Abstract
Activated ras causes increased activity of several signal transduction systems, including the mitogen-activated protein kinase kinase (MAPKK) pathway and the phosphoinositol-3-kinase (PI-3-K) pathway. We have previously shown that the PI-3-K pathway plays a major role in regulation of ras-mediated tumor angiogenesis in angiosarcoma cells. However, the contribution of the MAPKK pathway to tumorigenesis and angiogenesis is not fully understood. Overexpression of constitutively active forms of MAPKK has previously been shown to transform nonmalignant NIH3T3 fibroblasts, but the effect of down-regulation of MAPKK on tumorigenesis and angiogenesis in a well established tumor has not been fully explored. We introduced a dominant negative MAPKK gene into SVR murine angiosarcoma cells. Introduction of a dominant negative MAPKK causes a significant decrease in proliferation rate in vitro and morphological reversion. Cells expressing the dominant negative MAPKK have a greatly decreased ability to form colonies in soft agar compared with wild-type cells. Despite the decreased cell growth in vitro and inability to grow in soft agar, the cells were equally tumorigenic in nude mice. Our results suggest that the MAPKK pathway is required for soft agar growth of angiosarcoma cells, and separates the phenotypes of soft agar growth versus in vivo tumorigenicity. These findings have implications in the development of signal transduction modulators as potential antineoplastic agents.
Tumorigenesis due to activation of ras family oncogenes is a common event in solid tumors. Ras is known to activate several signal transduction pathways, including phosphoinositol-3-kinase (PI-3-kinase) and mitogen-activated protein kinase pathways. 1-3 Both of these pathways have been implicated in tumorigenesis and angiogenesis in transformation assays employing NIH3T3 cells. 4,5 Overexpression of PI-3 kinase has been shown to confer resistance to apoptosis in NIH3T3 cells that overexpress the c-myc oncogene and rescue epithelial cells from anoikis. 6,7 Expression of activated raf or constitutively active MAP kinase kinase (MAPKK) has been shown to convert NIH3T3 cells into aggressive fibrosarcomas. 4,5,8 In addition, transfection of activated ras and raf into NIH3T3 cells has been shown to cause increased expression of the potent angiogenic chemoattractant, vascular endothelial growth factor (VEGF). 9,10 However, the contribution of these pathways to the maintenance of angiogenesis and tumorigenesis in tumors other than transformed NIH3T3 cells has not been extensively studied.
We have established a two-step model for the development of malignant endothelial tumors by the sequential introduction of a temperature-sensitive SV40 large T antigen and activated H-ras into murine endothelial cells. The addition of activated H-ras causes a switch from cells that produce small quantities of angiogenic mediators to aggressive angiosarcomas, which produce high levels of angiogenic mediators. We have previously shown that treatment with wortmannin, a potent inhibitor of the PI-3-kinase pathway, resulted in down-regulation of the angiogenic mediators VEGF and matrix metalloproteinases, and decreased in vivo tumor size. However, inhibition of PI-3-kinase had no effect on the angiogenic antagonist tissue inhibitors of matrix metalloproteinases (TIMPs). 11 In this report, we describe the effect of inhibition of the MAP kinase signal transduction pathway by both introduction of a dominant negative MAPKK gene into angiosarcoma cells that express SV40 large T antigen and H-ras, and treatment of angiosarcoma cells with a chemical inhibitor of MAPKK, PD98059. These studies show that inhibition of the MAP kinase pathway leads to decreased proliferation and morphological reversion to the untransformed phenotype, as well as greatly decreased growth in soft agar, yet cells remain highly tumorigenic in vivo. These results point to a role of the MAP kinase pathway in mediating soft agar growth and a tissue-specific effect of the MAP kinase pathway on tumorigenesis.
Materials and Methods
Generation of Cell Lines
MS1 and SVR cells are derived from primary murine endothelial cells and were maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal calf serum. 11 SVR cells were infected with retroviruses encoding β-galactosidase (F. Boyce, Massachusetts General Hospital, Boston, MA) or a dominant negative MAPKK mutant A221 (C. Marshall, Institute of Cancer Research, London, UK). Both vectors encode puromycin resistance, and cells were selected in 2 μg/ml puromycin. β-Galactosidase expression was confirmed by histochemical staining with x-gal, 12 and expression of the dominant negative MAPKK gene was confirmed by growing individual clones and performing Western blot analysis with an antibody specific to rabbit MAPKK and phosphorylated MAPK. Protein extracts were prepared as described in Arbiser et al. 11 Two clones with reduced expression of phosphorylated MAP kinase were selected for further study and were named SVRA221a and SVRA221b. Expression of the dominant negative MAPKK was confirmed using antibody 177 to rabbit MAPKK (C. Marshall). This antibody cross-reacts with endogenous murine MAPKK, so an authentic clone that expresses the gene would be expected to have elevated levels of total MAPKK (rabbit transgene and endogenous murine MAPKK), but diminished phosphorylated MAP kinase. A clone expressing β-galactosidase was named SVRbag4 and was found to have identical expression of phosphorylated MAP kinase as parental SVR cells, and high level expression compared with MS1 cells, which do not contain activated H-ras. Both SVRA221a cells, which express the lowest quantity of phosphorylated MAP kinase, and SVRbag4 cells have been submitted to the American Type Culture Collection (Manassas, VA) for distribution.
In Vitro Proliferation Assays
We plated 10,000 cells of each cell type in 24-well dishes. The next day, the medium was replaced with fresh medium containing the inhibitors or vehicle controls. Cells were incubated at 37°C for 72 hours, and cell number was determined in triplicate using a Coulter Counter (Hialeah, FL). PD98059 13 and LY294002 14 were obtained from Calbiochem (San Diego, CA) and were reconstituted in dimethylsulfoxide (DMSO) to a final concentration of 5 mg/ml stocks.
