Abstract
Proteinases are important at several phases of physiological and pathological inflammation, mediating cellular infiltration, cytokine activation, tissue damage, remodeling, and repair. However, little is known of their role in the pathogenesis of inflammatory bowel disease. The aim of this study was to assess the role of tissue proteases in a mouse model of colitis. Proteolytic activity was analyzed, using gel and in situ zymography, in colonic tissues from severe combined immunodeficient mice with colitis induced by transfer of CD4+ T lymphocytes. Serine proteinase levels increased in colitic tissue, with major species of 23 kd, 30 kd, and 45 kd. Co-migration and inhibition studies indicated that the 23-kd proteinase was pancreatic trypsin and that the 30-kd species was neutrophil elastase. Matrix metalloproteinase (MMP)-9 expression, and MMP-2 and MMP-9 activation, was elevated in colitic tissues. Proteinase levels followed a decreasing concentration gradient from proximal to distal colon. Proteolysis was localized to infiltrating leukocytes in diseased severe combined immunodeficient mice. Transmural inflammation was associated with serine proteinase and MMP activity in overlying epithelium and with marked subepithelial proteolytic activity. The results demonstrate a clear elevation in the levels and activation of proteases in colitis, potentially contributing to disease progression through loss of epithelial barrier function.
The inflammatory bowel diseases (IBD), Crohn’s disease, and ulcerative colitis, probably share a common etiology, in terms of a dysregulation of mucosal T cell reactivity. 1 As disease progresses, the mucosal T-cell cytokine profile may be driven toward a Th1-type, characteristic of the transmural granulomatous inflammation seen in Crohn’s disease, or toward a Th2-type, characteristic of the mucosa-restricted lesions occurring in ulcerative colitis. 2 Their shared pathologies include acute and chronic inflammation, and connective tissue defects. The diseases are multiphasic with the initial inflammation leading to tissue damage and leukocyte infiltration characteristic of a wound-healing response, often with fibrotic hypertrophy, and typically following a recurrent disease cycle.
Pathogenesis in these diseases reflects complex interactions between regulatory and effector mechanisms of various infiltrating and stromal cell populations. These include pro-inflammatory and anti-inflammatory cytokines, some of which, for example, transforming growth factor-β (TGF-β), may perform either function depending on the stage of the pathology. 3 Also important are matrix proteinases, which facilitate lymphoid and myeloid cell infiltration, wound healing, and tissue remodeling 4,5 but which may cause tissue destruction if unregulated. The particular combination of these elements operating in response to the initial stimulus will determine subsequent pathology.
Recent analyses of established IBD lesions suggest extracellular matrix proteolysis as a putative marker of disease progression in IBD. In particular, matrix metalloproteinases (MMPs) -9 and -3 have been associated with mucosal damage and fistulae in Crohn’s disease patients, 6,7 MMPs 1, 3, and 13 with intestinal ulcer stroma, and MMPs 10 and 11 with the epithelium. 8,9 Furthermore, CD4+ T cell activation in fetal intestinal explants equated with increased levels of MMP-1 and MMP-3, and exogenous MMP-3 promoted tissue degradation 10 implying a causal role. Evidence of serine proteinases in IBD has been limited, with reports of undefined species associated with lesions from Crohn’s disease and ulcerative colitis patients, 7,11 and in hapten-induced ulcerative lesions in rats. 11
To evaluate therapies which mitigate unregulated tissue proteolysis, it is necessary to determine the role of specific proteinases at all stages of pathogenesis. This necessitates the use of model systems in which, like human disease, mucosal damage is a consequence of pathology rather than its cause.
When severe combined immunodeficient (SCID) mice are transplanted with syngeneic or congenic CD4+ T cells the transferred cells accumulate in mucosal tissues. 12 In the large intestine, these T cells proliferate, and induce colitis with many of the features of human IBD. 12-15 We have shown that mucosal macrophage activation occurs early in the pathogenesis of SCID colitis, along with tumor necrosis factor-α (TNF-α) induction in both macrophages and T cells, 16 similar to human Crohn’s disease. 17,18 Subsequent up-regulation of TGF-β transcription occurs in established disease (Whiting, Williams, Claesson, Reimann, and Bland, submitted).
It is known that TGF-β promotes activation of plasminogen and other serine proteinases, 19 down-regulates many MMPs (although not gelatinases), and promotes transcription of tissue inhibitors of MMPs, whereas TNF-α up-regulates most MMPs. 20,21 The potential for evaluating such changes in protease expression as a means of monitoring normal tissue repair processes 22,23 and evaluating healing in chronic ulcers 24 has been demonstrated.
