Abstract
Nephrin is a cell adhesion protein located at the slit diaphragm area of glomerular podocytes. Mutations in nephrin-coding gene (NPHS1) cause congenital nephrotic syndrome (NPHS1). We studied the developmental expression of nephrin, ZO-1 and P-cadherin in normal fetal kidneys and in NPHS1 kidneys. We used in situ hybridization and immunohistochemistry at light and electron microscopic levels. Nephrin and zonula occludens-1 (ZO-1) were first expressed in late S-shaped bodies. During capillary loop stage, nephrin and ZO-1 localized at the basal margin and in the cell-cell adhesion sites between developing podocytes, especially in junctions with ladder-like structures. In mature glomeruli, nephrin and ZO-1 concentrated at the slit diaphragm area. P-cadherin was first detected in ureteric buds, tubules, and vesicle stage glomeruli. Later, P-cadherin was seen at the basal margin of developing podocytes. Fetal NPHS1 kidneys with Fin-major/Fin-major genotype did not express nephrin, whereas the expression of ZO-1 and P-cadherin was comparable to that of control kidneys. Although early junctional complexes proved structurally normal, junctions with ladder-like structures and slit diaphragms were completely missing. The results indicate that nephrin is dispensable for early development of podocyte junctional complexes. However, nephrin appears to be essential for formation of junctions with ladder-like structures and slit diaphragms.
Congenital nephrotic syndrome of the Finnish type (NPHS1, CNF) is an autosomal recessive disease leading to fetal proteinuria and nephrotic syndrome soon after birth. 1,2 The gene mutated in this disease has recently been identified and named NPHS1. 3 NPHS1 codes for nephrin, a 1241-amino acid transmembrane protein of the immunoglobulin superfamily. 3 Nephrin is expressed in glomerular visceral epithelial cells (podocytes, GECs) and is located at the slit diaphragm area. 4-7
The slit diaphragm is a filamentous structure spanning the slit pore between the adjacent podocyte foot processes. 8 Recent findings indicate that the slit diaphragm is an essential structure in the glomerular filtration barrier for restricting the passage of plasma proteins into urine. 9,10 Based on electron microscopy, an isoporous, zipper-like structure for the slit diaphragm has been suggested. 11 A model for nephrin assembly into this isoporous filter was recently presented. 4,9 The molecular composition of the slit diaphragm is, however, still largely unknown. The tight junction protein, zonula occludens-1 (ZO-1), is found at the cytoplasmic side of the slit diaphragm. 12,13 The CD2-associated protein (CD2AP) is present in the podocyte foot processes, and it probably anchors nephrin to podocytes. 14 In addition, P-cadherin was recently localized at the slit diaphragm. 15
The slit diaphragm has been thought to originate from the subapical junctional complexes of immature visceral epithelial cells (primordial podocytes). 16 During glomerulogenesis, these junctions migrate along the lateral cell margins toward the basal surface to form mature slit diaphragms. 16 Based on the presence of the tight junction protein ZO-1 at the insertion site of the slit diaphragm, it has been assumed that the slit diaphragm represents a modified tight junction. 12 On the other hand, Reiser et al recently provided evidence that the slit diaphragm could be a P-cadherin-based adherens junction. 15
Here we studied the developmental expression of nephrin in human fetal kidneys and compared it to that of ZO-1 and P-cadherin. We also evaluated the morphogenesis of podocyte filtration slits in normal and NPHS1 kidneys lacking the nephrin molecule. The cytochemical and morphological data obtained indicate that nephrin is crucial for the final development of the slit diaphragm.
Materials and Methods
Tissue Samples
Tissue samples were collected at autopsy from human fetuses at 14, 16, 22, and 23 weeks of gestation obtained through prostaglandin-induced abortions due to anencephaly, gastroschisis, trisomy 18, and trisomy 21 (Department of Obstetrics and Gynecology, University of Helsinki). In these disorders, abnormalities have not been detected in chromosomes 15, 16, and 19, where the genes for ZO-1, P-cadherin, and nephrin, respectively, are located. 3,17,18 The autopsies were performed within a few hours after the abortion. These fetuses showed no macroscopic or histological abnormalities in kidneys or urinary tract. In addition, renal samples from two fetuses at 17 and 19 weeks of gestation aborted due to NPHS1 suspicion were collected. Both showed elevated levels of amniotic fluid α-fetoprotein and normal ultrasonography features. Mutation analysis of NPHS1 was performed as described earlier. 3 Adult control samples came from cadaver kidneys unsuitable for transplantation because of vascular abnormalities (from the IV Department of Surgery, University of Helsinki). Considering the suboptimal fixation conditions for specimen preparation (ie, autopsy samples obtained from abortions), we used only tissue blocks with adequate structural preservation for labeling.
For light microscopy, the tissue samples were snap frozen in cold isopentane cooled in liquid nitrogen, and fixed in 3.5% paraformaldehyde in phosphate buffer (0.1 mol/L, pH 7.3). In situ hybridization and immunoperoxidase studies were also performed on paraffin-embedded samples that had been fixed in 10% formalin in phosphate buffer (0.1 mol/L, pH 7.3). For immunoelectron microscopy, tissue blocks were cut into 1-mm 3 pieces and immersed in fixative (3.5% paraformaldehyde supplemented with 0.02–0.1% glutaraldehyde). Samples for morphological evaluation in electron microscopy were fixed in 2.5% glutaraldehyde followed by 1% OsO4.
This study was approved by the ethical committees of the Department of Obstetrics and Gynecology and The Hospital for Children and Adolescents of the University of Helsinki.
In Situ Hybridization
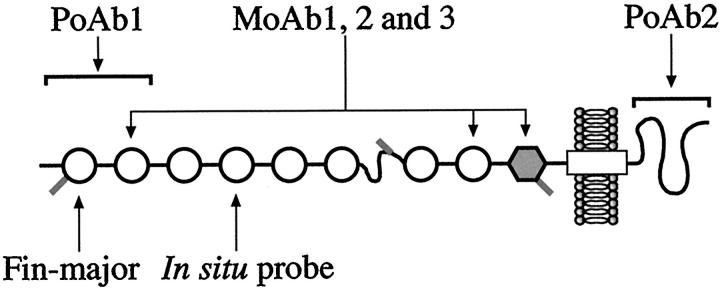
The probes were synthesized by subcloning a cDNA fragment of 287 bp corresponding to exon 10 in human NPHS1 gene into pBluescript, and antisense and sense RNAs produced using T3 and T7 RNA polymerases, respectively. 3 Exon 10 encodes for the fourth extracellular immunoglobulin (Ig) domain of nephrin (Figure 1) ▶ . Paraffin-embedded sections (10 μm) were deparaffinized in xylene, rehydrated in decreasing alcohol series, and treated with proteinase-K (Sigma Chemical, St. Louis, MO) before hybridization. Thereafter both the cryosections and paraffin-embedded sections were subjected to in situ hybridization, as previously described, 19 with some modifications. Briefly, the tissue sections were washed in phosphate buffered saline (PBS), acetylated, dehydrated, and then incubated with 1.2 × 10 6 of 33P-labeled antisense and sense riboprobes (1000 Ci/mmol; Amersham, Arlington Heights, IL) in a total volume of 80 μl at 60°C for 18 hours. After washes with standard saline citrate, RNA digestion, and dehydration, the sections were dipped in NTB2 nuclear emulsion (Kodak, Rochester, NY) and exposed in the dark at 4°C for 2 weeks. After developing, the sections were counterstained with hematoxylin and eosin. Microscopy was performed with standard Leica DM RX light microscope (Wetzlar, Germany).