Western Blotting
Cells were lysed in lysis buffer containing 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1% (v/v) Triton X-100, 10% glycerol, 1 mmol/L EDTA, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mmol/L benzamidine, 1 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L Na3VO4.
Protein concentration was determined by the Bradford assay using bovine serum albumin as a standard. Samples were treated with Laemmli sample buffer and heated to 90°C for 5 minutes before sodium dodecyl sulfate-polyacrylamide gel electrophoresis (National Diagnostics, Atlanta, GA) and transfer to nitrocellulose membranes. The membranes were then blocked with 5% nonfat dry milk in TBST and subsequently incubated with the appropriate antibody for immunoblotting. Anti-Erk2 monoclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-phospho-MAP kinase polyclonal antibody was from Promega (Madison, WI).
Assays of Matrix Metalloproteinase Bioactivity
Cells were grown to approximately 75% confluence in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal calf serum. After washing with phosphate buffered saline, medium was replaced with Cellgro Serumless medium (Mediatech, Herndon, VA). Wild-type cells were incubated in serum containing medium supplemented with 5 μg/ml PD98059 or an equal volume of DMSO before shifting into Cellgro medium containing either PD98059 or DMSO vehicle. In the case of SVRA221a or SVRbag4 cell lines, medium was shifted to Cellgro serumless medium for 24 hours before harvest.
Substrate gel electrophoresis (zymography) was conducted as described by us previously. 11 Briefly, Type 1 gelatin was added at a concentration of 1 mg/ml to the standard Laemmli acrylamide polymerization mixture. Conditioned medium from equivalent numbers of cells was diluted 3:1 with sample buffer (10% sodium dodecyl sulfate, 4% sucrose, 0.25 mol/L Tris (pH 6.8), 0.1% bromphenol blue) and electrophoresed as previously described. At the end of electrophoresis, gels were rinsed in 2.5% Triton X-100 for 30 minutes, then incubated overnight in substrate buffer (50 mmol/L Tris, pH 8, 5 mmol/L CaCl2, 0.02% NaN3). The gels were stained with 0.5% Coomassie blue R-250 in acetic acid:isopropyl alcohol:H2O (1:3:6) and destained in the same buffer in which the Coomassie blue was prepared. Densitometry of destained areas was quantified using a Datascopy GS Plus scanner connected to Macintosh II computer with Macimage software (Xerox Imaging Systems).
Assay of TIMP Bioactivity
TIMP bioactivity was quantified using conditioned media from equal numbers of cells treated with either PD98059 or vehicle control, or equal numbers of stable cells expressing the dominant negative MAPKK (SVRA221a) or vector control (SVRbag4). A solid collagen film assay was performed using C14-labeled collagen as previously described by us. 11
Assay of Anchorage-Independent Growth
Anchorage-independent growth was determined by adding equal numbers of viable cells to a plate containing 0.35% Noble agar in 10% fetal bovine serum containing media at a density of either 5 × 10 4 or 5 × 10 5 cells per 6-cm 2 plate. The number of colonies formed in agar was recorded 2 weeks after plating the cells. 15 The effect of the MAP kinase inhibitor PD98059 on soft agar formation of SVRbag4 cells was assessed by continuous treatment of SVRbag4 cells with either 50 μmol/L PD98059 or DMSO vehicle control for 4 days before trypsinization. Once trypsinized, the cells were placed into growth medium containing either 50 μmol/L PD98059 or DMSO vehicle for the entire soft agar assay.
In Vivo Tumorigenesis
SVRbag4 and SVRA221a,b (1 × 106) cell lines were injected into the flank of 6-week-old nude male mice obtained from Massachusetts General Hospital. Three weeks after tumors appeared, they were excised and fixed in both formalin and Carnoy’s fixative for hematoxylin and eosin staining. Tumor volume was measured using the formula (width × width × length) × 0.52, where width represents the shortest dimension. 11
To determine whether the inability to grow is soft agar was lost after in vivo growth, tumors of SVRbag4 and SVRA221a,b were explanted into tissue culture; once confluent, cells were assayed for their ability to grow in soft agar as described above. Cells were analyzed for growth in soft agar and expression of MAPKK by Western blot once explanted from mice.
Cell Cycle Analysis
Nuclei from cultured cells were isolated and stained with propidium iodide using the CycleTest Plus DNA Reagent Kit (Becton Dickinson, San Jose, CA). Data acquisition and analysis were performed on a FACSort flow cytometer (Becton Dickinson) using ModFitLT version 2.0 software (Becton Dickinson).
Results
Generation of Cells Expressing a Dominant Negative MAPKK
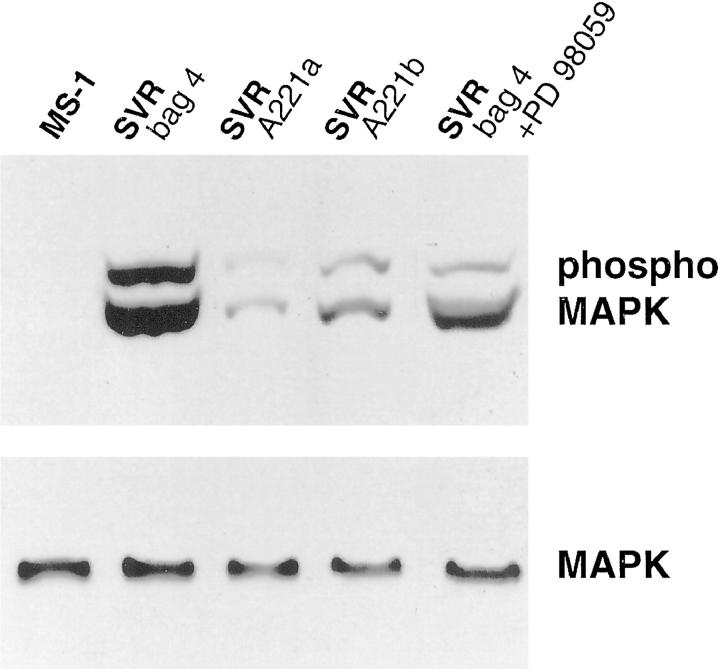
SVR cells were infected with recombinant retroviruses encoding either (i) a dominant negative MAPKK mutated at allele Ser-221 in the catalytic domain or (ii) β-galactosidase, and selected in puromycin. Resistant clones were screened by Western blot analysis for expression of phosphorylated MAP kinase and for expression of the rabbit MAPKK allele using antibody 177, which is specific for rabbit MAPKK. 16 Two clones expressing decreased levels of phosphorylated MAP kinase were selected for further study. A β-galactosidase-expressing clone was selected as a control and showed high levels of phosphorylated MAP kinase expression (Figure 1) ▶ . This clone had identical expression of phosphorylated MAP kinase compared with parental SVR cells (data not shown).
