Abstract
Breast cancer is the most frequent tumor type among women in the United States and in individuals with Li-Fraumeni syndrome. The p53 tumor suppressor gene is altered in a large proportion of both spontaneous breast malignancies and Li-Fraumeni breast cancers. This suggests that loss of p53 can accelerate breast tumorigenesis, yet p53-deficient mice rarely develop mammary tumors. To evaluate the effect of p53 loss on mammary tumor formation, the p53null allele was back-crossed onto the BALB/c genetic background. Median survival was 15.4 weeks for BALB/c-p53−/− mice compared to 54 weeks for BALB/c-p53+/− mice. Sarcomas and lymphomas were the most frequent tumor types in BALB/c-p53−/− mice, whereas 55% of the female BALB/c-p53+/− mice developed mammary carcinomas. The mammary tumors were highly aneuploid, frequently lost the remaining wild-type p53 allele, but rarely lost BRCA1. Although mammary tumors were rarely detected in BALB/c-p53−/− female mice, when glands from BALB/c-p53−/− mice were transplanted into wild-type BALB/c hosts, 75% developed mammary tumors. The high rate of mammary tumor development in the BALB/c background, but not C57Bl/6 or 129/Sv, suggests a genetic predisposition toward mammary tumorigenesis. Therefore, the BALB/c-p53+/− mice provide a unique model for the study of breast cancer in Li-Fraumeni syndrome. These results demonstrate the critical role that the p53 tumor suppressor gene plays in preventing tumorigenesis in the mammary gland.
The p53 tumor suppressor gene plays a complex and critical role in maintaining genome integrity. In cells with damaged DNA, p53 mediates the decision to arrest cells to allow for DNA repair or eliminate the cell by apoptotic pathways. Mutations in the p53 tumor suppressor gene (TP53) are the most common genetic abnormality being found in >50% of all human cancers, emphasizing the importance of p53 function for suppression of tumors. 1 Germline mutations in the p53 tumor suppressor gene are associated with Li-Fraumeni syndrome in which early-onset breast cancer is the most common cancer affecting women with Li-Fraumeni syndrome. 2-4 Although this suggests that the loss of p53 is a critical event in the progression of breast tumorigenesis, p53-deficient mice rarely developed mammary tumors. 5 Instead, mice lacking p53 died prematurely from a variety of other tumors. 6 The lack of mammary tumor formation in mice deficient for p53 suggested that loss of p53 alone was not sufficient for tumor development in the mammary gland or that its role as a tumor suppressor in this tissue was not essential.
Different strains of inbred mice have been shown to differ in their susceptibility to mammary tumorigenesis. 7 Female BALB/c mice were shown to be sensitive to radiation-induced mammary tumor development, whereas C57Bl/6 mice were resistant. 7,8 This difference was correlated with an increase in chromosomal instability in BALB/c mice compared to the C57Bl/6 mice rather than variations in hormonal levels. 7 In the analysis of families with Li-Fraumeni syndrome, it was shown that some women, in particular families, developed breast cancers more frequently than women in other families, 9 suggesting the presence of modifier loci that may also be involved in the predisposition to mammary tumor formation. Similarly, lack of mammary tumorigenesis in p53-deficient mice could have been because of the resistance of the genetic backgrounds.
Therefore, the p53null allele was transferred to the BALB/c genetic background to determine whether this altered the tumor spectrum. BALB/c-p53−/− mice displayed a similar tumor incidence and spectrum to that observed in other backgrounds with a predominance of lymphomas and hemangiosarcomas. An abnormal mammary phenotype, ranging from stromal alterations to preneoplastic changes in the mammary epithelium, was observed in the majority of the female BALB/c-p53−/− mice. In contrast, female BALB/c-p53+/− mice showed a prevalence of mammary carcinomas with a latency of 8 to 14 months. These data reveal a genetic predisposition to mammary tumor development in BALB/c mice, which is accelerated upon loss of p53. Furthermore, the prevalence of mammary tumors in BALB/c-p53+/− mice provides a model that more accurately reflects the tumor spectrum in individuals with Li-Fraumeni syndrome.
Materials and Methods
Mice and Tissues
C57Bl/6x129/Sv p53-deficient mice (generous gift from Tyler Jacks, Cambridge, MA) were mated with BALB/c mice. The p53null allele from these mice was back-crossed for nine generations onto the BALB/cMed strain. Individuals were genotyped by multiplexed PCR as described previously. 10,11 Mice were monitored weekly for 18 months and sacrificed when overt tumor development was detected or when signs of morbidity were evident. A total of 44 BALB/c-p53−/− mice (eight male, 36 female) and 45 BALB/c-p53+/− mice (seven male, 38 female) underwent necropsy. Five wild-type mice were sampled from age-matched animals in our colony (n = 50). Tumor tissues as well as the fourth inguinal mammary glands were excised and fixed for 8 to 12 hours in 10% neutral-buffered formalin. Tissues were stored in 70% ethanol before embedding in paraffin. Tissues were sectioned at a thickness of 4 μm and were stained with hematoxylin and eosin for evaluation by light microscopy. Additional samples of tumor tissue were snap-frozen and stored in liquid nitrogen.