Figure 1.
Nephrin molecule and its epitopes. Nephrin is a transmembrane adhesion protein with 1241 amino acid residues. The extracellular part contains eight immunoglobulin domains (open circles) and one fibronectin domain (gray hexagon). Polyclonal rabbit antibodies (pAb 1) were raised against the two terminal Ig-domains (residues 22–240). The other polyclonal antibody, pAb 2, reacted against the terminal intracellular part of nephrin (residues 1084–1241). The third antibody preparation contained three monoclonal antibodies (mAb 1, 2, and 3) reacting against the second and eighth Ig-domains and fibronectin domain. Fin-major mutation is a 2-bp deletion leading to a formation of only 90 amino acid residues. The probe used for in situ hybridization studies recognized exon 10, which encodes the fourth Ig domain of nephrin.
Antibodies
Three types of anti-nephrin antibodies were used: i) polyclonal rabbit antibodies against the extracellular part (pAb 1), ii) polyclonal rabbit antibodies against the intracellular part (pAb 2), and iii) a pool of three mouse monoclonal antibodies against the extracellular domains of nephrin (mAb 1, 2, and 3). The locations of the nephrin epitopes of these antibodies are shown in Figure 1 ▶ .
The preparation of pAb 1 directed against the two terminal extracellular Ig domains of nephrin (amino acids 22–240) has been described earlier. 4 The antigen for pAb 2 against the intracellular part of nephrin was prepared as follows. First, the intracellular portion of nephrin was expressed as six-histidine-tagged mouse dihyrofolate reductase fusion protein in Escherichia coli using QIAexpressionist expression system (Qiagen, Hilden, Germany). Then, a DNA fragment coding for amino acids 1084–1241 of human nephrin was amplified by polymerase chain reaction and cloned into vector PUC18 with Sureclone ligation kit (Pharmacia, Uppsala, Sweden). The fragment was cleaved from the vector using cleavage sites for BgIII and HindIII synthesized into polymerase chain reaction primers. The cleaved fragment was ligated into BgIII/HindIII-digested vector pQE-40. This expression plasmid was transformed into E. coli strain XL-1 blue. The fusion protein was expressed at high levels only as insoluble inclusion bodies. Protein purification was initiated by lysing bacterial cells by brief sonication and pelleting the inclusion bodies at 10,000 × g for 30 minutes. The pellet was washed with 0.2% sodium deoxycholate in Tris-buffered saline and recentrifugated. Insoluble proteins were solubilized and the fusion protein was purified in 8 mol/L urea using affinity chromatography. The purified intracellular nephrin was used to raise polyclonal antibodies in three New Zealand White rabbits using standard protocols (SVA, Uppsala, Sweden). Antisera titers were measured in enzyme-linked immunosorbent assay using the antigen as capture reagent.
Generation and characterization of the mouse monoclonal anti-nephrin antibodies (mAb 1, 2, and 3) is described in detail elsewhere (Ruotsalainen V, Putaala H, Tryggvason K, manuscript in preparation). These antibodies were derived from a mouse immunized with a recombinant nephrin produced in human embryonic kidney A293 cell line. The antigen contained the whole extracellular portion of the molecule (amino acids 1–1059 fused to a sequence of six histidines). The epitopes of the three antibodies were characterized. They recognize the second and eighth extracellular Ig domains and the fibronectin domain, as shown in Figure 1 ▶ . A pool of these antibodies was used in immunohistochemistry.
The specificity of the anti-nephrin antibodies was confirmed by Western blotting. All preparations (pAb 1, pAb 2, and the pool of mAb 1, 2, and 3) gave an immunoreactive band at the size of nephrin (180 kd) when glomerular extract was used as an antigen (data shown elsewhere). 4,20
Rabbit polyclonal antibody, directed against the cytoplasmic slit diaphragm protein ZO-1 was purchased from Zymed Laboratories (San Francisco, CA). Mouse monoclonal anti-P-cadherin antibodies were purchased from Transduction Laboratories (Lexington, KY).
The polyclonal antibodies against the extracellular part of nephrin (pAb 1) were used exclusively for immunoelectron microscopy. The polyclonal antibodies against the intracellular part of nephrin (pAb 2), as well as ZO-1 antibodies, were used in immunoperoxidase and immunofluorescence stainings and in immunoelectron microscopy. The pool of three monoclonal anti-nephrin antibodies (mAb 1, 2, and 3), as well as P-cadherin antibodies, were used only in immunoperoxidase and immunofluorescence stainings.
Immunoperoxidase Staining
Cryosections and deparaffinized sections (3 μm) were incubated in 3% hydrogen peroxide to quench endogenous peroxidase activity, followed by microwave treatment exclusively for paraffin sections in 10 mmol/L citric acid for 10 minutes to improve antibody penetration. Primary antibodies were diluted to PBS and incubated for 2 to 3 hours at room temperature. Amplification of the primary antibody reaction was achieved by incubating the sections with biotinylated anti-rabbit-IgGs and anti-mouse-IgGs (Vector Laboratories, Burlingame, CA) for 30 minutes. This was followed by a complex of avidin and biotinylated peroxidase (Vector Laboratories) for 30 minutes. Each incubation was followed by three 5-minute washes in PBS. The binding was visualized using diaminobenzidine substrate (Dako Corp., Carpinteria, CA), and the tissues were counterstained with Harris’s hematoxylin. Controls included replacement of the primary antibodies with PBS or nonimmune rabbit or mouse IgGs. Microscopic observations were carried out with a standard Leica DM RX light microscope.
Double Immunofluorescence Staining
The free aldehyde groups from cryosections (5 μm) were blocked with glycine-PBS and nonspecific binding sites with 3% bovine serum albumin (BSA) and 1% gelatin in PBS (blocking solution). The antibodies were diluted in the blocking solution and incubations were performed sequentially. The monoclonal anti-nephrin antibodies were incubated overnight at 4°C, and anti-ZO-1 antibodies 2 to 3 hours at room temperature. P-cadherin antibodies were incubated overnight at 4°C, followed by polyclonal anti-nephrin antibodies for 2 to 3 hours at room temperature. Cyanine- (Cy2, Cy3) and rhodamine-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were incubated for 2 hours. Each incubation was followed by three 5-minute washes with PBS; finally, sections were mounted in Mowiol (Calbiochem, La Jolla, CA). For controls, sections were incubated in PBS or nonimmune rabbit or mouse IgGs instead of a primary antibody. The sections were examined either with a standard Leica DM RX light microscope or Leica TCS NT confocal laser scanning microscope.