Figure 1.
Analysis of phospho MAP kinase and total MAP kinase expression in immortalized (MS1) and transformed endothelium (SVRbag4) in the presence and absence of a chemical MAPKK inhibitor (PD98059), and cell lines (SVRA221a,b,c) expressing a dominant negative MAPKK gene. The top gel represents an immunoblot with an antibody specific for phosphorylated MAP kinase, and the bottom gel represents an immunoblot with an antibody for total MAP kinase.
Introduction of Dominant Negative MAPKK into SVR Cells Induced Morphological Change
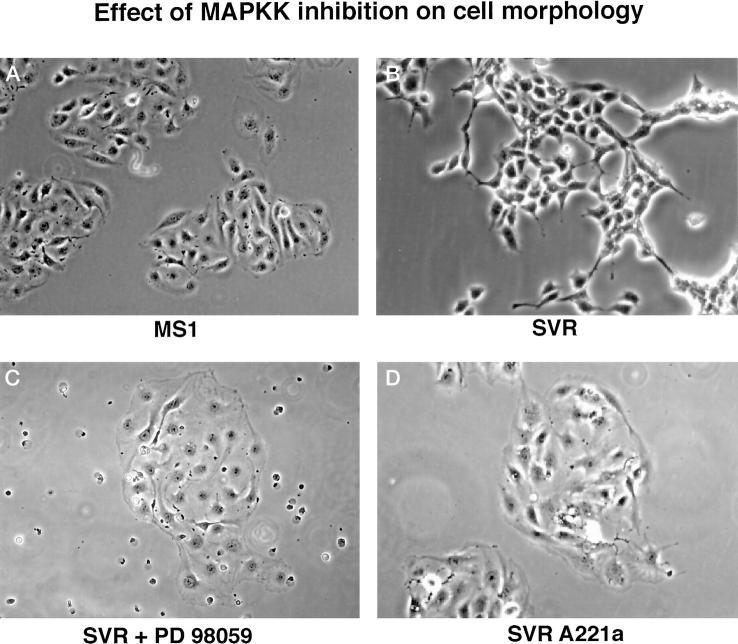
SVR cells have a spindle-type morphology, which is retained on introduction of β-galactosidase (Figure 2B) ▶ , but treatment of parental SVR cells with PD98059 led to increased cellular size and width (Figure 2C) ▶ . Similar morphological changes were seen with introduction of the MAPKK mutant Ser-221 (Figure 2D) ▶ . These morphological changes are similar to endothelial cells (MS1 cells), which lack activated ras activity (Figure 2A) ▶ . Treatment of cells with the phosphoinositol-3-kinase inhibitor LY294002 had no noticeable morphological effect on SVR or SVRbag4 cells.
Figure 2.
Effect of inhibition of MAPKK by a dominant negative MAPKK gene or by the chemical inhibitor PD98059 on morphology of endothelial cells. MS1 represents endothelial cells containing only SV40 large T antigen; SVR represents MS1 cells transformed with ras; SVR+ PD98059 represents SVR cells treated with PD98059 (5 μg/ml); and SVRA221a represents cells stably expressing the dominant negative A221 allele of MAPKK. The morphology of SVRAnd SVRbag4 cells are identical. Original magnifications, ×40.
Effect of Signal Transduction Inhibitors on Proliferation of SVRbag4 and SVRA221a Cells
Both cell lines were grown in the presence of a MAPKK inhibitor, PD98059, and a phosphatidylinositol-3-kinase inhibitor LY294002 in doses ranging from 0 to 10 μg/ml, and cell counts were performed 72 hours later. Introduction of the 221 allele of MAPKK led to a decrease of nearly 75% in growth rate. Both cell types were susceptible to growth inhibition by LY294002, the phosphatidylinositol-3-kinase inhibitor, in a dose-dependent fashion, but the SVRA2221a cells were relatively resistant to growth inhibition by PD98059. In contrast, growth of control SVRbag4 cells was highly inhibited by PD98059 (Figure 3) ▶ . No cytotoxicity was observed as a result of treatment with either PD98059 or LY294002.
Figure 3.