Ploidy Analysis
Single cell suspensions were obtained by digesting five 50-μm paraffin sections in pepsin. The suspensions were filtered, stained with propidium iodide, and analyzed using a FACScan flow cytometer. A total of 20,000 events were collected from each sample as described previously. 12 DNA histograms were produced using ModFit LT software (Verity Software House, Topsham, ME).
Cytogenetic Analysis
Fresh tumor tissue was removed at necropsy, rinsed with Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) to remove cellular debris, minced, and digested for 3 hours in 10 μg/ml of collagenase III (Sigma, St. Louis, MO). The cell suspension was washed with 5% adult bovine serum in phosphate-buffered saline and cultured in a 100-mm dish in Dulbecco’s modified Eagle’s medium/F12 media supplemented with sodium bicarbonate, HEPES buffer, 2% adult bovine serum, insulin (10 μg/ml), and epidermal growth factor (5 ng/ml) until 60% confluent. The actively dividing culture was treated with colcemid (Life Technologies, Inc.) for 18 hours, then harvested by trypsin digestion. Cells were lysed with 0.068 mol/L KCl and fixed with a 3:1 methanol and glacial acetic acid solution. Interphase and metaphase nuclei were trypsinized and stained with Giemsa (BDH Chemicals, Poole, UK) for visualization.
Southern Blot Analysis
Fresh-frozen tumor samples taken at the time of necropsy were homogenized in 100 mmol/L Tris, 5 mmol/L ethylenediaminetetraacetic acid, 0.2% sodium dodecyl sulfate, 200 mmol/L NaCl, and digested with 100 μg/ml proteinase K. Genomic DNA was extracted and purified with phenol/chloroform (1:1; v/v). Ten micrograms of DNA were digested with EcoRI and StuI, then separated on a 0.7% agarose gel. The DNA was transferred to a nylon membrane, then hybridized to a genomic clone (probe B, a generous gift from Tyler Jacks) spanning the region from exon 7 to exon 9 of the p53 gene. 11 The blot was stripped and hybridized sequentially with probes for BRCA1 (exon 11, provided by Roger Wiseman, Research Triangle Park, Durham, NC), and β-casein cDNA (exons 1 to 9). Southern blot was performed in duplicate and quantitation was performed using a phosphorimager (Molecular Dynamics, Sunnyvale, CA). Hybridization values for p53 and BRCA1 were normalized to β-casein to control for loading variation. Hybridization values were compared to the genomic DNA of corresponding genotypes isolated from tail biopsies. Allelic loss was scored if the hybridization value of the tumor was less than or equal to 50% of the value obtained from p53+/− tail DNA.
Mammary Transplants
Whole gland transplants were performed by surgically removing the fourth inguinal mammary glands from mature (>8weeks) BALB/c-p53−/− (n = 16) or BALB/c-p53+/+ (n = 8) females and suturing them onto the abdominal fascia of age-matched BALB/c wild-type recipients. Reconstituted mammary gland transplants were prepared with modifications of previous procedures. 13 The fourth inguinal mammary glands from 21- to 24-day-old BALB/c-p53+/+ females were cleared of mammary epithelium. Single ducts from mature BALB/c-p53−/− donors were dissected and transplanted into the cleared fat pads from BALB/c-p53+/+ recipients (n = 14). Two weeks after the initial surgery, the reconstituted glands were removed and then transplanted onto the abdominal fascia of age matched BALB/c wild-type females. All transplant recipients were palpated weekly for tumor formation and sacrificed when tumor masses were detected. Tissues were processed and sectioned for histological evaluation as above.
Results
Tumor Incidence and Survival Rate of BALB/c-p53-Deficient Mice
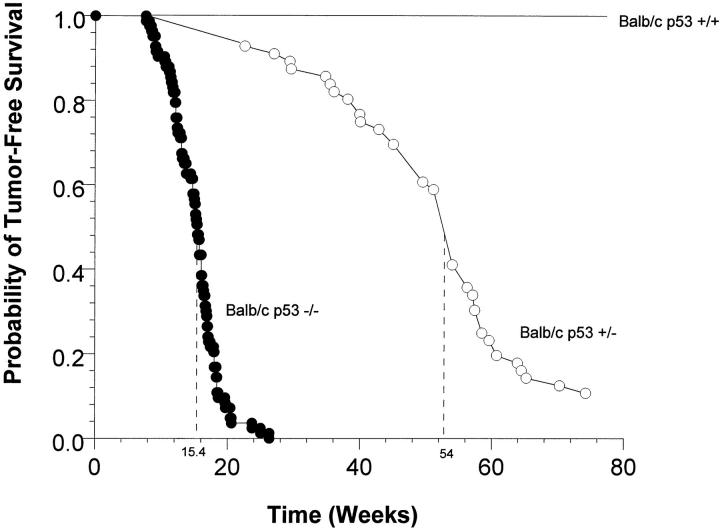
Mice deficient for p53 showed heightened tumor susceptibility compared to wild-type animals. BALB/c-p53−/− mice developed tumors earlier and at higher frequency than did BALB/c-p53+/− mice. All of the BALB/c-p53−/− mice had developed tumors or died by 26 weeks of age with a median of 15.4 weeks (Figure 1) ▶ . Among the BALB/c-p53+/− mice, 50% developed tumors by 54 weeks and only 10% of the animals were tumor-free throughout the duration of the study. No wild-type animals developed tumors within the 80-week period of observation.
Figure 1.