Immunoelectron Microscopy
Immunoelectron microscopy was performed on heat-cured LR-white resin sections (London Resin, Besingstoke, UK) as described earlier. 4 Briefly, thin sections were collected on Pioloform-carbon-coated nickel grids (Agar Scientific, Stansted, UK) and incubated in the primary antibodies diluted in blocking solution. After incubation in diluted 5- or 10-nm gold-conjugated secondary antibodies in 0.3% BSA-PBS, the sections were post-stained in 1% uranyl acetate. Double labeling for nephrin (visualized by 10 nm gold goat anti-rabbit IgG) and ZO-1 (visualized by 5 nm gold goat anti-rabbit IgG) was performed sequentially on different sides of thin resin sections, as described earlier. 21 For this, thin sections were collected into uncoated grids. Thus, any possible interaction between the two sets of labeling reactions were avoided. Post-staining with 1% uranyl acetate was performed on only one side of the grid. Controls included incubation of grids after the same procedure but incubating with a nonimmune rabbit IgG as a primary antibody. The sections were examined with a Jeol 1200 EX electron microscope (Tokyo, Japan) at 60 kV. Note that the fixation and embedding protocol used yielded satisfactory labeling density but very low image contrast. Therefore, intracellular structures were hardly visible, except for the nucleus.
Morphological Electron Microscopy
Electron microscopy was performed according to standard procedures (fixation in 2.5% glutaraldehyde, followed by OsO4 and Epon embedding). Thin sections were post-stained with 1% uranyl acetate and lead. The sections were examined with a Jeol 1200 EX electron microscope at 60kV.
Results
Nephrin in Normal Glomerulogenesis
Nephrin mRNA was first detected in late S-shaped bodies as studied by in situ hybridization of 13- to 23-week human fetal kidney samples (Figure 2, C and D) ▶ . The signal was localized to the area next to vascular cleft. Earlier stages of glomerulogenesis (vesicle, comma-shaped, and early S-shaped bodies) remained negative for nephrin mRNA (Figure 2, A and B) ▶ . All glomeruli of capillary loop and maturing stages were strongly positive for nephrin mRNA. The signal was most intense at early capillary loop stage (Figure 2, E and F) ▶ . In situ hybridization experiments with nephrin sense riboprobe, used as controls, revealed only background staining (data not shown).
Figure 2.
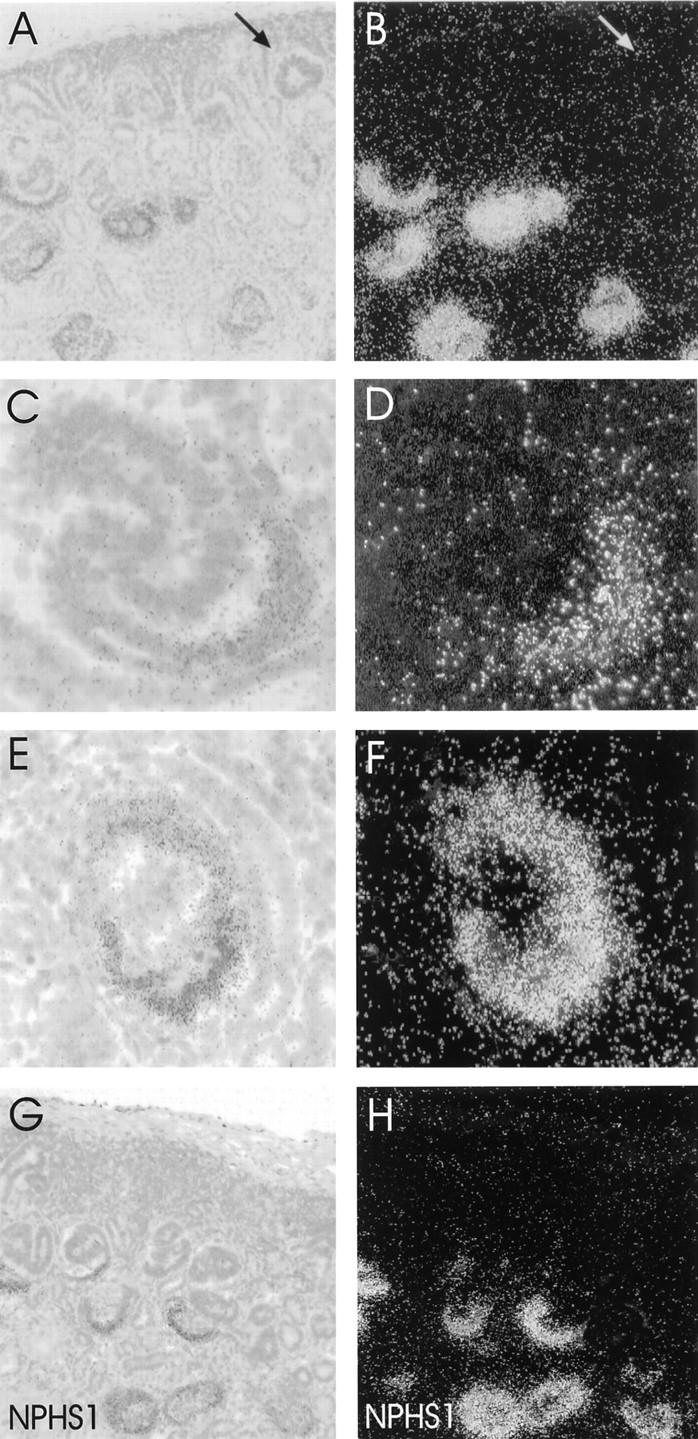
In situ hybridization for nephrin mRNA. Brightfield hematoxylin-eosin and respective darkfield views. A and B: Normal human fetal kidney cortex. Early stages of glomerular development beneath the capsule remain negative for nephrin mRNA. Arrow indicates a vesicle stage glomerulus. C and D: The synthesis of nephrin mRNA begins in late S-shaped glomeruli. Signal for nephrin mRNA is found adjacent to vascular cleft. E and F: Capillary loop stage glomeruli showing strong synthesis of nephrin mRNA. G and H: Fetal NPHS1 kidney with Fin-major/Fin-major genotype. The expression pattern for nephrin mRNA is similar to that seen in normal fetal kidney. Original magnifications, ×80 (A, B, G, H) and ×500 (C−F).