In this study, we have used the SCID mouse colitis model to define serine and metalloproteinase expression in established colitis, to localize proteinase activity within the tissue, and to co-localize with infiltrating and stromal cell types.
Materials and Methods
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich, Poole, UK.
Mice and Cell Transfer
C.B-17+/+ mice and congenic C.B-17scid/scid (SCID) mice were bred and housed under identical specific pathogen-free conditions. Experimental mice were injected intraperitoneally with 5 × 10 5 nonfractionated CD4+ splenic T cells and monitored for signs of disease, as previously described. 25 Colonic tissue samples were taken at autopsy from eight CD4+ T cell-transplanted, diseased SCID, and two nontransplanted age- and sex-matched control SCID mice, 3 months after T cell transfer, and four C.B-17+/+ mice. Specimens from all mice were processed for gel and in situ zymographic analysis. Tissues were scored blind (0, no pathology to 3, severe pathology) for six disease parameters: tissue hypertrophy, CD3+ T cell infiltration, crypt hyperplasia, crypt distortion and branching, crypt abscesses, and ulceration.
Preparation of Tissue Samples
Colons were divided into three equal segments, distal, mid, and proximal. One centimeter from each was embedded in OCT (R. A. Lamb, London, UK) and snap-frozen in isopentane cooled over liquid nitrogen. Fecal material was removed from the remaining segments which were then flushed with 1 ml of phosphate-buffered saline, snap-frozen in liquid nitrogen, and stored at −80°C.
Specimens for biochemical analyses were pulverized to a powder using a freezer mill in liquid nitrogen, lyophilized, and the powder weighed. Brij 35 (0.1%) in triethanolamine (20 mmol/L) was added to the powder at 20 μl/mg and extraction performed for 16 hours at 4°C. Insoluble material was removed by centrifugation, and extracts mixed with nonreducing sodium dodecyl sulfate sample buffer. Uniform volumes were loaded for electrophoretic analyses, results therefore being normalized to original specimen dry weight.
Gelatin Substrate Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (Zymography)
Gelatin zymography was used to quantify MMP-2 and -9, as previously described. 22 In brief, gelatin was co-polymerized in 10% polyacrylamide gels, and run in a Bio-Rad Mini Protean II apparatus (Bio-Rad, Hemel Hempstead, Herts, UK), with samples, prestained molecular weight markers (sodium dodecyl sulfate-7B), and MMP-2 standard (Biogenesis, Bournmouth, Dorset, UK). MMP proteolysis buffer was prepared from 50 mmol/L Tris/HCl, pH7.8, 50 mmol/L CaCl2, 0.5 mol/L NaCl, and 1 mmol/L amino phenyl mercuric acetate, with addition of a cocktail of serine proteinase inhibitors, comprising phenylmethyl sulfonyl fluoride (PMSF), leupeptin, and soybean trypsin inhibitor, at the concentrations detailed below. Gels were washed in 2.5% (v/v) Triton X-100 (BDH, Poole, Dorset, UK), incubated in the proteolysis buffer at 37°C for 18 hours, and stained with 0.2% Coomassie blue R250.
Casein Substrate Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (Zymography)
Casein zymography was used in the identification and quantitation of caseinolytic serine proteinases as previously described. 24 In brief, 12.5% polyacrylamide gels were cast and run with Hammersten casein (BDH) as the substrate, and human neutrophil elastase, prepared from rheumatoid lung lavage, 26 as enzyme standard. Rat neutrophil elastase, prepared from tissue from an excisional wound model, 23 was also used for comparison. Proteolytic clarification of the electrophoresed gels was performed as appropriate for serine proteinases in 100 mmol/L phosphate buffer (pH 6.8) supplemented with 8 mmol/L ethylenediaminetetraacetic acid (EDTA) and 0.2% (v/v) Triton X-100.