Survival curves of BALB/c p53-deficient mice. The probability of tumor-free survival of BALB/c-p53−/− mice (n = 85; closed circles), and BALB/c-p53+/− mice (n = 56; open circles) was monitored for 80 weeks. No wild-type animals died during this study (n = 5). Dashed lines represent the median time at which 50% of the animals had developed tumors.
Tumor Spectrum and Mammary Phenotype in BALB/c- p53−/− Mice
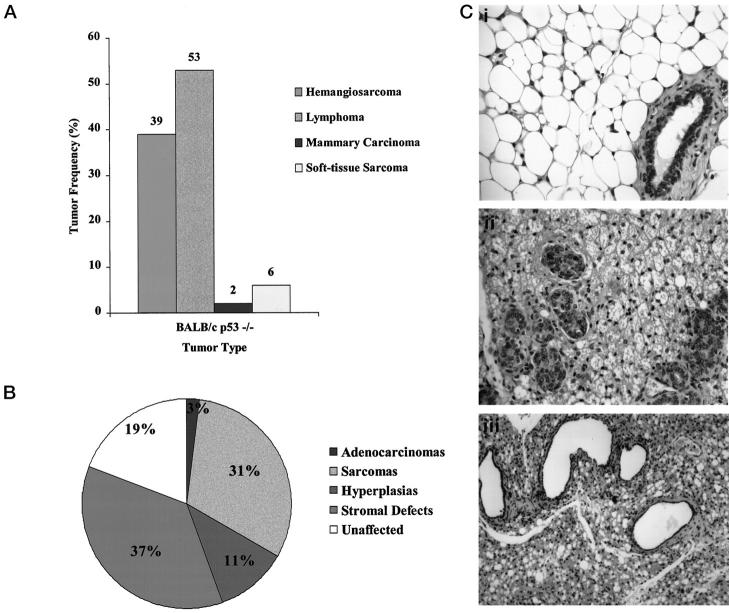
There were four predominant cancers detected in BALB/c-p53−/− mice (Figure 2A) ▶ . Multiple tumor types were often present in individual mice. Lymphomas were the most frequent tumor type, affecting 53% of the BALB/c-p53−/− mice and involved primarily the thymus, lymph nodes, or other secondary lymphoid tissues. Hemangiosarcomas were the second most frequently detected tumor, affecting 39% of the animals. These tumors usually were present in the subcutaneous and soft tissues on the limbs or head, or in the mammary glands. Although the focus of the study was on primary tumors, metastases were detected in the lung and liver in several cases. Only one mammary carcinoma developed in the BALB/c-p53−/− mice.
Figure 2.
Tumor distribution and mammary abnormalities in BALB/c-p53−/− mice. A: Tumor spectrum from BALB/c-p53−/− mice. Frequency histogram of tumor types observed in both male and female mice (n = 44). B: Mammary phenotype of female BALB/c-p53−/− mice. Relative frequency of abnormalities detected in the inguinal or thoracic gland from nulliparous and breeder females (n = 36). C: H&E-stained sections of mammary tissue from BALB/c-p53−/− females. The normal murine mammary gland architecture is characterized by large adipocytes in the stroma surrounding a sparse population of ducts (i, ×40 objective). Typical stromal changes observed in BALB/c-p53−/− females (ii and iii). Stroma characterized by microvesicular adipocytes and proliferation of mammary ducts (ii, ×40 objective). Hypercellular stroma and dilated ducts with attenuation of the mammary epithelium (iii, ×20 objective).
Previous studies have shown that mating of p53 heterozygous animals yielded a reduced number of p53-null female offspring because of exencephaly. 14 Similarly, BALB/c-p53−/− female offspring were not born at the expected Mendelian ratios indicating that there were gestational defects resulting in embryonic lethality. BALB/c-p53−/− females were primarily normal, but microscopic lesions were detected in 78% of the mammary glands. These included sarcomas, epithelial hyperplasia at 2 to 5 months of age, and alterations in stromal morphology. Stromal abnormalities and sarcomas were the predominant mammary lesions present in 68% of the BALB/c-p53−/− female mice. The stromal changes were characterized by adipocytes with microvesicular fat droplets and a hypercellularity of the stroma (Figure 2C ▶ , ii). Periductal stromal tissue was thickened and many of the mammary ducts were markedly dilated with attenuated epithelial lining (Figure 2C ▶ , iii). In addition to the morphological changes observed in the mammary glands of nulliparous BALB/c-p53−/− mice, abnormal stromal and glandular architecture were also seen in glands of pregnant, lactating, and postinvoluting mice (data not shown).
Tumor Spectrum and Mammary Phenotype in BALB/c-p53 Heterozygous Mice
Although BALB/c-p53+/− mice developed tumors frequently, there was a delay in their appearance and a difference in the tumor spectrum compared to the BALB/c-p53−/− mice. Hemangiosarcomas, lymphomas, and osteosarcomas commonly found in some other strains of p53-heterozygous mice 6,11 were less frequent in BALB/c-p53+/− mice, but still accounted for 36% of all of the tumors (Figure 3A) ▶ .
Figure 3.