Polyclonal antibodies against the intracellular part of and monoclonal antibodies against the extracellular part of nephrin gave a similar staining pattern in immunoperoxidase and immunofluorescence stainings of human fetal kidneys. Nephrin gave a faint but clear reactivity in columnar epithelial cells adjacent to vascular cleft in the late S-shaped bodies (Figure 3A) ▶ . At capillary loop stage, nephrin was present along the basal margins and also as discrete lines between the developing podocytes (Figure 3B) ▶ . Moreover, intensely stained dots could be detected at lateral surfaces of adjacent developing podocytes (Figure 3C) ▶ . These were found in 36% (25/70) of the analyzed capillary stage glomeruli. In maturing stage glomeruli, nephrin reactivity was present as a strong continuous line at the basal margin of podocytes (Figure 3D) ▶ . Nonimmune rabbit and mouse sera, used as controls, did not show glomerular reactivity (Figure 3E) ▶ .
Figure 3.
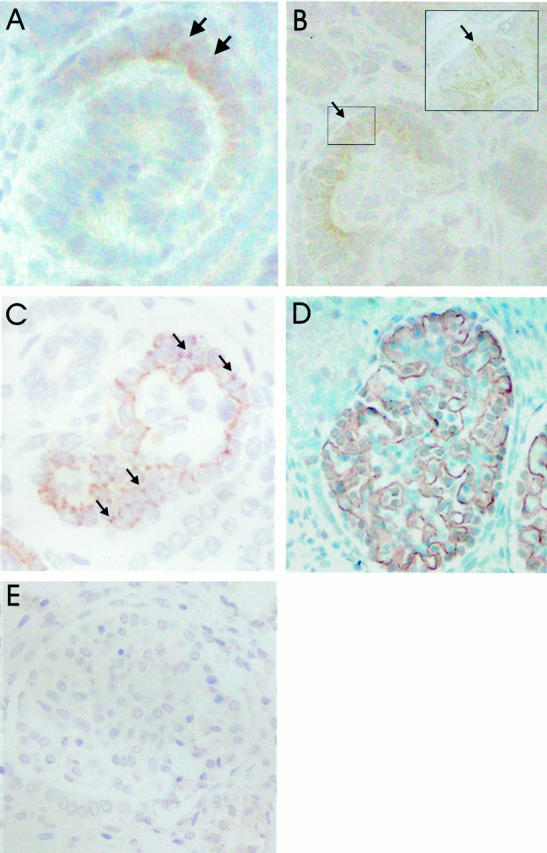
Immunoperoxidase staining for nephrin in normal glomerulogenesis using antibodies directed against intracellular part of nephrin. A: Weak nephrin signal is first observed in late S-shaped bodies, specifically in columnar epithelial cells adjacent to vascular cleft (arrow). B and C: In capillary stage glomeruli, nephrin is found along the basal margin and between developing podocytes as dots or discrete lines (arrows). Inset shows two discrete lines of nephrin-spesific peroxidase between developing podocytes. D: In maturing stage glomeruli, nephrin is distributed exclusively along the glomerular basement membrane. E: No glomerular reactivity was observed with the incubation of rabbit nonimmune sera. Original magnifications, ×500 (A) and ×400 (B−E).
Immunoelectron microscopy revealed nephrin in junctions with ladder-like structures between the differentiating podocytes at capillary loop stage (Figure 4, A and B) ▶ . In more mature glomeruli, nephrin was concentrated at the slit diaphragm area (Figure 4, E and F) ▶ . Earlier stages, ie, S-shaped bodies, could not be identified in these samples.
Figure 4.

Immunoelectron microscopy of nephrin and ZO-1 during normal glomerulogenesis. Nephrin antibodies were directed against the extracellular part of the molecule. A and B: In glomerular cross-section, nephrin is found in the ladder-like structure between developing podocytes of capillary stage glomerulus. Nucleus shows some background labeling. C and D: Colocalization of nephrin and ZO-1 in developing glomeruli (cross-section). Nephrin labeling visualized with 10 nm gold (long arrows), and ZO-1 with 5 nm gold (small arrows). Both proteins are found in the ladder-like structure of capillary stage glomeruli. RBC, red blood cell in the capillary lumen; GBM, glomerular basement membrane. E and F: In maturing stage glomeruli, nephrin labeling is concentrated in the slit diaphragm area (arrows). Note that the localization of gold label in the slit pore area varies due to alterations in the angle of the cross-section.
Glomerulogenesis in NPHS1 Kidneys
The two fetuses aborted due to NPHS1 suspicion were homozygous for Fin-major mutation. This mutation is a 2-bp deletion in exon 2, causing a frameshift and a translational stop at the end of exon 2, leading to a truncated nephrin protein of only 90 amino acids (Figure 1) ▶ . 3 Although nephrin mRNA was detected in these kidneys (Figure 2, G and H) ▶ , immunostaining with both polyclonal antisera failed to show nephrin reactivity (Figure 5, A and B) ▶ .
Figure 5.
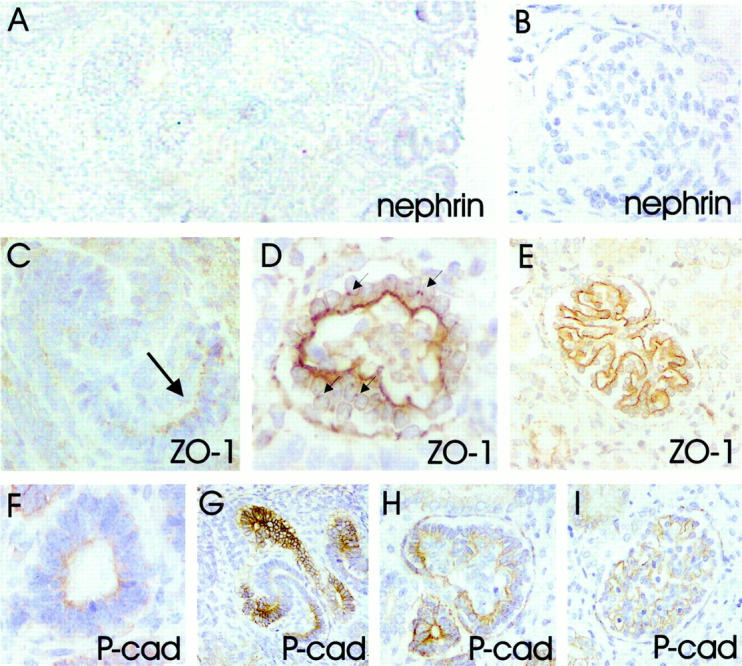
Immunoperoxidase staining of fetal NPHS1 kidney with Fin-major/Fin-major genotype. A and B: Nephrin is not detected in the developing glomeruli with polyclonal antibodies directed against the intracellular part of the molecule. C: S-shaped bodies give ZO-1-positive staining in columnar epithelial cells adjacent to vascular cleft (arrow). ZO-1 is also detected in developing tubular cells. D: In early capillary stage glomeruli, ZO-1 is found preferentially along the basal margin, and between developing podocytes as spots (arrows) or discrete lines. E: Later, ZO-1 is found exclusively along the glomerular basement membrane in capillary tufts of mature stage glomeruli. F: P-cadherin is first observed in vesicle stage glomeruli at the apical border of developing epithelial cells. G: In the S-shaped bodies, P-cadherin is seen in columnar epithelial cells adjacent to vascular cleft. P-cadherin is also found in ureteric buds and developing tubuli. H: In the capillary loop stage glomeruli, P-cadherin is detected at the basal margin of developing podocytes, and faint reactivity is also observed on occasion between the developing podocytes. I: In more mature glomeruli, the staining intensity for P-cadherin is reduced, and P-cadherin is found along the glomerular basement membrane. Original magnifications, ×125 (A), ×400 (B, D, G), ×500 (C, F), and ×300 (E, H, I).
