured in 70% formamide/2× standard saline citrate, pH 7, at 73°C for 10 minutes. After dehydration in an ethanol series, 10 μl of the probe (LSI HER-2/CEP17; Vysis Inc., Downers Grove, IL) was denatured (73°C for 5 minutes) and applied onto slides. The hybridization was performed overnight at +37°C in a moist chamber. Excess of the probes was washed in 0.4× standard saline citrate (at 73°C, 2 minutes), followed by 0.4× standard saline citrate/0.1% Nonidet P-40 (2 minutes at room temperature). Nuclei were counterstained with 4′,6-diamino-2 phenylindole dihydrochloride (DAP1) (1 μg/ml) in an antifade embedding solution (p-phenylene-diamine dihydrochloride).
Hybridization signals were enumerated in at least 150 to 250 morphologically intact and nonoverlapping nuclei. We used a Leica DMRB epifluorescence microscope equipped with a ×100 oil immersion objective and a triple bandpass filter for simultaneous detection of Spectrum Green, Spectrum Orange, and 4′,6-diamino-2 phenylindole dihydrochloride (filter from ChromaTechnology, Tucson, AZ). Her-2/neu amplification was determined as a ratio of HER-2 and chromosome 17 centromere signal counts. Ratios <2 were determined as no amplification, those between 2 and 5 as low-level amplification, and those >5 as high-level amplification.
CISH
CISH was done on 5-μm-thick archival formalin-fixed paraffin-embedded tissue sections at the University of Tampere. In brief, the sections were deparaffinized and incubated in pretreatment buffer in a temperature-controlled microwave oven at 92°C for 10 minutes, using a Spot-Light FFPE reagent kit (Zymed Inc., South San Francisco, CA). The sections were then allowed to cool down for 20 minutes and then washed with phosphate-buffered saline (PBS). Enzymatic digestion was done by applying 100 μl of FFPE digestion enzyme on to slides (10 to 15 minutes at room temperature). The slides were then washed with PBS and dehydrated with graded ethanols. The ready-to-use digoxigenin-labeled HER-2/neu probe (consisting of two contig BAC clones; Zymed) was applied onto slides which were covered under 14 × 14-mm coverslips (10 μl probe mixture/slide). The slides were denatured on a hot plate (94°C) for 3 minutes, and the hybridization was performed overnight at 37°C. After hybridization, the slides were washed with 0.5× standard saline citrate for 5 minutes at 75°C, followed by three washes in PBS/0.2% Tween 20 at room temperature. The HER-2/neu probe was detected with sequential incubations with anti-digoxigenin fluorescein, anti-fluorescein peroxidase, and diaminobenzidine according to the manufacturer’s instructions (Zymed). Tissue sections were lightly counterstained with hematoxylin and embedded.
The CISH hybridizations were evaluated using an Olympus BX50 microscope equipped with ×40 and ×60 dry objectives and using 10 × 22 wide-field oculars. Unaltered gene copy number was defined as one to five signals per nucleus. Low-level amplification was defined as six to 10 signals per nucleus in >50% of cancer cells, or when a small gene copy cluster was found. Amplification of HER-2 was defined when a large gene copy cluster in >50% of carcinoma cells or numerous (>10) separate gene copies were seen. Images were captured using a Pixera PVC100C digital camera (Pixera Corp., Los Gatos, CA).
IHC
IHC of HER-2/neu protein (p185HER2) was done on tissue sections adjacent to those used in CISH at the University of Tampere. The sections were deparaffinized followed by antigen-retrieval in 0.01 mol/L citrate buffer, pH 7.3, at 94°C for 20 minutes, using a temperature-controlled microwave oven. After blocking for nonspecific antibody binding (using the blocking reagent Histostain Plus kit, Zymed), the sections were incubated overnight (at 4°C) with a monoclonal antibody to the intracellular domain of HER-2/neu protein (clone CB-11; Novocastra Laboratories, Newcastle UK). A standard avidin-biotin-peroxidase complex technique was used for visualization, with diaminobenzidine as the chromogen (Histostain Plus kit). Intense cell membrane immunoreaction present in >50% of cancer cells was considered as 3+ staining and was considered as overexpression of p185HER-2. Staining present in a smaller proportion of cells or that with lower intensity was considered as 2+ staining. The controls consisted of three cell lines (SK-BR-3, >30 gene copies of HER-2/neu; MDA-MB-453, eight gene copies; and ZR-75–1, two gene copies) were fixed overnight with 10% formalin and pelleted as a normal paraffin block.