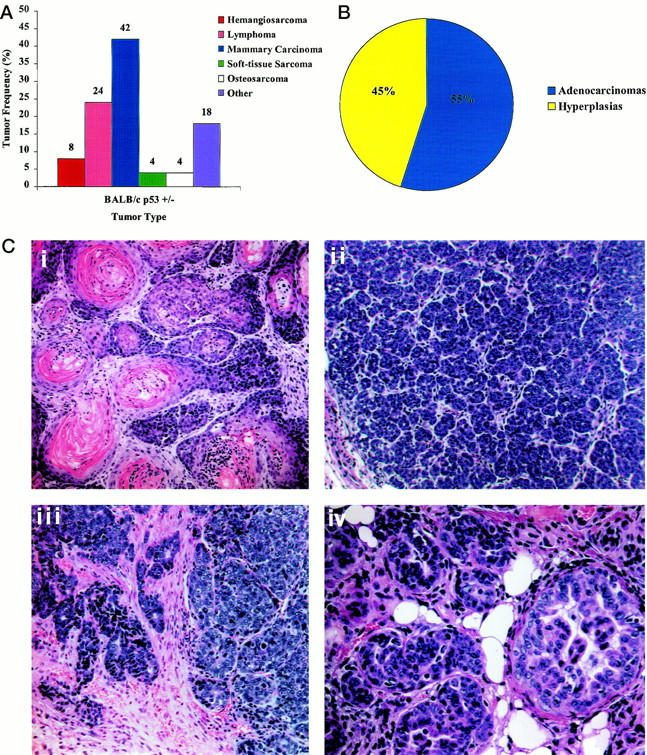
Tumor distribution and mammary abnormalities in BALB/c-p53+/− mice. A: Tumor spectrum from BALB/c-p53+/− mice. Frequency histogram of tumor types observed in both male and female mice (n = 45). B: Mammary phenotype of female BALB/c-p53+/− mice. Relative frequency of abnormalities detected in the inguinal or thoracic mammary glands from nulliparous and breeder females (n = 38). Mammary hyperplasia was observed either alone or in association with tumors in all glands analyzed. C: H&E-stained sections of mammary tumor tissue from BALB/c-p53+/− females (×20 objective). A typical adenoacanthoma characterized by keratin formation (i) and an adenocarcinoma with small acinar structures (ii). H&E section of a mammary carcinoma infiltrating adjacent stroma. (iii, ×20 objective). Ductal hyperplasia commonly seen in BALB/c-p53+/− female mice (iv, ×40 objective).
Although mammary carcinomas were rare in BALB/c-p53−/− mice, they were the most prevalent tumor type in BALB/c-p53+/− mice, accounting for 42% of the tumors (Figure 3A) ▶ . All of the female heterozygous mice developed mammary abnormalities, which were either overt mammary carcinomas (55%) or hyperplasias (45%) (Figure 3B) ▶ . Mammary tumors originated in both the inguinal and thoracic glands with a latency of 8 to 14 months. These tumors were generally adenoacanthomas or acinar-type adenocarcinomas (Figure 3C) ▶ with occasional poorly differentiated carcinomas identified. One animal developed two separate primary mammary tumors in different glands that were of different histological types. Many of the mammary carcinomas had infiltrating growth patterns, however metastases were not detected. Mammary carcinomas were present in nulliparous animals and breeder females. Epithelial hyperplasia was detected in all of the female BALB/c-p53+/− mice that did not develop mammary tumors (Figure 3C, iv) ▶ and was also present in the ductal epithelium of those mice that developed mammary carcinomas.
Frequent Loss of p53 but Not BRCA1 in Mammary Tumors from BALB/c-p53+/− Mice
Flow cytometry was performed to determine whether the tumors contained a population of aneuploid cells (Figure 4A) ▶ . Seventy-one percent (10 of 14) of the mammary tumors analyzed exhibited rates of aneuploidy ranging from 17 to 89%. Tumors that maintained a diploid state had relatively high S-phase fractions ranging from 5 to 21%. Cytogenetic analysis was performed to examine the types of chromosomal abnormalities present in these tumors (Figure 4B) ▶ . Chromosomal translocations, strand breaks, and aberrant mitotic exchanges were common abnormalities associated with mammary tumors from BALB/c-p53+/− mice.
Figure 4.