The light microscopy of the NPHS1 kidneys lacking nephrin showed tubular dilatation but no major changes in glomeruli, as reported previously. 22 In electron microscopy, the junctions between developing podocytes of the late capillary loop stage glomeruli appeared normal (Figure 6) ▶ . However, junctions with ladder-like structures were missing in capillary stage glomeruli of both NPHS1 kidneys. In total, 12 (5 and 7) capillary stage glomeruli were analyzed in two kidneys. In four normal fetal kidneys, the analysis of 12 capillary stage glomeruli (3 glomeruli/kidney) revealed 9 junctions with ladder-like structures (1, 2, 3, and 3 junctions/kidney).
Figure 6.
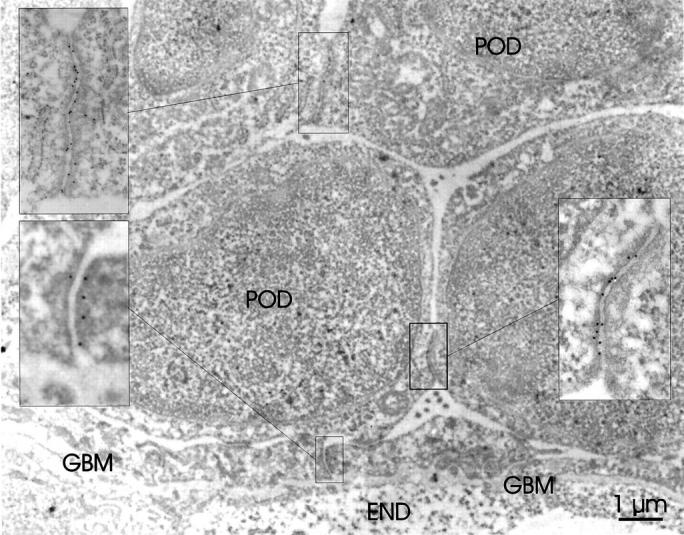
Immunoelectron microscopy study of ZO-1 in developing glomeruli with Fin-major/Fin-major genotype. ZO-1 labeling is found at the junctional complexes (insets) between developing podocytes (POD). ZO-1 labeling is also observed at filtration slit areas (inset) just above the glomerular basement membrane (GBM). Endothelial cell (END) layer is marked in the glomerular capillary wall.
In maturing stage glomeruli, fusion of the foot processes, and irregular podocyte foot processes were observed in NPHS1 kidneys (Figure 7A) ▶ . The slit pores between the foot processes were of various sizes. The filamentous structure of the slit diaphragm, however, was completely missing in 100 filtration slits analyzed (Figure 7, B and C) ▶ . This is in sharp contrast to what is seen in normal fetal kidneys, where these filaments were present in over 60% (71/110) of the mature filtration slits studied (Figure 7, D and E) ▶ .
Figure 7.
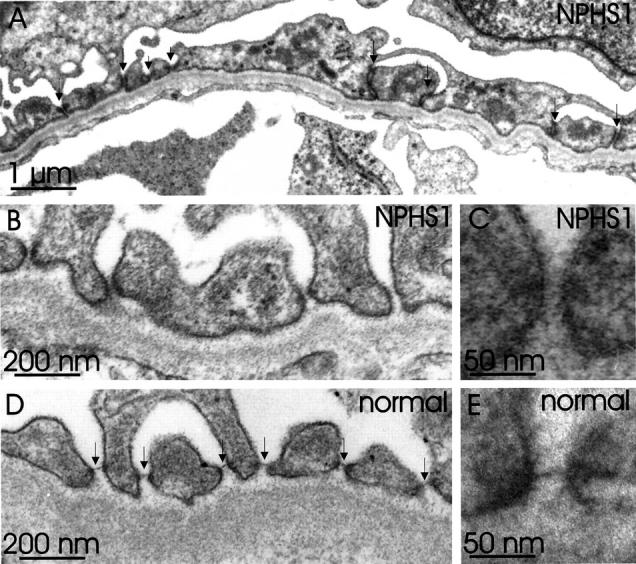
Ultrastructure of the capillary wall in mature stage glomeruli, as seen with conventional electron microscopy. A: NPHS1 fetal kidney. Broadening of the foot processes is observed. Occluding junctions (long arrows) are often found between irregularly shaped foot processes. Normal looking filtration slits (short arrows) are also present. B and C: The spanning filamentous structure of the slit diaphragm is not found in filtration slits of NPHS1 kidney. Instead, only varying amounts of fuzzy cell surface material similar to that seen on urinary surface of podocytes is observed. D and E: For comparison, glomerular capillary wall of normal fetal kidney shows intact foot processes adjoined by slit diaphragms (arrows).
Comparison of Nephrin to ZO-1 and P-Cadherin
In normal fetal kidney, ZO-1 was found in visceral and parietal epithelial cells (Bowman’s capsule) of glomeruli, as well as in tubular epithelial cells (Figure 8) ▶ . The expression of ZO-1 in NPHS1 kidneys lacking nephrin was similar to that of normal fetal kidneys (Figures 5, 6, and 8) ▶ ▶ ▶ . During glomerulogenesis, ZO-1 was first detected in late S-shaped glomeruli, colocalizing with nephrin (Figure 8C) ▶ . At capillary loop stage, ZO-1 colocalized with nephrin along the basal margins and also between the developing podocytes (Figure 8D) ▶ . Abundant localization of ZO-1 to cell junctions at the lateral margins of developing podocytes was evident in immunoperoxidase staining (Figure 5D) ▶ . The intense dots at junctions of developing podocytes were observed in over 70% (36/51) of the capillary loop stage glomeruli.
Figure 8.