Inhibition Studies
The proteinase class responsible for the clarified zones in each analysis was identified by incorporating, as appropriate: MMP inhibitors, EDTA (50 mmol/L, and omitting CaCl2), or peptidyl hydroxamate (1 μmol/L; Calbiochem, Nottingham, Notts, UK); serine proteinase inhibitors PMSF (1 mmol/L), or 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (Pefabloc, 4 mmol/L; Boehringer Mannheim, Lewes, E. Sussex, UK); trypsin-like enzyme inhibitors, soybean trypsin inhibitor (20 μg/ml), or leupeptin (50 μmol/L); elastase inhibitor, elastatinal (50 μmol/L); or cysteine proteinase inhibitor trans-epoxysuccinyl-l-leucylamido(4-guanidino)-butane (E64, 25 μmol/L), in the proteolysis buffers. The identity of MMP-2 and -9 was further corroborated by demonstrating gelatin affinity, as previously described. 22
Densitometric Analysis
Quantitative densitometry was performed as previously described. 27,28
In Situ Zymography
Localization of active enzymes was performed by in situ zymography based on the method of Galis et al. 29 Fluorescein isothiocyanate-casein (1 mg/ml) was dissolved in incubation buffer (50 mmol/L Tris-HCl, pH 7.5, 10 mmol/L CaCl2, 0.05% Brij 35), filtered, and spread on glass slides. After air-drying, substrate uniformity was confirmed by fluorescence microscopy. Frozen sections (8 μm) were transferred to substrate-coated slides (experimental and control specimens on the same slide), air-dried, and covered with incubation buffer and coverslip, secured with nail varnish. Slides were incubated at 37°C and 100% humidity, to allow substrate:label proteolysis, and monitored every 2 to 3 days. Control slides were incubated either at 4°C, or at 37°C in the presence of appropriate proteinase inhibitors. Released fluorescein was washed from beneath the coverslip with incubation buffer. Areas of active enzyme were identified by clearance of labeled substrate.
To characterize the nature of the active proteinases involved in substrate degradation, EDTA (20 mmol/L), peptidyl hydroxymate MMP inhibitor (10 μmol/L), aprotinin (50 μg/ml), soybean trypsin inhibitor (100 μg/ml), PMSF (1 mmol/L), pepstatin (1 μmol/L), or E64 (25 μmol/L), were added at the start of incubation and replenished every 2 to 3 days.
Immunohistochemistry, Antibodies, and Reagents
Immunohistochemistry was performed as previously described, 16 using rat monoclonal antibodies raised against CD3 (clone KT3; Serotec, Oxford, UK), mouse macrophage (clone A3–1, F4/80 antigen; Serotec), mouse activated macrophage (clone 158.2, ATCC, HB8466), and mouse CD11b (M1/70; Serotec). Secondary antibody was biotin-conjugated goat anti-rat IgG (Harlan Sera-Lab, Loughborough, Leics, UK). StreptABComplex/HRP peroxidase complex (DAKO A/S, Glostrup, Denmark), and diaminobenzidine-4HCl, was used to visualize the staining.
Statistical Analysis
The Mann-Whitney U test of the quantitative zymography data were performed using the Genstat statistics package. A P value of 0.05 or less was regarded as significant. In all plots, means and standard errors are shown.
Results
Disease Severity
Tissues from eight diseased SCID mice, two nontransplanted SCID mice, and four C.B-17+/+ mice were stained for CD3 to determine levels of T cell infiltration, or for CD11b to assess polymorph infiltration and the development of crypt abscesses. At least two sections from proximal, mid, and distal colon tissues from each mouse were scored in a blinded manner for pathological changes. The results are presented in Table 1 ▶ . Pathology ranged from mild (mouse 3) to severe (mouse 8), and was in accordance with previous findings. 30
Table 1.
Pathology Scores for Colitis Induced in SCID Mice by Transfer of CD4+ T Cells
| Mouse no. | Scores | ||
|---|---|---|---|
| Proximal | Mid | Distal | |
| 1 | 2 (0–1) {0} | 2 (0–1) {0} | 2 (0–1) {1} |
| 2 | 6 (0–2) {0} | 4 (0–2) {0} | 2 (0–1) {0} |
| 3 | 1 (0–1) {0} | 1 (0–1) {0} | 1 (0–1) {0} |
| 4 | 3 (0–1) {2} | 3 (0–1) {2} | 2 (0–1) {2} |
| 5 | 3 (0–1) {1} | 2 (0–2) {1} | 3 (0–2) {1} |
| 6 | 5 (0–2) {1} | 4 (0–2) {0} | 2 (0–1) {0} |
| 7 | 2 (0–1) {0} | 5 (0–2) {0} | 6 (0–2) {0} |
| 8 | 3 (0–1) {0} | 7 (0–2) {0} | 10 (0–3) {3} |
SCID mice reconstituted with CD4+ T cells were scored for colitis pathology in proximal, mid, and distal colon. At least two sections were scored blind for each tissue on a 0–3 scale where 0 = no pathology to 3 = severe pathology for six criteria: tissue hypertrophy, mononuclear cell infiltration, crypt hyperplasia, crypt distortion and branching, crypt abscesses, and ulceration. Scores represent the sum of the scores for all criteria for each tissue with the range in parentheses. Scores for neutrophil involvement are given separately in brackets.