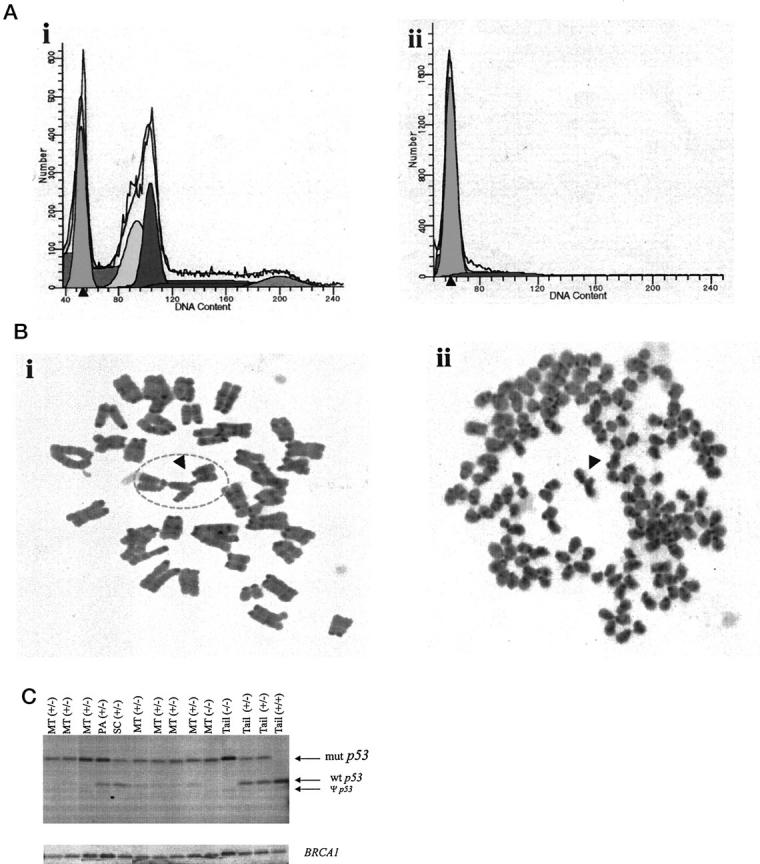
Analysis of DNA from BALB/c-p53+/− mammary carcinomas. A: Representative DNA histograms determined by FACS analysis from mammary tumors (n = 14). Seventy-one percent of the tumors were aneuploid with DNA content >40 (i), and the remaining 29% of the tumor were diploid (ii). The solid line represents the distribution of cells. Subpopulations of cells were calculated using the ModFit LT software and are represented by the shaded peaks. B: Representative Geimsa-stained (original magnification, ×100) metaphase spreads of mammary carcinomas from BALB/c-p53+/− females. Typical abnormalities such as quadriradial chromosomes (i) and chromosome breaks commonly found in aneuploid karyotypes (ii) marked by arrows, respectively. C: Southern blot analysis of p53 and BRCA1 in BALB/c-p53+/− mammary tumors. Genomic DNA from mammary tumors (MT), a prepucial adenoma (PA), a salivary gland carcinoma (SC), and tails (tail) were digested with EcoRI and StuI. Loss of heterozygosity was determined using a genomic DNA clone spanning exon 7 to exon 9 of the p53 gene. Three bands were detected in tail DNAs from BALB/c-p53+/− mice (tail +/−). These fragments represent the wild-type allele (wt p53), the mutant allele (mut p53), and the pseudogene (ψ p53). The blot was reprobed with exon 11 of BRCA1 to determine whether there was loss of BRCA1 in the mammary tumors from BALB/c-p53+/− mammary tumors.
Because of the high degree of aneuploidy and chromosomal abnormalities detected in the mammary tumors, Southern blot analysis was performed on mammary carcinomas to analyze the status of the wild-type p53 allele in the BALB/c-p53+/− mice (Figure 4C) ▶ . In all seven of the mammary tumors analyzed, there was partial or complete loss of the wild-type allele. In contrast, the wild-type p53 allele was retained in a prepucial adenoma and a salivary gland carcinoma from BALB/c-p53+/− mice. Tail DNA wild-type, p53+/−, and p53−/− animals were used as positive controls for DNA levels. There was no correlation between ploidy status of the mammary carcinoma and loss of heterozygosity.
Because the genes for BRCA1 and p53 lie 21 centimorgans apart on chromosome 11 in mice, it was possible that BRCA1 may also have been lost in the in the mammary tumors of BALB/c-p53+/− mice. Therefore, Southern blot analysis for BRCA1 was performed on those mammary carcinomas that were analyzed for p53 loss of heterozygosity (Figure 4C ▶ , bottom). In all of the mammary tumors analyzed, little or no loss of BRCA1 was detected compared to tail DNA. The β-casein locus was also analyzed to control for the possibility of random losses because of chromosomal instability. Similar to the BRCA1 locus, there was no evidence of significant loss of alleles at the β-casein locus compared to tail DNA (data not shown). Therefore, preferential loss of the wild-type p53 allele in these mammary tumors was not a random event, but seems to be selected for during tumor progression.
Development of Mammary Tumors from BALB/c-p53−/− Epithelium
Because mammary tumors developed in only one of the BALB/c-p53−/− mice, it was possible that early mortality masked the development of mammary carcinomas. Therefore, whole glands from BALB/c-p53−/− mice were transplanted into wild-type (BALB/c-p53+/+) recipients and monitored for tumor formation. Of the whole gland transplants that were accepted, 75% developed mammary carcinomas (Table 1) ▶ , which were first observed by 7 months after transplantation (Figure 5A) ▶ . Several BALB/c-p53−/− whole gland transplants failed to engraft in the BALB/c wild-type recipients (25%). No tumors developed in the whole gland transplants derived from wild-type BALB/c mice.
Table 1.
Tumor Incidence in BALB/c-p53−/− Mammary Gland Transplants
| Transplant genotype | n | Accepted (%) | Tumors (%) |
|---|---|---|---|
| Whole-gland transplants* | |||
| +/+ whole gland | 8 | 8 (100) | 0 (0) |
| −/− whole gland | 16 | 12 (75) | 9 (75) |
| Reconstituted gland transplants† | |||
| p53null/wt† | 14 | 11 (79) | 6 (55) |
| p53wt/wt‡ | 4 | 100 | 0 (0) |
*Whole glands and reconstituted glands were transplanted into BALB/c wild-type recipients.
†p53−/−epithelium was transplanted into wild-type stroma.
‡Wild-type epithelium transplanted into wild-type stroma.
Figure 5.