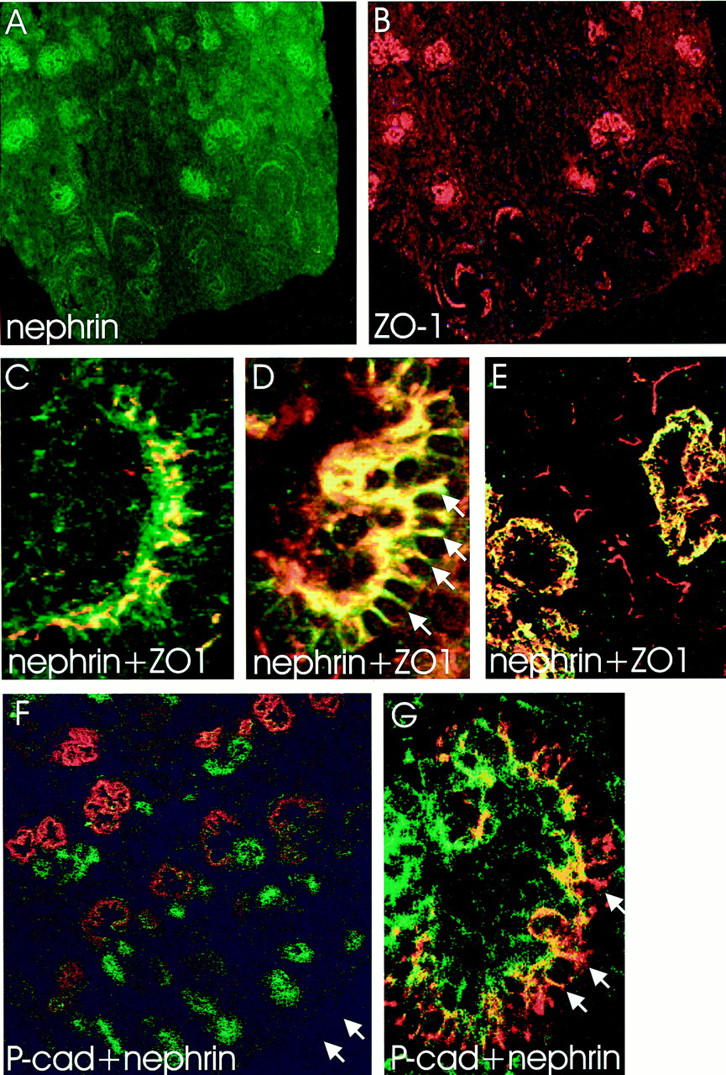
A−E: Nephrin and ZO-1 in double immunofluorescence staining of normal fetal kidney. Nephrin (green, A) and ZO-1 (red, B) are detected in similar patterns in developing glomeruli. C−E: Confocal laser scanning microscopy shows overlapping (yellow) of two signals. C: In late S-shaped body, nephrin and ZO-1 are found in columnar epithelial cells adjacent to vascular cleft. D: In capillary stage glomeruli, nephrin and ZO-1 colocalize along the glomerular basement membrane, and between developing podocytes as dots and thin lines (arrows). E: The colocalization in mature stage glomeruli appears as a sheet-like, granular yellow fluorescence, whereas ZO-1 (red) is also detected in the Bowman’s capsule. F and G: Double immunofluorescence staining for P-cadherin (green) and nephrin (red). Confocal laser scanning microscopy showing the partial overlapping (yellow) of signals. F: P-cadherin stains intensively for ureter buds and developing tubuli, whereas only weak signal is seen in developing glomeruli. Nephrin is in contrary strongly expressed in developing capillary tufts. Arrows point to the capsule of the fetal kidney. G: In capillary stage glomeruli, the overlapping of P-cadherin and nephrin signals is observed along the glomerular basement membrane. In addition, nephrin is found between the developing podocytes as red spots and discrete lines (arrows). Original magnifications, ×60 (A, B, F), ×600 (C, D, G), and ×400 (E).
In immunoelectron microscopy, ZO-1 was first seen at the junctions between developing podocytes and later at the filtration slit area (Figure 6) ▶ . Double-labeling immunoelectron microscopy revealed colocalization of nephrin and ZO-1 in junctions with the ladder-like structures (Figure 4, C and D) ▶ .
Monoclonal anti-P-cadherin antibodies gave strong reactivity against ureteric buds and developing tubuli (Figure 8F) ▶ . Weaker expression of P-cadherin was detected in developing glomeruli (Figure 5, F ▶ -I, and Figure 8F ▶ ). The expression of P-cadherin in NPHS1 kidney was similar to that of normal fetal kidney (Figure 5, F ▶ -I). P-cadherin was first seen at vesicle stage, staining the apical margins of the developing epithelial cells (Figure 5F) ▶ . In the late S-shaped bodies, P-cadherin colocalized with nephrin and ZO-1 (Figure 5G) ▶ . At capillary loop stage, staining for P-cadherin was observed at the basal margins, and to some extent, also between the developing podocytes (Figure 5H) ▶ . However, spot-like staining between developing podocytes, as seen in nephrin and ZO-1 staining, was not observed (Figure 8G) ▶ . In mature stage glomeruli, intensity for P-cadherin staining was reduced in immunoperoxidase staining, giving a linear staining pattern along the glomerular basement membrane (Figure 5I) ▶ . In immunofluorescence staining, P-cadherin was not detected in mature stage glomeruli (data not shown).
Discussion
Recent identification of nephrin as a key component of the podocyte filtration slit has opened new possibilities for studying the development of the glomerular filtration barrier. 3,9,23 Here, we describe the expression of nephrin during normal glomerulogenesis and how its absence affected the structural differentiation of the filtration slit in NPHS1 kidneys. The expression of nephrin was also compared to that of ZO-1 and P-cadherin.