Evaluation of CISH, FISH, and IHC were done in a blinded manner, ie, unaware of the results of the other assays.
Results
CISH was successful in 157 of 160 (98%) formalin-fixed paraffin-embedded breast cancer samples attempted. Gene copies visualized by CISH were clearly distinguishable using a ×40 objective in tissue sections which were lightly counterstained with hematoxylin (Figure 1A) ▶ . Amplified gene copies appeared typically as large peroxidase-positive intranuclear gene copy clusters (Figure 1B) ▶ or as numerous individual peroxidase-positive small signals (Figure 1C) ▶ . Tumors with no amplification showed typically 1 to 2 spots per nucleus (when diploid), or two to four spots in chromosomally aneuploid cases.
Figure 1.
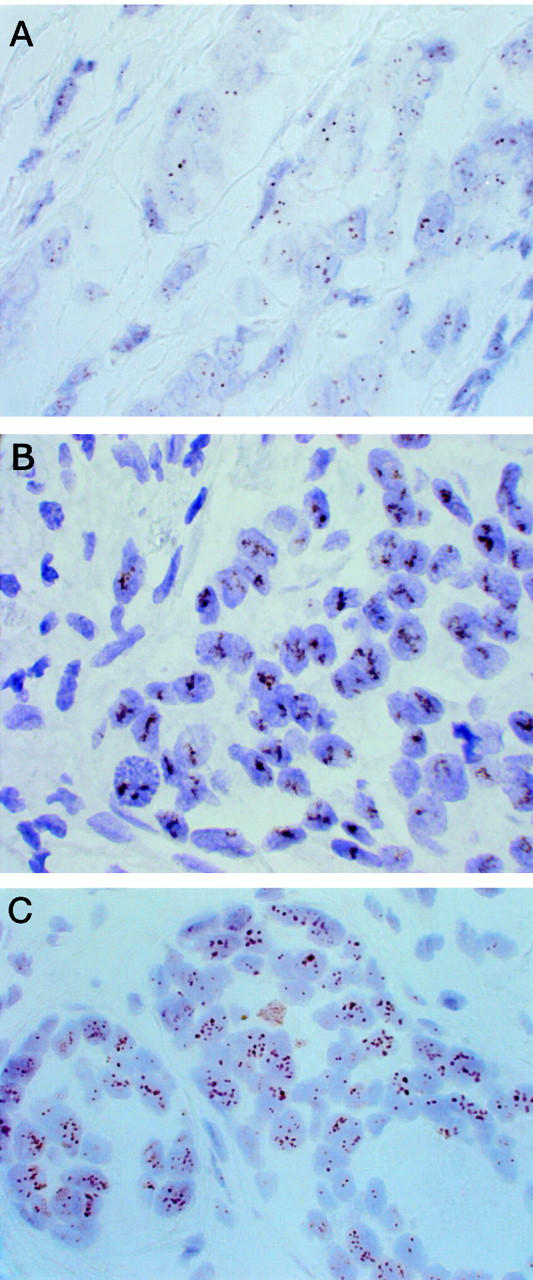
Examples of CISH of HER-2 oncogene in breast cancer. A: A tumor with one to two clearly identifiable copies of HER-2/neu gene (no amplification). A typical HER-2/neu amplification appears either as a peroxidase-positive cluster of gene copies (B), or as multiple individual gene copies (C). Original magnification, ×600. Counterstained with hematoxylin.
Comparison of CISH, FISH, and IHC
We correlated the results obtained by CISH to those by FISH made on cells prepared from a fresh tumor sample. In a series of 157 unselected breast cancers the prevalence of HER-2 amplification was 23.6% by FISH and 17.2% by CISH. There were 120 tumors with no amplification and 27 with amplification by both methods (Table 1) ▶ . FISH found HER-2 amplification in 10 tumors which were negative by CISH (five gene copies or less) (Table 1) ▶ . The kappa coefficient, measuring agreement between the methods (0, no agreement; 1, perfect agreement) was 0.81 (95% confidence interval, 0.69 to 0.92).
Table 1.