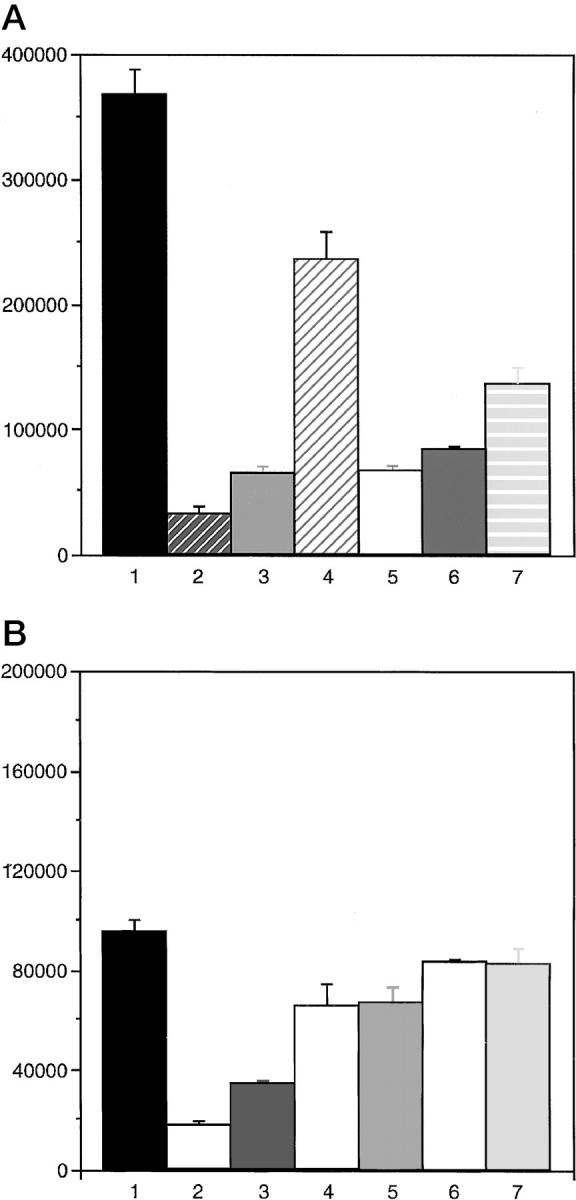
A: Dose response of SVRbag4 cells to the MAPKK inhibitor PD98059 and the PI-3-K inhibitor LY294002. The first lane represents cell counts of untreated cells; the second lane, cells treated with 10 μg/ml LY294002; the third lane, 5 μg/ml LY294002; the fourth lane, 1 μg/ml LY294002; the fifth lane, 10 μg/ml PD98059; the sixth lane, 5 μg/ml PD98059; the seventh lane, 1 μg/ml PD98059. Cells were treated with inhibitor for 72 hours, and cells were counted. All experiments were repeated in triplicate, and the error bar represents SEM. All doses of inhibitors resulted in significant inhibition compared with untreated cells, P < 0.05. B: Dose response of SVRA221a cells to the MAPKK inhibitor PD98059 and the PI-3-K inhibitor LY294002. The lanes are labeled as in A. Cells were treated as in A. All experiments were performed in triplicate, and the error bar represents SEM, P < 0.05. Treatment of SVRA221a cells with the PI-3-K inhibitor led to significant decreases in cell proliferation (P < 0.05), but there was no significant decrease in proliferation as a result of treatment with the MAPKK inhibitor PD98059.
Total RNA was extracted from SVRA221a cells, which stably express the dominant negative A221 allele of MAPKK, or SVRbag4, which serve as wild-type control. No significant differences were observed in VEGF mRNA expression (data not shown).
Effect of MAPKK Inhibition on MMP and TIMP Bioactivity
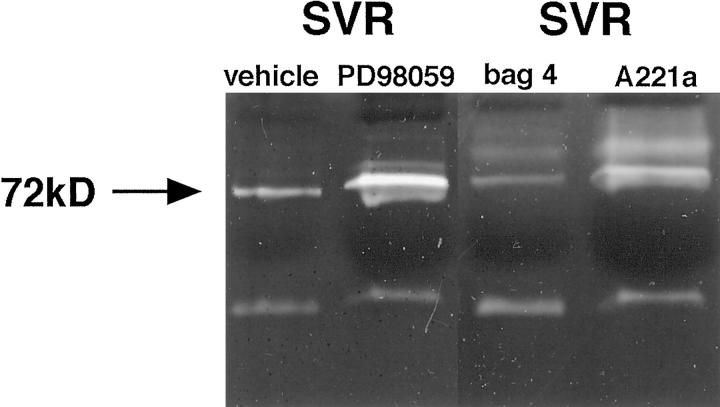
Treatment of SVR cells with the low molecular weight inhibitor PD98059 caused a modest increase in gelatinase activity in conditioned media (Figure 4) ▶ . A similar pattern of enhancement of MMP bioactivity was observed in SVRA221a, which expresses the dominant negative MAPKK gene, compared with the vector control cells SVRbag4 (Figure 4) ▶ .
Figure 4.
Effect of MAPKK inhibition on metalloproteinase bioactivity in SVR-conditioned media. Conditioned media from equal numbers of cells were electrophoresed through an acrylamide gel containing gelatin. White bands represent gelatinolysis. The first two lanes represent SVR cells treated with vehicle (DMSO) or PD98059; the second two lanes compare a vector control (SVRbag4) with a clone expressing a dominant negative (SVRA221a). Experiments were repeated in triplicate.
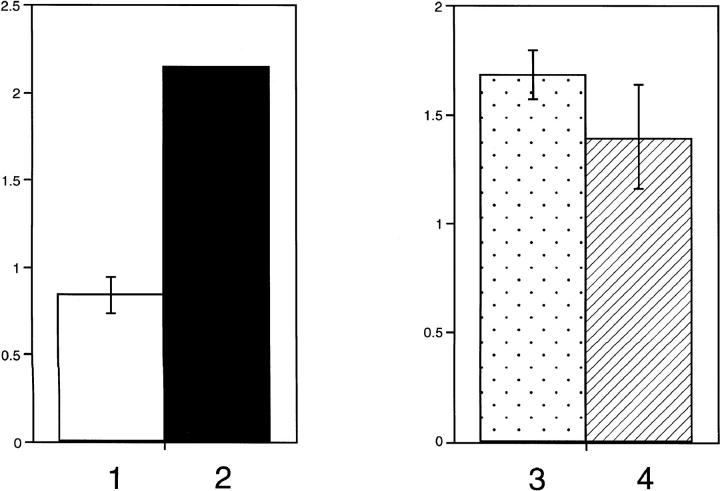
Conditioned media from equivalent numbers of SVRA221a and control SVRbag4 cells were tested in a radiometric enzyme assay for TIMP activity. As shown in Figure 5 ▶ , acute down-regulation of MAPKK by PD98059 resulted in up-regulation of TIMP bioactivity, whereas stable down-regulation of MAPKK resulted in no change in TIMP bioactivity (Figure 5) ▶ .