Glomerulogenesis is morphologically divided into four stages: the vesicle, S-shaped body, capillary loop, and maturing stages. 16,24 S-shaped glomerulus is the earliest stage at which the presumptive podocytes can be identified. 25 At this stage, the developing podocytes are connected at the apical-lateral cell membrane interface by junctional complexes, which subsequently are believed to form the slit diaphragms. The junctions migrate down the cell membrane and reach their most basal location near the glomerular basement membrane during the capillary loop stage. After interdigitation of basolateral surfaces of adjoining cells, the slit diaphragms are formed in maturing stage glomeruli. These electron microscopic findings are supported by data showing that the tight junction protein, ZO-1, first appears in apically located junctions and then migrates down to the level of slit diaphragm. 12
The developmental expression of nephrin in rat and mouse has been reported. 6,26 The mAb 5–1-6, recognizing an epitope of the extracellular domain of rat nephrin, 27 detected nephrin in the S-shaped bodies. 26 According to this study, nephrin was present below the tight junctions on the basal and lateral surfaces of the epithelial cells in capillary loop stage glomeruli. Later, the reactivity concentrated at the filtration slits. Kawachi et al 26 suggested that nephrin and ZO-1 would reach the slit diaphragm from opposite directions. In mouse, nephrin first appeared in the early capillary loop stage as studied by immunofluorescence staining. 6 Using immunoelectron microscopy, nephrin was observed on or near the intercellular junctions between the forming foot processes of the epithelial cells. In contrast, labeling was not found on lateral and apical surfaces above these regions or on basal surfaces facing the underlying glomerular basement membrane. 6
In the present work, we found that human nephrin was first expressed in the late S-shaped bodies simultaneously with ZO-1. Interestingly, the signal intensity for nephrin mRNA was strongest in the developing podocytes at the capillary loop stage, before the appearance of foot processes or slit diaphragms. At this stage, nephrin was mostly localized at the basal margin of the developing podocytes, but also on the lateral surfaces as strings and dots. This pattern resembled that of ZO-1. However, in double immunofluorescence staining, the reactivities for nephrin and ZO-1 did not show complete overlapping. The expression of ZO-1 was more abundant at the cellular junctions (the dots) as compared to nephrin. This was also seen in immunoelectron microcopy where ZO-1 localized to junctional complexes as previously reported. 12
An interesting finding in immunoelectron microscopy was that both nephrin and ZO-1 were localized at junctions with the ladder-like structures, which are commonly found between the developing podocytes in capillary loop stage glomeruli. 16 These structures typically disappear before the appearance of the slit diaphragms in maturing stage glomeruli. The ladder-like structures have also been found in aminonucleoside nephrosis and immune complex-mediated nephritis in animals. 28,29 In the nephrotic model, the ladder-like structures were suggested to be redundant slit diaphragms. Absence of ZO-1 in the ladder-like structures in rat nephrosis has also been reported, 30 suggesting that ladder-like structures could represent something else. The relationship between these two structures is still unclear.
We found that the slit diaphragms were completely missing in the fetal NPHS1 kidneys lacking the nephrin molecule. Recently, we found the same in kidneys of NPHS1 children nephrectomized at the age of 1 to 2 years. 7 The lack of the slit diaphragm already in the fetal NPHS1 kidneys of Fin-major homozygotes strongly favors an idea that this is a primary disorder, not secondary to the long-lasting proteinuria. Interestingly, the early junctions between the developing podocytes at the capillary loop stage seemed normal in electron microscopy. ZO-1 was also normally expressed in these junctions. Thus, nephrin was clearly not needed for the early development and migration of junctional complexes at the S-shaped and capillary loop stages. On the other hand, the formation of the ladder-like structures, as well as the slit diaphragms, seemed to be dependent on the expression of nephrin. It is tempting to speculate that the ladder-like structures represent a fragmented form of tight junctions, which, in normal kidney, differentiate into mature slit diaphragm.
Although nephrin and the slit diaphragms were completely missing in the NPHS1 kidneys, the broadened and irregular podocyte foot processes remained attached to the GBM. This morphological alteration is observed also in other human nephroses and in animal models. 31 The molecular basis of this phenomenon is not known. The actin-rich cytoskeleton of the foot processes plays a major role in the maintenance of the podocyte foot process structure. 8 These microfilaments are associated with integrins that anchor the foot processes to the glomerular basement membrane. The expression of integrins and the glomerular basement membrane components in NPHS1 kidneys is quite normal 32 and could contribute to the maintenance of the podocyte structure in NPHS1. The lateral surfaces of the podocyte foot processes are rich in negatively charged glycoproteins, which probably help to keep the foot processes apart from each other. 8 The relative role of the slit diaphragm components and the cell surface glycocalyx for the normal foot process ultrastructure is not known. It is perhaps surprising that lack of nephrin, or even minor mutations in this molecule, have such dramatic effects on the foot process organization.
ZO-1 is a tight junction protein; its presence at the slit diaphragm suggests that this structure is a modified tight junction. 12 Recently, however, Reiser et al suggested that the slit diaphragm is a modified adherens junction. 15 They located P-cadherin at the slit diaphragm area in podocyte cultures and presented a model, where P-cadherin served as the core protein of the slit diaphragm. Homophilic interactions of P-cadherin would make the bridge between the podocytes. This is in contrast to the model of Ruotsalainen et al, 4,9 suggesting that nephrin forms the porous substructure of the slit diaphragm.
In this work, strong reactivity for P-cadherin was observed in ureteric buds, tubular structures, and vesicle stage glomeruli, as reported previously. 33,34 All these structures were negative for nephrin. In the S-shaped bodies and capillary stage glomeruli, nephrin, and P-cadherin colocalized to the basal margin of developing podocytes. The staining intensity for P-cadherin was reduced at these stages as compared to vesicle stage glomeruli, and, in contrast to nephrin, P-cadherin did not show dots of staining at the lateral surfaces of developing podocytes. These findings are in line with the immunofluorescence study of human and pig fetal kidneys by Tassin et al. 34 The findings suggested that during the polarization process of developing podocytes, P-cadherin was not concentrated to cellular junctions in the same way as nephrin. So far, this conclusion has not been verified at the ultrastructural level, because none of the antibody preparations has given a reliable signal for P-cadherin in immunoelectron microscopy.
The major observation on P-cadherin, however, was that it was expressed normally in NPHS1 kidneys missing the slit diaphragms. This finding favors the idea that nephrin, not P-cadherin, forms the backbone of the slit diaphragm. This is supported by the fact that knockout mice lacking P-cadherin do not develop nephrosis, 35 whereas mice not expressing nephrin die due to nephrosis in the first day (Putaala H, Tryggvason K, unpublished data). The fact that point mutations in NPHS1 gene cause massive proteinuria in newborn babies 36 further supports the importance of nephrin.
To summarize our results, nephrin is first expressed in late S-shaped body during human glomerulogenesis. Nephrin colocalizes with ZO-1 in the ladder-like structures between differentiating podocytes before the formation of foot processes and mature slit diaphragms. In the kidneys with Fin-major/Fin-major genotype, filamentous ladder-like structures and slit diaphragms are completely missing, whereas ZO-1 and P-cadherin are expressed normally.
Acknowledgments
The skillful technical assistance of Mirka Parkkinen, Sinikka Heikkilä, Eeva-Liisa Eskelinen, Sirpa Juvonen, Tuire Koro, Pirkko Leikas-Lazanyi, Mervi Lindman, Arja Strandell, and Seija Ylönen is gratefully acknowledged.
Footnotes
Address reprint requests to Hannu Jalanko M.D., Hospital for Children and Adolescents, University of Helsinki, 00029 HYKS, Finland. E-mail: hannu.jalanko@hus.fi.
Supported by The Sigrid Jusélius Foundation, The Ulla Hjelt Foundation, Helsinki University Central Hospital Research Fund, The Swedish Medical Research Council, Novo Nordisk Foundation, and NIH grant DK54724.