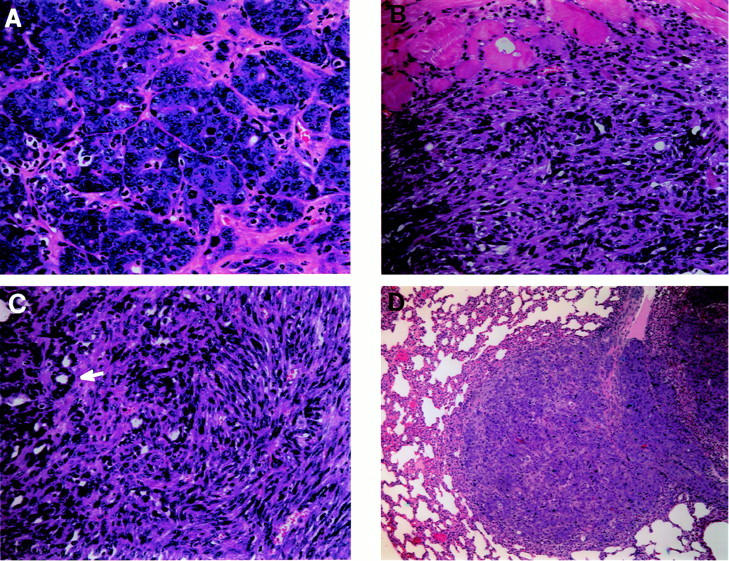
Histology of mammary carcinomas derived from transplants. A: The transplantation of a BALB/c-p53−/− whole gland into a wild-type recipient yielded mammary tumors as early as 7 months. Typical adenocarcinoma with structures reminiscent of mammary ducts and several mitotic figures (H&E, ×40 objective). B: Adenocarcinomas also developed from reconstituted glands consisting of p53-deficient epithelium in a wild-type fat pad. A poorly differentiated mammary carcinoma invading the adjacent skeletal muscle (H&E, ×20 objective). C: An adenocarcinoma arising from a p53null/wt transplant that has both acinar structures (white arrow) and a spindle cell component (×40 objective). D: Lung metastasis from a mammary carcinoma derived from a reconstituted gland consisting of p53−/− epithelium in a wild-type fat pad (H&E, ×10 objective).
Morphological changes in the mammary stroma were observed frequently in BALB/c-p53−/− mice. To determine whether loss of p53 in the mammary epithelium was sufficient for the development of mammary carcinomas without the contribution of the p53-deficient stroma, reconstituted gland transplants were performed. Reconstituted mammary glands composed of either p53−/− epithelium and p53+/+ stroma (p53null/wt) or p53+/+ epithelium and p53+/+ stroma (p53w/w) were transplanted into wild-type BALB/c hosts and monitored for tumor formation. Of the p53null/wt-reconstituted glands that were accepted, 55% developed tumors (Table 1) ▶ . Many of these tumors were histologically similar to the tumors derived from the BALB/c-p53−/− whole-gland transplants. Mammary tumors from p53null/wt-reconstituted glands often exhibited sarcomatoid differentiation, invasive components, and distant metastases (Figure 5, A–C) ▶ . The p53wt/wt transplants, which consisted of wild-type epithelium and stroma, did not develop tumors. Although the number of tumors that developed in the whole-gland transplants was greater than in p53null/wt-reconstituted gland transplants, there was no statistical difference in latency or frequency between the transplant experiments (P > 0.05). Similar to the BALB/c-p53−/− whole gland transplants, 21% of the p53null/wt-reconstituted gland transplants failed to engraft. This seemed to be dependent on the lack of p53 in the epithelium because all of the p53wt/wt transplants were accepted and is consistent with recent experiments with transplants of p53-deficient mammary epithelium. 13
Discussion
Given the large number of breast cancers with p53 mutations and the prevalence of breast tumors in women with Li-Fraumeni syndrome, 15-17 it was surprising that the p53-deficient mice rarely developed mammary tumors. 18 Therefore, we sought to examine the effects of p53 loss on the BALB/c strain that has been widely used for the study of the mammary gland and has been shown to be more susceptible to induction of mammary tumors. 7 BALB/c-p53−/− female mice did not develop mammary carcinomas, but stromal changes were frequently observed in the mammary gland. Early mortality because of lymphomas was likely to have precluded the development of mammary tumors. This is supported by the development of mammary carcinomas when p53-deficient mammary glands were transplanted into wild-type hosts (Table 1) ▶ . Furthermore, transplantation of p53-deficient epithelium into wild-type fat pads also produced mammary carcinomas, suggesting that p53 loss in the mammary epithelium is a critical step in tumorigenesis (Table 1) ▶ . 13 The histology of mammary tumors observed in the BALB/c-p53-deficient mammary tissue is typical of spontaneous mammary tumors in mice 19 and does not exhibit unique histological features that have been observed in other genetically engineered mice. 20 Therefore, this knockout model of mammary tumorigenesis seems to reflect the acceleration of sporadic mammary tumors and their progression that is found in otherwise genetically normal mice.