Figure 5.
Effect of MAPKK inhibition on TIMP bioactivity. The first graph examines the effect of acute inhibition of MAPKK by addition of PD98059 on TIMP bioactivity. This experiment was repeated in triplicate. Lane 1 represents vehicle-treated cells; lane 2 represents cells treated with PD98059. The second graph examines the effect of chronic inhibition of MAPKK (SVRA221a) by stable expression of dominant negative MAPKK. Lane 3 represents SVRbag4 (vector control cells), and lane 4 represents conditioned media from an equivalent number of SVRA221a cells.
Effect of MAPKK Inhibition on Anchorage-Independent Growth
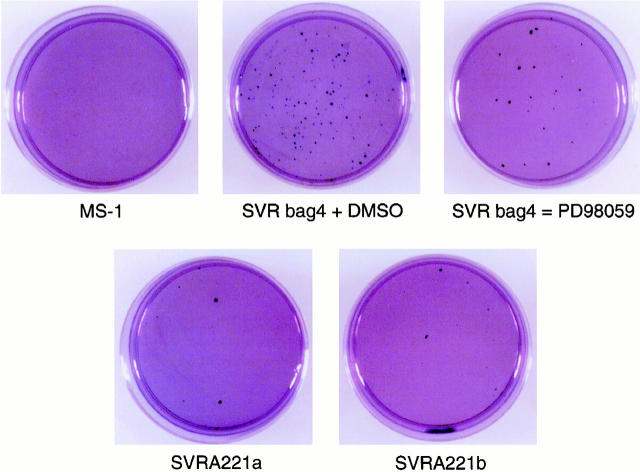
The overexpression of a dominant negative mutant form of MAPKK suppressed ras-induced anchorage-independent growth in angiosarcoma cells. MS1 endothelial cells transformed by H-ras and containing a vector control (SVRbag4) display anchorage-independent growth as measured by their ability to form colonies in soft agar (Figure 6 ▶ and Table 1 ▶ ). Ectopic expression of a dominant negative mutant form of MAPKK in the ras-transformed angiosarcoma cell lines (SVRA221a,b) dramatically decreased the number of colonies observed in soft agar, as compared with SVRbag4 (Figure 6 ▶ and Table 1 ▶ ). Similarly, treatment of wild-type SVR cells with PD98059 suppressed the ability of these cells to grow in soft agar. The ability of the cell lines to grow in soft agar correlated with the expression levels of phosphorylated MAP kinase. These data suggest that activation of MAPKK is required for ras-induced anchorage-independent growth of angiosarcoma cells in soft agar.
Figure 6.
A representative soft agar assay stained with 0.2% Giemsa 2 weeks after plating. MS1, SVRbag4 treated with 50 μmol/L PD98059 or vehicle control, and SVRA221a,b cells were seeded at either 5 × 10 4 cells per 6-cm 2 dish. Macroscopic colonies were counted 14 days after plating the cells in agar.
Table 1.
Cell Line Data
| Cell line | Number of colonies |
|---|---|
| MS1 | 0 |
| SVRbag4 | 2039 ± 74 |
| SVRbag4/PD98059 | 837 ± 66 |
| SVRA221a | 21 ± 3 |
| SVRA221b | 54 ± 11 |
Data represent the mean ± standard error of the number of colonies observed in three independent experiments in cells before injection into animals, each done in triplicate. The difference in colony formation between SVR bag4 and SVR A221a,b are significant (P < 0.05).
Effect of MAPKK Inhibition on in Vivo Tumorigenesis
One million cells of each type were injected subcutaneously into the right flank of nude male mice. The tumors derived from the cells expressing the dominant negative MAPKK gave rise to rapidly growing tumors that did not vary significantly in size from wild-type SVRbag4 tumors (Figure 7) ▶ . The decreased phosphorylation of MAPKK was also observed after in vivo growth, thus ruling out the possibility that tumor growth in animals was secondary to loss of the transfected gene (data not shown). The finding of equal tumorigenesis of the clones of SVR cells is surprising, in light of the greatly impaired ability of the SVRA221a,b cell lines to form colonies in soft agar. The decreased ability of cells containing the dominant negative MAPKK was preserved after in vivo growth, as cells explanted from rapidly growing SVRA221a,b tumors showed diminished growth in soft agar compared with wild-type SVRbag4 cells. The average volume of the SVRbag4 tumors was 1225 mm3, of SVRA221a tumors, 2783 mm3, and of SVRA221b tumors, 1468 mm3. The tumor volume of SVRA221a and SVRA221b did not differ significantly from that of SVRbag4.
Figure 7.
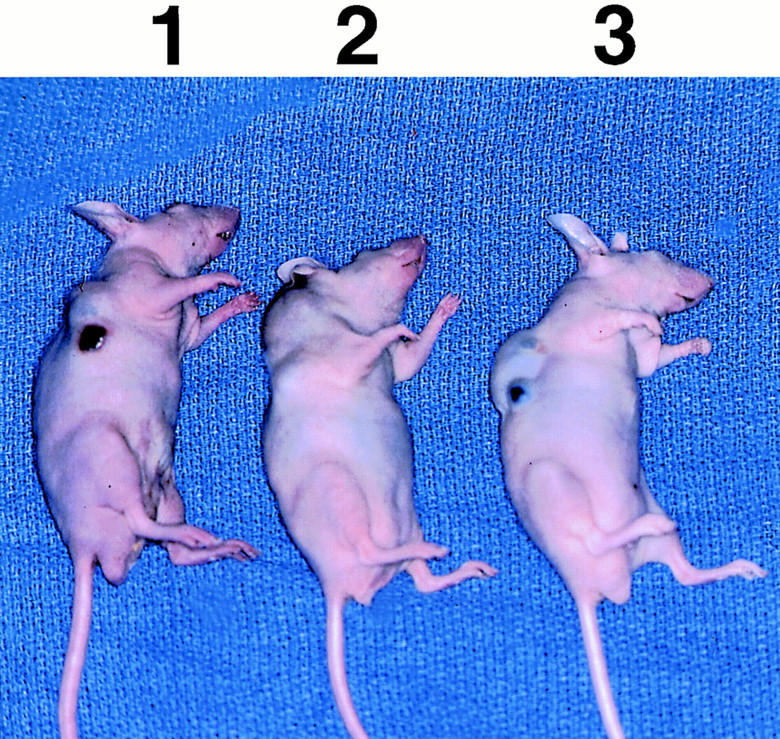
Effect of dominant negative MAPKK expression on in vivo tumorigenesis in nude mice; n = 5 mice per group. The differences in tumor volume are not significant (P > 0.05). The mouse under 1 contains a representative SVRbag4 tumor; the mouse under 2 contains a representative SVRA221a tumor; and the mouse under 3 contains a representative SVRA221b tumor.