Serine Proteinases Were Up-Regulated in Colitis
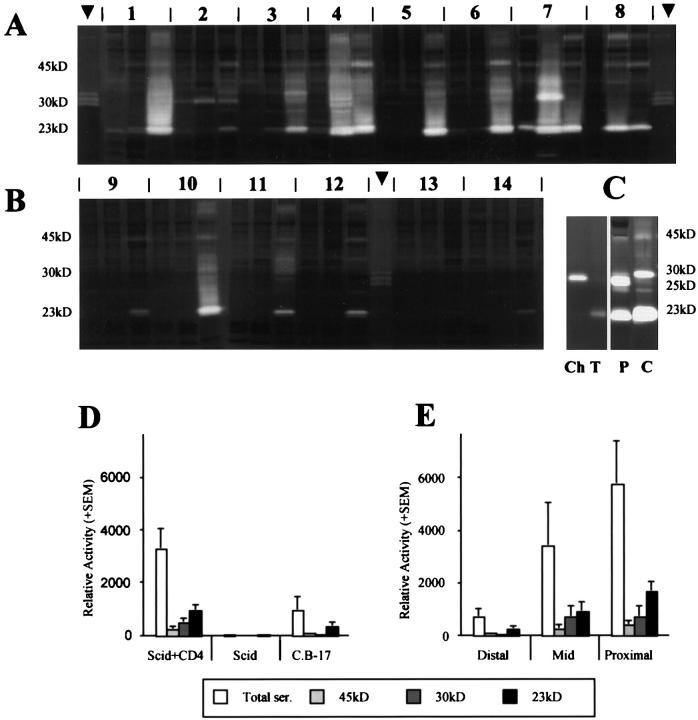
There was a clear up-regulation in serine proteinase activities in the colitic mice specimens (Figure 1, A and D) ▶ , the majority of which displayed substantial levels of serine caseinolytic activity, whereas only one C.B-17+/+ sample, and no nontransplanted SCID samples, showed comparable activity (Figure 1, B and D) ▶ .
Figure 1.
Up-regulation of tissue serine proteinases in SCID mice with colitis. Zymography was performed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels containing casein as substrate and incubated overnight at 37oC in the presence of EDTA. A: Colonic extracts from eight SCID mice transplanted with CD4+ T cells. B: Colonic extracts from four C.B-17 mice 9–12 and two nontransplanted SCID mice. 13,14 Arrowheads depict rat neutrophil elastase marker. Three samples per mouse: distal colon, left; mid-colon, middle; proximal colon, right. Relative mobility of the major protease species is given. C: Comparison of proteases in an extract from a colitic colon from a transplanted mouse (C) with those in a mouse pancreatic extract (P), with trypsin (T) and chymotrypsin (Ch) markers. D: Relative activity of total serine proteases and of the three major protease species (23 kd, 30 kd, and 45 kd) in the three groups of mice, mean densitometry values of all samples in each group + SE. E: Relative activity of total and individual serine proteases in distal, mid, and proximal regions of the colon in CD4+ T-cell-transplanted SCID mice only, mean of densitometry values from all eight mice + SE.
Quantitative analysis (Figure 1D) ▶ demonstrated significantly higher levels of total serine proteinase activity in the transplanted SCID samples compared with nontransplanted SCID (P < 0.01) and C.B-17+/+ (P = 0.01) control mice. The major species were 45-kd, 23-kd, and 30-kd proteinases. Levels of each of these proteinases were elevated in the transplanted SCID mice, compared with negligible levels in the SCID controls (45 kd, P < 0.01; 30 kd, P = 0.03; 23 kd P < 0.01), and intermediate levels in C.B-17+/+ mice (45 kd, P = 0.04; 30 kd, P = 0.03; 23 kd P < 0.01).
In those mice with high levels of serine proteinases, expression was generally greatest in the proximal colon samples (Figure 1E) ▶ . Total serine proteinase activity in the IBD mice increased eightfold between the distal and proximal regions (P < 0.01), fourfold for the 45-kd proteinase (P < 0.01), 10-fold for the 30-kd species (P < 0.01), and sixfold for the 23-kd proteinase (P < 0.01).
A 23-kd serine proteinase was found in the colon lumen contents of all mice, elevated in IBD compared with C.B-17+/+ and SCID controls, and was evident throughout the small intestinal lumen (data not shown). However, there was no evidence of either the 45-kd or 30-kd proteinase in the lumen.