Comparison between CISH and FISH in the Detection of HER-2/neu Amplification in 157 Breast Cancers
| FISH | CISH | |
|---|---|---|
| No amplification (%) | Amplification (%) | |
| No amplification | 120 (76.4) | 0 (0) |
| Amplification | 10 (6.4) | 27 (17.2) |
Kappa coefficient, 0.81 (95%. C??i, 0.69 to 0.92).
HER-2 gene amplification by CISH and FISH was also compared with p185HER2 protein overexpression detected by IHC (using monoclonal antibody CB-11) (Table 2) ▶ . IHC was somewhat less sensitive but generally in good agreement with FISH and CISH. The prevalence of HER-2 overexpression was 19.7%. There were 11 tumors positive by FISH but negative by IHC, but only two such tumors positive by CISH. Only one of the immunohistochemically weakly positive (2+) tumors was found amplified by CISH or FISH. Table 3 ▶ lists the results of CISH, FISH, and IHC of the 10 tumors in which CISH and FISH were in disagreement.
Table 2.
Relationship between HER-2/neu Amplification Determined by FISH and CISH and p185HER2 Overexpression by Immunohistochemistry
| Immunohistochemistry with monoclonal antibody CB-11* | |||
|---|---|---|---|
| Negative (0 or 1+) | Weakly positive (2+) | Positive (3+) | |
| FISH | |||
| No amplification | 115 | 4 | 1 |
| Amplification | 11 | 1 | 25 |
| CISH | |||
| No amplification | 124 | 5 | 1 |
| Amplification | 2 | 0 | 25 |
*Using standard microwave oven heating for antigen retrieval.
Table 3.
Results of HER-2/neu CISH, FISH and Immunohistochemistry in Cases with Disagreement between the Methods
| Tumor no. | FISH | CISH | Immunohistochemistry |
|---|---|---|---|
| 22 | Low-level amplification* | Not amplified | Negative (0 or 1+) |
| 41 | Low-level amplification | Not amplified | Negative (0 or 1+) |
| 52 | Low-level amplification | Not amplified | Negative (0 or 1+) |
| 54 | Low-level amplification | Not amplified | Negative (0 or 1+) |
| 88 | High-level amplification† | Not amplified | Negative (0 or 1+) |
| 106 | Low-level amplification | Not amplified | Weakly positive (2+) |
| 123 | Low-level amplification | Not amplified | Negative (0 or 1+) |
| 126 | Low-level amplification | Not amplified | Negative (0 or 1+) |
| 127 | Low-level amplification | Not amplified | Negative (0 or 1+) |
| 135 | Low-level amplification | Not amplified | Negative (0 or 1+) |
*Ratio between HER-2/neu and 17 centromere signals 2 to 5.
†Ratio between HER-2/neu and 17 centromere signals >5.
Discussion
The present study demonstrated the utility of enzymatic (peroxidase) detection in the determination of HER-2 amplification in paraffin-embedded tumor samples. To the best of our knowledge oncogene amplification has been detected with enzymatic DNA in situ hybridization only in one previous study. 13 The main reason for preferring fluorescence detection has been the superior signal-to-noise ratio of the hybridization signal when modern epifluorescence microscopes are used. Although bright-field detection is relatively simple and straightforward with chromosome centromere probes, 14 the difficulty to obtain bright enough signals with gene-specific cosmid, P1, PAC, and BAC clones has impaired the detection of unique sequence probes with bright-field microscopy techniques. The key solution to the problem was to use a contig of two BAC clones, after removing their repetitive DNA sequences (eg, Alu and LINE elements), which cause unspecific hybridization. 15 According to our experience, the modified probe gives much brighter and more crisp signals than ordinary P1 and BAC probes in CISH, and yet satisfactory signals are obtained by both probe types in FISH. Another advance in the present CISH method is the pretreatment of tissue sections with a combination of heating in a microwave oven and a short enzyme digestion. After microwave treatment the need for individual adjustment of enzymatic digestion was not needed for a vast majority of tissue sections, which is often the case when using sodium bisulphite treatment or enzymatic digestion alone. 16 The detection system used in CISH (anti-digoxigenin-fluorescein isothiocyanate plus anti-fluorescein-isothiocyanate horseradish peroxidase plus diaminobenzidine chromogen) was found to have superior sensitivity over direct (conventional) immunodetection (anti-digoxigenin horseradish peroxidase plus diaminobenzidine). Tyramide-based signal enhancement was not found useful in this application.