Cell Cycle Analysis
To determine whether the decreased growth rate of SVRA221a cells was due to decreased plating efficiency or was secondary to changes in cell cycle progression, cells were stained with propidium iodide and analyzed by flow cytometry. Wild-type SVRbag4 cells showed the following percentages of cells in G0-G1: 53.6%, G2-M: 4.3%, and S: 42.1%, whereas SVRA221a cells showed G0-G1: 59.4%, G2-M: 15.7%, and S: 24.9%. Thus, SVRA221a cells showed a decreased percentage of cells in S phase, suggesting that the decreased growth in vitro is due in part to slowing of cell cycle progression.
Maintenance of Phenotype after Tumorigenesis in Mice
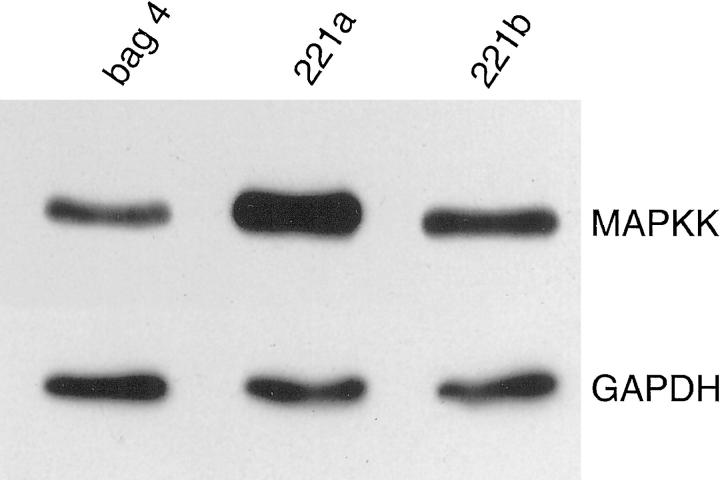
To determine whether the dissociation of soft agar growth and in vivo tumorigenesis was maintained, we explanted tumors from animals bearing SVRbag4 and SVRA221a,b into tissue culture. Cells were cultured from tumors, and the ability of these cells to form colonies in soft agar was analyzed. Consistent with the phenotype before implantation, SVRA221a,b were significantly impaired in their ability to form colonies in soft agar, even after in vivo growth (Table 2) ▶ . In addition, high level expression of transgene was observed in explanted cells, with A221a exhibiting a sixfold increase and A221b showing a twofold increase in total (transgene and endogenous) MAPKK compared with SVRbag4 (endogenous murine MAPKK; Figure 8 ▶ ). This is consistent with maintenance of the phenotype after in vivo growth.
Table 2.
Soft Agar Assay from Cells Derived from Tumors
| Cell line | Number of colonies |
|---|---|
| SVRbag4 | 159 ± 27 |
| SVRA221a | 16 ± 3 |
| SVRA221b | 111 ± 12 |
Data represent the mean ± standard error of the number of colonies observed in two independent experiments in cells explanted from mice bearing tumors, each done in triplicate. The difference in colony formation between SVRbag4 and SVRA221a,b are significant (P < 0.05).
Figure 8.
Western blot analysis of expression of dominant negative MAPKK after in vivo growth. The upper band represents total MAPKK (transgene plus endogenous murine MAPKK), and the bottom lane represents GAPDH, a control for loading. A sixfold increase is noted in SVRA221a and a twofold increase is noted in SVRA221b compared with SVRbag4.
Discussion
Mutations in ras are associated with numerous forms of human malignancy, but the individual contributions of the signal transduction pathways activated by ras is less well understood. Ras has been shown to activate multiple signal pathways including MAPKK and PI-3-K. Most of the studies involving ras and MAPKK have used NIH3T3 cells, an immortalized fibroblast cell line deficient in the tumor suppressor gene p16. 17 Introduction of dominant negative MAPKK into ras transformed NIH3T3 fibroblasts causes loss of tumorigenesis, thus MAPKK plays an important role in ras transformation of NIH3T3 cells. 18 However, recent evidence suggests that the findings in NIH3T3 fibroblasts may not be generalized to other cell types. For example, oncogenic raf, which causes activation of MAPKK, is insufficient to transform immortalized intestinal epithelial cells. 19,20 This may be due to either the cell type (intestinal epithelium versus fibroblast) or the genetic background of the cell line being transformed by ras. We previously described a two-step system of transformation in endothelial cells to study the contribution of these signal transduction pathways in promoting tumorigenesis and angiogenesis. Introduction of H-ras into immortalized endothelium results in increased in vivo proliferation relative to apoptosis and increased production of the angiogenic mediators VEGF and MMP, as well as decreased production of the angiogenesis inhibitor TIMP. 11 We have previously shown that the PI-3-K pathway regulates the production of the angiogenic mediators VEGF and MMP, and treatment of animals with the PI-3-K inhibitor wortmannin resulted in a significant decrease in tumor size. Consistent with our results, expression of the p110 catalytic subunit of PI-3-K caused transformation of chicken endothelium into angiosarcomas. 21 The effect of MAPKK modulation in this system has not been fully explored. Because of this, we initially sought to introduce a constitutively active MAPKK into large T immortalized, nontumorigenic endothelial cells. We were unable to obtain any stable clones overexpressing a constitutively active MAPKK. This result may be secondary to a growth inhibitory effect of raf-MAPKK pathway overexpression observed in certain cell types. 22-24
Primary animal cells and untransformed cell lines require a solid surface on which to attach, spread, and multiply, a phenomenon known as anchorage-dependent growth. These cells, if detached from solid supports, undergo apoptosis through a process termed anoikis. 25 Researchers have taken advantage of the property of transformed cells to grow in suspension, using agar, to show the ability of viruses to transform cells in an anchorage-independent assay. 26-29
A correlation exists between anchorage-independent growth and tumorigenicity in mice. 15,26-29 Shin et al have demonstrated that other cellular phenotypes of transformation, such as cell density and growth in reduced serum are not strongly correlated with neoplastic growth in vivo. 29 The ability of cells to grow in the absence of adhesion presumably reflects the ability of transformed cells to survive and grow in inappropriate locations in vivo.