In humans, Li-Fraumeni syndrome is an inherited predisposition to cancer development and more than half of affected families carry germline mutations in the p53 tumor suppressor gene. 2 Tumors typically associated with this syndrome are early-onset breast carcinomas, osteosarcomas, soft-tissue sarcomas, brain tumors, and leukemias. 2,3,15-17 The BALB/c-p53+/− mice developed mammary carcinomas and other malignancies in a pattern and frequency similar to that reported for the Li-Fraumeni syndrome in humans (Figure 3A) ▶ . 2-4,11,16 The breast cancers that arise in women from Li-Fraumeni families typically develop at mid-life, are highly aneuploid, and frequently lose the remaining wild-type p53 allele. 9,17,18 Similarly, the mammary tumors in BALB/c-p53+/− mice developed near mid-life (latency of 8 to 14 months), were highly aneuploid, and frequently lost the wild-type p53 allele (Figure 4) ▶ . This frequent loss of the wild-type allele in mammary carcinomas from the BALB/c-p53+/− mice differs from the situation in lymphomas where more than half of lymphomas that developed in p53-heterozygous mice retained the wild-type allele. 21 Hence, loss of the wild-type p53 allele may be required for tumor development in the mammary epithelium, but not in other tissues.
The loss of BRCA1 has been linked to heightened risk of breast cancer, yet mutations in this tumor suppressor gene are infrequent in sporadic breast cancers. In BRCA1 conditional knockout mice, where mammary tumors developed after 10 months, three out of four mammary tumors had also lost p53 22 suggesting that loss of both genes may contribute to tumorigenesis. However, none of the mammary carcinomas from BALB/c-p53+/− mice lost BRCA1, supporting the concept that loss of p53 alone is a central and perhaps early event for initiation and progression of mammary tumors. Given that the p53 and BRCA1 genes lie 21 centimorgans apart on chromosome 11, it was surprising that loss of BRCA1 was not seen in some of the tumors if genetic losses occurred randomly. Although the exact mechanism by which p53 was deleted cannot be determined by this analysis, these data suggest that the tumor suppressive effects of p53 occur independent of BRCA1, and that loss of BRCA1 was not required for the development of mammary tumors.
The high rate of mammary tumor development in the BALB/c p53-deficient mice compared to other p53-knockout strains is intriguing. Differences in tumor spectrum were reported between C57Bl/6x129/Sv, which primarily developed lymphomas, and pure 129/Sv, which readily developed testicular tumors. 18 It was suggested that the difference in tumor spectrums was related to the difference in strain-specific tumor susceptibilities. The 129/Sv mice have a relatively high incidence of teratocarcinomas 23 whereas the C57Bl/6 do not. Although the normal BALB/c inbred strain has a low incidence of spontaneous mammary tumors, it has been demonstrated that the BALB/c mammary epithelium is susceptible to genetic instability and radiation-induced mammary tumorigenesis. 7,8,24 A pivotal role of p53 in determining susceptibility of the mammary epithelium to tumor development is suggested by recent experiments demonstrating that p53 function is compromised in the normal BALB/c mammary epithelium. 25 The heightened susceptibility of BALB/c mice to mammary tumor formation supports the existence of genetic modifiers that interact with p53-deficiency epistatically and predisposes to tissue-specific tumorigenesis. The BALB/c strain possesses a variant allele for Cdkn2a (p16/INK4a, p19/ARF) 26 that increases susceptibility of the BALB/c strain to plasmacytomas. P19/ARF-deficient mice are predisposed to tumor development, yet mammary carcinomas are rare. 27 This suggests that other unidentified modifier loci may be responsible for the mammary tumor phenotype in BALB/c-p53+/− mice. Identification of the genetic modifier gene(s) responsible for the heightened development of mammary carcinomas in BALB/c mice will be of particular importance because it may explain the variation in susceptibility to tumor development and tumor spectrum in different individuals with the same germline mutation in p53.
Acknowledgments
We thank S. Marconi for tissue embedding and staining; R. Holden and R. Naeem for assistance in cytogenetics; and R. Bronsen for histopathological analysis of murine tumors.
Footnotes
Address reprint requests to D. Joseph Jerry, Department of Veterinary and Animal Sciences, University of Massachusetts, Amherst, MA 01003. E-mail: jjerry@vasci.umass.edu.
Supported in part by grants from the Massachusetts Department of Public Health (34088PP1017 to D. J. J.), the National Cancer Institute (CA66670 to D. J. J.; CA25215 to D. M.), and from the United States Department of Agriculture (MAES707).