After solving the issues related to lower sensitivity of peroxidase-based probe detection, the advantages of CISH over FISH in routine diagnostics of HER-2 amplification are obvious. First, CISH hybridization is much faster to analyze than FISH, and verification of histopathology can be done simultaneously from the tissue section that is counterstained with hematoxylin. In FISH the representativity of the cells selected for signal enumeration is based on nuclear DAP1 staining, which does not allow sufficient histopathological evaluation of the cells. Thus, in routine diagnostics FISH is prone to sampling error, although this can be minimized by using adjacent H&E-stained slides.
The current version of HER-2 CISH is based on single color detection of one probe, similarly as in the Food and Drug Administration-approved FISH test (INFORM HER-2/neu test, Ventana Medical Systems, Tucson, AZ). We used a similar definition for amplification, ie, more than five gene copies in at least 50% of the cancer cells. In our material approximately half of the nonamplified tumors showed one to two signals per cell, and half showed three to five copies/nucleus. The latter is because of chromosomal aneuploidy and should be interpreted as no HER-2 amplification. Because of DNA replication during S and G2/M phases of the cell cycle, a small proportion (10 to 30%) of aneuploid cancer cells may contain five to eight signals/nucleus. This should also be regarded as a negative finding (no HER-2 amplification). The theoretical advantage of two-color FISH (Vysis) is its ability to distinguish chromosomal amplification from aneuploidy using a differentially labeled reference probe (chromosome 17 centromere). However, as yet there are no large comparisons between Vysis’ two-color FISH and the CISH-like single-color FISH which could confirm the advantage of the two-color system.
In our series, the agreement between CISH with FISH was generally very good. However, there were 10 tumors (6.4%) defined as amplified by FISH but not amplified by CISH. This suggests that CISH may have a lower sensitivity. However, an alternative explanation is the difference in the sample materials. FISH was done on entire nuclei derived from freshly frozen tissue material, whereas CISH used paraffin-embedded tissue sections, which are technically more difficult to hybridize. When one examines the discordant tumors in detail (Table 3) ▶ , it appears that all but one tumor was scored as having a borderline low-level amplification in FISH (copy number ratio, 2 to 5). Moreover, eight of these tumors were negative by CB-11 IHC (one had 2+ staining). Thus the discordancies may simply reflect the fact that the threshold for calling low-level amplification may have been too low, and that it may not always associate with gene amplification which causes p185HER2 overexpression. Moreover, unbiased assessment of sensitivity and specificity of CISH (versus FISH) would require a much larger set of tumors, where the effect of a few individual tumors would not be as large as in this material. The superiority of one HER-2/neu test over another can best be solved by using the clinical response to trastuzumab therapy as reference.
In terms of feasibility and accuracy, CISH provides a tempting alternative to HER-2 IHC, the specificity and sensitivity problems of which have been demonstrated in several studies. 3-8 In IHC it is not possible to identify a false-negative staining result or to discriminate whether a 2+ staining result is because of HER-2 gene amplification (and a likely response to Herceptin), or because of amplification-unrelated p185HER2 expression or nonspecific staining. In CISH, the diagnosis of unaltered HER-2 gene status is based on the presence of one to five gene copies in cancer cells. If CISH shows no hybridization signals, the hybridization had failed because of technical reasons. A typical gene amplification by CISH is a peroxidase-positive cluster of multiple gene copies, which is easy to identify in the microscope with a ×40 objective magnification. The exact enumeration of gene copies is not always possible, but at least in routine diagnostics enumeration of gene copies exceeding 10 is not needed. The most difficult category in CISH is the low-level amplification (six to 10 gene copies/cell), in which accurate enumeration of the gene copies is necessary. However, even in these cases the microscopic evaluation is much faster than that of FISH.
In conclusion, the present results provide a proof-of-principle of CISH in the detection of HER-2 amplification in archival paraffin-embedded breast tumor samples. Because most pathologists are not familiar with fluorescence microscopy but are experienced in evaluating CISH-like peroxidase-based immunostainings, the time and effort needed for learning evaluation of CISH is much shorter than that of FISH. Moreover, CISH does not require any equipment that doesn’t already exist in routine histopathology laboratories. Although larger tumor materials need to be tested before the exact performance of CISH can be assessed, the present results already make CISH as a tempting alternative to FISH as a secondary test to clarify the 2+ results of immunohistochemical staining. Alternatively, because of the feasibility and relative low cost, combined use of IHC and CISH can be considered instead of FISH in the primary tumor screening of HER-2/neu oncogene amplification status.