V. R. and J. P. contributed equally to this work.
References
- 1.Hallman N, Hjelt L, Ahvenainen EK: Nephrotic syndrome in newborn and young infants. Ann Paediatr Fenn 1956, 2:227-241 [PubMed] [Google Scholar]
- 2.Holmberg C, Jalanko H, Tryggvason K, Rapola J: Congenital nephrotic syndrome. Barratt TM Avner ED Harmon WE eds. Pediatric Nephrology. 1999, :pp 765-778 Lippincott Williams & Wilkins, Baltimore [Google Scholar]
- 3.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 1998, 1:575-582 [DOI] [PubMed] [Google Scholar]
- 4.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 1999, 96:7962-7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holthöfer H, Ahola H, Solin M-L, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D: Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 1999, 155:1681-1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holzman LB, St. John PL, Kovari IA, Verma R, Holthöfer H, Abrahamson DR: Nephrin localizes to the slit pore of the glomerular epithelial cells. Kidney Int 1999, 56:1481-1491 [DOI] [PubMed] [Google Scholar]
- 7.Patrakka J, Kestilä M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Männikkö M, Visapää I, Holmberg C, Rapola J, Tryggvason K, Jalanko H: Congenital nephrotic syndrome (NPHS1): features resulting from different mutations in Finnish patients. Kidney Int 2000, 58:972-980 [DOI] [PubMed] [Google Scholar]
- 8.Mundel P, Kriz W: Structure and function of podocytes: an update. Anat Embryol 1995, 192:385-397 [DOI] [PubMed] [Google Scholar]
- 9.Tryggvason K: Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 1999, 10:2440-2445 [DOI] [PubMed] [Google Scholar]
- 10.Somlo S, Mundel P: Getting a foothold in nephrotic syndromes. Nat Genet 2000, 24:333-335 [DOI] [PubMed] [Google Scholar]
- 11.Rodewald R, Karnowsky MJ: Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 1974, 60:423-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnabel E, Anderson JM, Farquhar MG: The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 1990, 111:1255-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurihara H, Anderson JM, Farquhar MG: Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci USA 1992, 89:7075-7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagava O, Miner JH, Shaw A: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999, 286:312-315 [DOI] [PubMed] [Google Scholar]
- 15.Reiser J, Kriz W, Kretzler W, Mundel P: The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 2000, 11:1-8 [DOI] [PubMed] [Google Scholar]
- 16.Reeves W, Caulfield JP, Farquhar MG: Differentation of epithelial foot processes and filtration slits. Lab Invest 1978, 39:90-100 [PubMed] [Google Scholar]
- 17.Hoover KB, Liao SY, Bryant PJ: Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentation and loss of heterozygosity. Am J Pathol 1998, 153:1767-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bussemakers MJ, van Bokhoven A, Voller M, Smit FP, Schalken JA: The genes for the calcium-dependent cell adhesion molecules P- and E-cadherin are tandemly arranged in the human genome. Biochem Biophys Res Comm 1994, 203:1291-1294 [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson DG: Whole mount in situ hybridization of vertebrae embryos. Wilkinson DG eds. In Situ Hybridization: A Practical Approach. 1992, :pp 75-83 IRL Press, Oxford [Google Scholar]
- 20.Patrakka J, Ruotsalainen V, Ketola I, Holmberg C, Heikinheimo M, Tryggvason K, Jalanko H: Expression of nephrin in pediatric kidney diseases. J Am Soc Nephrol 2000 (in press) [DOI] [PubMed]
- 21.Bendayan M: Double immunocytochemical labeling applying the protein A-gold technique. J Histochem Cytochem 1982, 30:81-85 [DOI] [PubMed] [Google Scholar]
- 22.Autio-Harmainen H, Rapola J: Renal pathology of fetuses with congenital nephrotic syndrome of the Finnish type. Nephron 1981, 29:158-163 [DOI] [PubMed] [Google Scholar]
- 23.Wickelgren I: First components found for key kidney filter. Science 1999, 286:225-226 [DOI] [PubMed] [Google Scholar]
- 24.Abrahamsom DR: Glomerulogenesis in the developing kidney. Semin Nephrol 1991, 11:375-389 [PubMed] [Google Scholar]
- 25.Schnabel E, Dekan G, Miettinen A, Farquhar MG: Biogenesis of podocalyxin–the major glomerular sialoglycoprotein in the newborn rat kidney. Eur J Cell Biol 1989, 48:313-326 [PubMed] [Google Scholar]
- 26.Kawachi H, Abrahamson DR, St. John PL, Goldstein DJ, Shia MA, Matsui K, Shimizu F, Salant DJ: Developmental expression of the nephritogenic antigen of monoclonal antibody 5–1-6. Am J Pathol 1995, 147:823-833 [PMC free article] [PubMed] [Google Scholar]
- 27.Topham PS, Kawachi H, Haydar SA, Chugh S, Addona TA, Charron KB, Holzman LB, Shia M, Shimizu F, Salant DJ: Nephritogenic mAb 5–1-6 is directed at the extracellular domain of rat nephrin. J Clin Invest 1999, 104:1559-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneeberger EE, Grupe WE: The ultrastructure of the glomerular slit diaphragm in autologous immune complex nephritis. Lab Invest 1976, 34:298-305 [PubMed] [Google Scholar]
- 29.Ryan GB, Rodewald R, Karnowsky MJ: An ultrastructural study of the glomerular slit diaphragm in aminonucleoside nephrosis. Lab Invest 1975, 33:461-468 [PubMed] [Google Scholar]
- 30.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG: The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 1992, 141:805-815 [PMC free article] [PubMed] [Google Scholar]
- 31.Smoyer WE, Mundel P: Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med 1998, 76:172-183 [DOI] [PubMed] [Google Scholar]
- 32.Ljungberg P, Virtanen I, Holmberg C, Jalanko H: Distribution of renal integrin receptors and their ligands in congenital nephrotic syndrome of the Finnish type. Virch Arch 1996, 428:333-346 [DOI] [PubMed] [Google Scholar]
- 33.Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintler C, Dressler GR: Differential expression and function of cadherin-6 during renal epithelium development. Development 1998, 125:803-812 [DOI] [PubMed] [Google Scholar]
- 34.Tassin M-T, Beziau A, Gubler M-C, Boyer B: Spatiotemporal expression of molecules associated with junctional compelexes during the in vivo maturation of renal podocytes. Int J Dev Biol 1994, 38:45-54 [PubMed] [Google Scholar]
- 35.Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, Hynes RO: Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol 1997, 139:1025-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenkkeri U, Männikkö M, McCready P, Lamerdin J, Gribouval O, Niaudet P, Antignac C, Kashtan CE, Holmberg C, Olsen A, Kestilä M, Tryggvason K: Structure of the gene for congenital nephrotic syndrome of the Finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 1999, 64:51-61 [DOI] [PMC free article] [PubMed] [Google Scholar]