References
- 1.Hollstein M, Rice K, Greenblatt M, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris C: Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acid Res 1994, 22:3551-3555 [PMC free article] [PubMed] [Google Scholar]
- 2.Malkin D, Li F, Strong L, Fraumeni J, Nelson C, Kim D, Kassel J, Gryka M, Bischoff F, Tainsky M, Friend S: Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990, 250:1233-1238 [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, Schauble B, zur Hausen A, Esteve J, Ohagaki H: Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol 1997, 150:1-13 [PMC free article] [PubMed] [Google Scholar]
- 4.Akashi M, Koeffler HP: Li-Fraumeni syndrome and the role of the p53 tumor suppressor gene in cancer susceptibility. Clin Obstet Gynecol 1998, 41:172-199 [DOI] [PubMed] [Google Scholar]
- 5.Donehower LA: The p53-deficient mouse: a model for basic and applied cancer studies. Cancer Biol 1996, 7:269-278 [DOI] [PubMed] [Google Scholar]
- 6.Donehower L, Harvey M, Slagle B, McArthur M, Montgomery C, Butel J, Bradley A: Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature 1992, 356:215-221 [DOI] [PubMed] [Google Scholar]
- 7.Ullrich RL, Bowles ND, Satterfield LC, Davis CM: Strain-dependent susceptibility to radiation-induced mammary cancer is a result of differences in epithelial cell sensitivity to transformation. Radiat Res 1996, 146:353-355 [PubMed] [Google Scholar]
- 8.Ponnaiya B, Cornforth MN, Ullrich RL: Radiation-induced chromosomal instability in BALB/c and C57Bl/6 mice: the difference is as clear as black and white. Radiat Res 1997, 147:121-125 [PubMed] [Google Scholar]
- 9.Varley JM, McGown G, Thorncroft M, Santibanez-Koref MF, Kelsey AM, Tricker KJ, Evans DG, Birch JM: Germ-line mutations of TP53 in Li-Fraumeni families: an extended study of 39 families. Cancer Res 1997, 57:3245-3252 [PubMed] [Google Scholar]
- 10.Jerry DJ, Kuperwasser C, Downing SR, Pinkas J, He C, Dickinson E, Marconi S, Naber SP: Delayed involution of the mammary epithelium in BALB/c-p53 null mice. Oncogene 1998, 17:2305-2312 [DOI] [PubMed] [Google Scholar]
- 11.Jacks T, Remington L, Williams B, Schmitt E, Halachmi S, Bronson R, Weinberg R: Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994, 4:1-7 [DOI] [PubMed] [Google Scholar]
- 12.Li B, Murphy KL, Luacirica R, Kittrell F, Medina D, Rosen JM: A transgenic mouse model for mammary carcinogenesis. Oncogene 1998, 16:997-1007 [DOI] [PubMed] [Google Scholar]
- 13.Jerry DJ, Kittrell FS, Kuperwasser C, Laucirica R, Dickenson ES, Bonilla PJ, Butel JS, Medina D: A mammary-specific model demonstrates the role of p53 tumor suppressor gene in tumor development. Oncogene 2000, 19:1052-1058 [DOI] [PubMed] [Google Scholar]
- 14.Sah V, Attardi L, Mulligan G, Willams B, Bronson R, Jacks T: A subset of p53-deficient embryos exhibit exencephaly. Nat Genet 1995, 10:175-180 [DOI] [PubMed] [Google Scholar]
- 15.Birch JM, Blair V, Kelsey AM, Evans DG, Harris M, Tricker KJ, Varley JM: Cancer phenotype correlates with constitutional TP53 genotype in families with the Li-Fraumeni syndrome. Oncogene 1998, 17:1061-1068 [DOI] [PubMed] [Google Scholar]
- 16.Varley JM, Thorncroft M, McGown G, Appleby AM, Tricker KJ, Evans DGR, Birch JM: A detailed study of loss of heterozygosity on chromosome 17 in tumors from Li-Fraumeni patients carrying a mutation to the TP53 gene. Oncogene 1997, 14:865-871 [DOI] [PubMed] [Google Scholar]
- 17.Varley JM, Evans DG, Birch JM: Li-Fraumeni syndrome—a molecular and clinical review. Br J Cancer 1997, 76:1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey M, McArthur M, Montgomery C, Bradley A, Donehower L: Genetic background alters spectrum of tumors that develop in p53-deficient mice. FASEB J 1993, 7:938-943 [DOI] [PubMed] [Google Scholar]
- 19.Squartini F, Pingitore R: Tumours of the mammary gland. Pathology of Tumours in Laboratory Animals, Tumors of the Mouse, vol 2. Edited by V Turusov, U Mohr. Oxford University Press, New York, 1994, pp 47–100
- 20.Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE: The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 2000, 19:968-988 [DOI] [PubMed] [Google Scholar]
- 21.Venkatachalam S, Shi Y, Jones SN, Vogel H, Bradley A, Pinkel D, Donehower LA: Retention of wild-type p53 in tumors from p53 heterozygous mice: reduction of p53 dosage can promote cancer formation. EMBO J 1998, 17:4657-4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Wagner K, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng C: Conditional mutation of BRCA1 in mammary epithelial cells results in blunted ductal morphogenesis and tumor formation. Nat Genet 1999, 22:37-43 [DOI] [PubMed] [Google Scholar]
- 23.Altman PL, Katz DD: Inbred and genetically defined strains of laboratory animals, Part I. Mouse and Rat. 1979, :pp 207-208 Federation of American Societies of Experimental Biology, Bethesda [Google Scholar]
- 24.Ullrich RL, Davis CM: Radiation-induced cytogenetic instability in vivo. Radiat Res 1999, 152:170-173 [PubMed] [Google Scholar]
- 25.Kuperwasser C, Pinkas J, Hurlbut GD, Naber SP, Jerry DJ: Cytoplasmic sequestration and functional repression of p53 in the mammary epithelium is reversed by hormonal treatment. Cancer Res 2000, 60:2723-2729 [PubMed] [Google Scholar]
- 26.Zhang S, Ramsay ES, Mock BA: Cdk2a, the cyclin-dependent kinase inhibitor encoding p16/INK4a and p19/ARF, is a candidate for the plasmacytoma susceptibility locus, Pctr1. Proc Natl Acad Sci 1998, 95:2429-2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ: Tumor spectrum in ARF-deficient mice. Cancer Res 1999, 59:2217-2222 [PubMed] [Google Scholar]