Acknowledgments
The skillful technical assistance of Mrs. Anne Luuri, Mrs. Sari Toivola, and Ms. Päivi Järvinen is greatly appreciated.
Footnotes
Address reprint requests to Prof. Jorma Isola, University of Tampere, Institute of Medical Technology, P.O. Box 607, FIN-33101 Tampere, Finland. E-mail: jorma.isola@uta.fi.
Supported by The Finnish Academy of Sciences, The Finnish Cancer Society, The Sigrid Jusélius Foundation, Maud Kuistila Foundation, Finnish Cultural Foundation, the Tampere University Hospital Research Foundation, and “les Amis de l’Institut Bordet” Brussels, Belgium.
References
- 1.Ross JS, Fletcher JA: HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol 1999, 112:S53-S67 [PubMed] [Google Scholar]
- 2.Shak S: Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Herceptin Multinational Investigator Study Group. Semin Oncol 1999, 6:71-77 [PubMed] [Google Scholar]
- 3.Press MF, Hung G, Godolphin W, Slamon DJ: Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res 1994, 54:2771-2777 [PubMed] [Google Scholar]
- 4.Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ: Specificity of Hercep Test in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol 1999, 17:1983. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ: Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol 1999, 17:1974. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell MS, Press MF: The role of immunohistochemistry and fluorescence in situ hybridization for HER2/neu in assessing the prognosis of breast cancer. Semin Oncol 1999, 26:108-116 [PubMed] [Google Scholar]
- 7.Jimenez RE, Wallis T, Tabasczka P, Visscher DW: Determination of Her-2/Neu status in breast carcinoma: comparative analysis of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol 2000, 13:37-45 [DOI] [PubMed] [Google Scholar]
- 8.Espinoza F, Anguiano A, Roche PC, Ingle JN: The HercepTest assay: another perspective. J Clin Oncol 1999, 17:2293B. [DOI] [PubMed] [Google Scholar]
- 9.Kallioniemi OP, Kallioniemi A, Kurisu W, Thor A, Chen LC, Smith HS, Waldman FM, Pinkel D, Gray JW: Amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci USA 1992, 89:5321-5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauletti G, Godolphin W, Press MF, Slamon DJ: Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996, 13:63-72 [PubMed] [Google Scholar]
- 11.Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, Hung G, Robinson RA, Harris C, El-Naggar A, Slamon DJ, Phillips RN, Ross JS, Wolman SR, Flom KJ: HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 1997, 15:2894-2904 [DOI] [PubMed] [Google Scholar]
- 12.Gancberg D, Lespagnard L, Rouas G, Paesmans M, Piccart M, Di Leo A, Nogaret JM, Hertens D, Verhest A, Larsimont D: Sensitivity of HER-2/NEU antibodies in archival tissues samples of unselected breast carcinomas: correlation with oncogene amplification in 160 cases. Am J Clin Pathol 2000, 113:675-682 [DOI] [PubMed] [Google Scholar]
- 13.Vos CB, Ter Haar NT, Peterse JL, Cornelisse CJ, van de Vijver MJ: Cyclin D1 gene amplification and overexpression are present in ductal carcinoma in situ of the breast: J Pathol 1999, 187:279-284 [DOI] [PubMed] [Google Scholar]
- 14.Hopman AH, Claessen S, Speel EJ: Multi-colour brightfield in situ hybridisation on tissue sections: Histochem Cell Biol 1997, 108:291-298 [DOI] [PubMed] [Google Scholar]
- 15.Davison JM, Morgan TW, Hsi BL, Xiao S, Fletcher JA: Subtracted, unique-sequence, in situ hybridization: experimental and diagnostic applications. Am J Pathol 1998, 153:1401-1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull JH, Harnden P: Efficient nuclear FISH on paraffin-embedded tissue sections using microwave pretreatment. BioTechniques 1999, 26:416-422 [DOI] [PubMed] [Google Scholar]