In this report, using the various endothelial cell lines, we tested their transforming ability in vitro by a colony formation assay in soft agar. These results suggest that the endothelial cell line MS1, which is normally anchorage-dependent, requires the activation of MAPKK downstream of ras to be transformed in vitro. In contrast, SVRA221a,b cells, which demonstrate low levels of MAP kinase activation and poor growth in soft agar, are fully tumorigenic in vivo. A potential predictor of the effect of signal transduction inhibition in a tumor cell line may be the bioactivity of MMPs, enzymes required for tumor growth and metastasis. 30 Inhibition of MAPKK in our system, by both the small molecular weight inhibitor PD98059 and a dominant negative MAPKK led to an increase in MMP bioactivity. Another MAPKK-related gene, MAPKK-4, has been implicated as a tumor suppressor gene in gastrointestinal malignancy, 31 so pharmacological inhibition of MAPKK may be beneficial in some malignancies, but not in others.
Our findings, that introduction of a dominant negative MAPKK slows growth in vitro and inhibits soft agar growth, but not tumorigenesis, are surprising. However, one must take into account the different environments of these assays. Growth in vitro is partially a function of the cell to proliferate in two dimensions attached to a plastic surface. Growth in soft agar requires three-dimensional growth without attachment to physiologically relevant basement membrane proteins and host integrins. Growth in vivo involves three-dimensional growth in the presence of host basement membrane proteins and integrins. Our results suggest that down-regulation of MAP kinase may play a role in two- and three-dimensional growth in a solid area in the absence of host proteins and integrins, but is not required for in vivo growth. Reliance on the soft agar assay alone may lead researchers to overlook potentially important oncogenic events.
Our results have several implications. First, MAPKK is not likely to be the major signal transduction pathway in regulating tumorigenesis and angiogenesis in ras-transformed angiosarcoma. Recent data showing that overexpression of the catalytic subunit of PI-3-kinase results in avian angiosarcomas suggest that PI-3-kinase mediates the angiogenic switch in angiosarcoma. 21 Second, growth rate in vitro cannot always be correlated with in vivo tumorigenicity. Control SVRbag4 cells grow much more rapidly than MAPKK-deficient SVRA221a cells in vitro, but are about equally tumorigenic. Consistent with our studies in mice, we have demonstrated that phosphorylated MAP kinase is elevated in benign endothelial neoplasms (hemangiomas and pyogenic granulomas), but shows very low level expression in malignant angiosarcoma. 32 These findings suggest that high level expression of phosphorylated MAP kinase is not required for human angiosarcoma growth in vivo, consistent with the findings of this study. Examination of expression of phosphorylated MAP kinase may prove diagnostically useful in endothelial neoplasms. Finally, prior studies have concluded that anchorage-independent growth is synonymous with tumorigenesis, and, conversely, that loss of soft agar growth means cells are incapable of forming tumors. Our results suggest that this correlation is not perfect, and that in vivo studies are required to fully assess tumorigenesis. Knowledge of the effect of MAPKK inhibition on in vivo growth will be necessary to determine the type of tumor that will be most responsive to MAPKK inhibition. Finally, MAPKK inhibitors may be pharmacologically useful for some malignancies but may fail to suppress the growth of other malignancies, depending on tissue type.
Acknowledgments
We acknowledge Dr. Judah Folkman for his discussion and critical reading of this manuscript and Dr. Chris Marshall for his gift of antibody and helpful discussion.
Footnotes
Address reprint requests to Jack L. Arbiser, Department of Dermatology, Emory University School of Medicine, WMB 5309, Atlanta, GA 30322. E-mail: jarbise@emory.edu.
Supported by the Society for Pediatric Dermatology, the Dermatology Foundation, and the Thomas B. Fitzpatrick KAO Award (to J. L. A.), American Skin Association and National Institute of AMS grants R03AR44947 (to J. L. A.), and Emory Skin Disease Research Core Center P30 AR 42687 and KO8 AR02030 (to J. L. A.). M. A. M. is supported by American Cancer Society grant 83821. K. R. L. is supported by the Garrett B. Smith Foundation and the Leukemia and Lymphoma Society.
References
- 1.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J: Phosphoinositol-3-OH kinase as a direct target of ras. Nature 1994, 370:527-532 [DOI] [PubMed] [Google Scholar]
- 2.Joneson T, White MA, Wigler MH, Bar-Sagi D: Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of ras. Science 1996, 271:810-812 [DOI] [PubMed] [Google Scholar]
- 3.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ: Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for ras transformation. Mol Cell Biol 1995, 15:6443-6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowley S, Paterson H, Kemp P, Marshall CJ: Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation and for transformation of NIH 3T3 cells. Cell 1994, 77:841-852 [DOI] [PubMed] [Google Scholar]
- 5.Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG: Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 1994, 265:966-970 [DOI] [PubMed] [Google Scholar]
- 6.Kauffman-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G: Suppression of c-myc induced apoptosis by ras signalling through PI(3)K and PKB. Nature 1997, 385:544-548 [DOI] [PubMed] [Google Scholar]
- 7.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J: Matrix adhesion and ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J 1997, 16:2783-2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troppmair J, Bruder JT, Munoz H, Lloyd PA, Kyriakis J, Banerjee P, Avruch J, Rapp UR: Mitogen activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires raf and is necessary for transformation. J Biol Chem 1994, 269:7030-7035 [PubMed] [Google Scholar]
- 9.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS: Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995, 55:4575-4580 [PubMed] [Google Scholar]
- 10.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D: Both v-Ha-ras and v-raf stimulate the expression of the vascular endothelial growth factor in NIH3T3 cells. J Biol Chem 1995, 270:25915-25919 [DOI] [PubMed] [Google Scholar]
- 11.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers HR, Folkman J: Oncogenic H-Ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA 1997, 94:861-866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagasawa T, Suzuki S, Takeda T, DeGroot LJ: Thyroid hormone receptor b1 expression in developing mouse limbs and face. Endocrinology 1997, 138:1276-1281 [DOI] [PubMed] [Google Scholar]
- 13.Kim B, Leventhal PS, Saltiel AR, Feldman EL: Insulin-like growth factor-1 mediated neurite outgrowth in vitro requires mitogen-activated protein kinase activation. J Biol Chem 1997, 272:21268-21273 [DOI] [PubMed] [Google Scholar]
- 14.Vlahos CJ, Matter WF, Hui KY, Brown RF: A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one. J Biol Chem 1994, 269:5241-5248 [PubMed] [Google Scholar]
- 15.LaMontagne KR, Jr, Hannon G, Tonks NK: Protein tyrosine phosphatase PTP1B suppresses p210 bcr-abl induced transformation of Rat1 fibroblasts and promotes differentiation of K562 cells. Proc Natl Acad Sci USA 1998, 95:14094-14099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH: Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 1998, 93:605-615 [DOI] [PubMed] [Google Scholar]
- 17.Linardopoulos S, Street A, Quelle DE, Parry D, Peters G, Sherr CJ, Balmain A: Deletion and altered regulation of p16ink4a and p15ink4b in undifferentiated mouse skin tumors. Cancer Res 1995, 55:5168-5172 [PubMed] [Google Scholar]
- 18.Qiu RG, Chen J, Kirn D, McCormick F, Symons M: An essential role for Rac in ras transformation. Nature 1995, 374:457-459 [DOI] [PubMed] [Google Scholar]
- 19.Oldham SM, Cox AD, Reynolds ER, Sizemore NS, Coffey RJ, Jr, Der CJ: Ras, but not src transformation of RIE-1 epithelial cells is dependent on activation of the mitogen-activated protein kinase cascade. Oncogene 1998, 16:2565-2573 [DOI] [PubMed] [Google Scholar]
- 20.Oldham SM, Clark GJ, Gangarosa LM, Coffey RJ, Jr, Der CJ: Activation of the Raf-1/MAP kinase cascade is not sufficient for Ras transformation of RIE-1 epithelial cells. Proc Natl Acad Sci USA 1996, 93:6924-6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK: Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 1997, 276:1848-1850 [DOI] [PubMed] [Google Scholar]
- 22.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M: Raf-induced proliferation or cell cycle arrest is determined by the level of raf activity with arrest mediated by p21Cip1. Mol Cell Biol 1997, 17:5598-5611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pumiglia KM, Decker SJ: Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA 1997, 94:448-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson NL, Gardner AM, Diener KM, Lange-Carter CA, Gleavy J, Jarpe MB, Minden A, Karin M, Zon LI, Johnson GL: Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J Biol Chem 1996, 271:3229-3237 [DOI] [PubMed] [Google Scholar]
- 25.Frisch SM, Ruoslahti E: Integrins and anoikis. Curr Opin Cell Biol 1997, 9:701-706 [DOI] [PubMed] [Google Scholar]
- 26.Lago TG, Witte ON: The bcr-abl oncogene transforms rat-1 cells and cooperates with v-myc. Mol Cell Biol 1989, 9:1263-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacPherson I, Montagnier L: Suspension culture for the selective assay of cells transformed by polyoma virus. Virology 1964, 23:291-294 [DOI] [PubMed] [Google Scholar]
- 28.Stoker M, O’Neill C, Berryman S, Waxman V: Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer 1968, 3:683-693 [DOI] [PubMed] [Google Scholar]
- 29.Shin S, Freedman VH, Risser R, Pollack R: Tumorigenicity of virus transformed cells in nude mice is correlated specifically with anchorage-independent growth in vitro. Proc Natl Acad Sci USA 1975, 72:4435-4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stetler-Stevenson WG: Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 1999, 103:1237-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng DHF, Perry WL, Hogan JK, Baumgard M, Bell R, Berry S, Davis T, Frank D, Frye C, Hattier T, Hu R, Jammulapati S, Janecki T, Leavitt A, Mitchell JT, Pero R, Sexton D, Schroeder M, Su P-H, Swedlund B, Kyriakis JM, Avruch J, Bartel P, Wong AKC, Oliphant A, Thomas A, Skolnick MH, Tavtigian SV: Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res 1997, 57:4177-4182 [PubMed] [Google Scholar]
- 32.Arbiser JL, Weiss SW, Arbiser ZK, Bravo F, Govindajaran B, Caceres-Rios H, Cotsonis G, Recavarren S, Swerlick RA, Cohen C: Differential expression of active MAP kinase in cutaneous endothelial neoplasms: implications for biologic behavior and response to therapy. J Am Acad Dermatol (in press) [DOI] [PubMed]