Inhibition with leupeptin, soybean trypsin inhibitor, and PMSF demonstrated that the three major species were trypsin-like serine proteinases. Co-migration of the 30-kd species with rat neutrophil elastase, and its inhibition by elastatinal (data not shown), indicate this proteinase to be neutrophil elastase.
Analysis of pancreatic extracts demonstrated strong bands at 23 and 25 kd corresponding to trypsin and chymotrypsin, respectively, according to comparisons with standards, and at 45 kd (Figure 1C) ▶ . The trypsin co-migrated with the 23-kd protease found in colitic tissue and gut lumen, and the 45-kd band with that found in colitic tissue, implying identity in each case. However, there was no clear evidence of species corresponding with chymotrypsin in colitic tissue, or neutrophil elastase in the pancreatic samples.
Immunoblotting studies using antiserum to plasmin/plasminogen indicated that the 45-kd proteinase was not plasmin (data not shown).
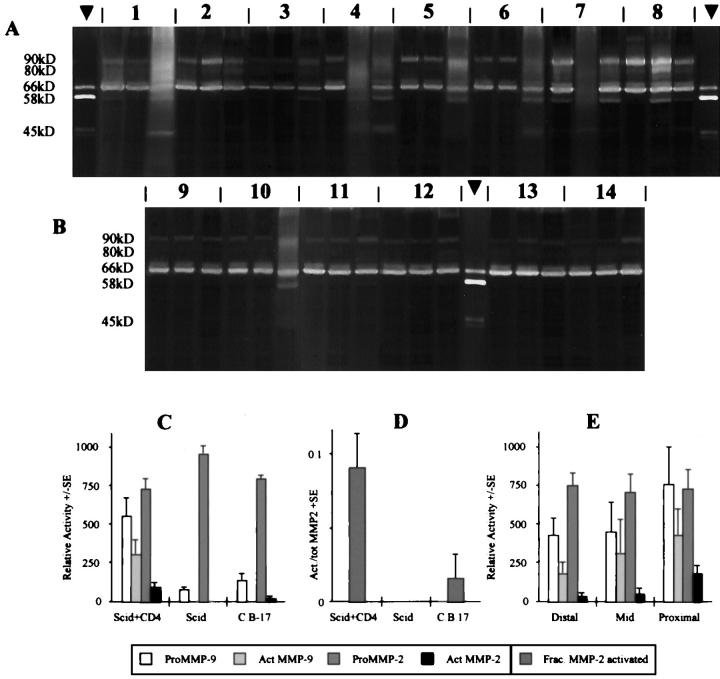
MMPs Were Up-Regulated and Activated in Colitis
Quantitative zymography demonstrated a marginal decrease in the level of proMMP-2 in IBD samples, as compared with the SCID (P = 0.06) and C.B-17+/+ (P = 0.24) control samples (Figure 2, A–C) ▶ . However, this may be because of conversion to the activated form, which occurred (P < 0.03) in the IBD samples (Figure 2D) ▶ .
Figure 2.
Up-regulation of matrix MMPs in SCID mice with colitis. Zymography was performed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels containing gelatin as substrate and incubated overnight at 37oC in the presence of PMSF, leupeptin, and soybean trypsin inhibitor. A: Colonic extracts from eight SCID mice transplanted with CD4+ T cells. B: Colonic extracts from four C.B-17 mice 9–12 and two nontransplanted SCID mice. 13,14 Arrowheads depict MMP-2 standard. Three samples per mouse: distal colon, left; mid colon, middle; proximal colon, right. Relative mobility of the major protease species is given. C: Relative activity of total MMPs and of pro- and active forms of MMP-2 and MMP-9 in the three groups of mice, mean densitometry values of all samples in each group + SE. D: Proportion of MMP-2 in its activated form in colon tissues from mice transplanted with CD4+ T cells (n = 8), nontransplanted SCID mice (n = 2) and C.B-17 mice (n = 4), + SE, as determined by densitometry from gelatin sodium dodecyl sulfate-polyacrylamide gel electrophoresis zymography. E: Relative activity of total MMPs and of pro- and active forms of MMP-2 and MMP-9 in distal, mid, and proximal regions of the colon in CD4+ T-cell-transplanted SCID mice only, mean of densitometry values from all eight mice + SE.
ProMMP-9 was up-regulated by a factor of 5 in the IBD samples (Figure 2, A–C) ▶ , with respect to both control groups (P < 0.01), and the activated forms of MMP-9, absent in all of the control specimens, were also evident in colitis (P < 0.01). In two of the transplanted SCID samples (no. 2 proximal and no. 8 distal) a 130-kd neutrophil-associated complex between MMP-9 and lipocalin 22,31 was identified.
Regional differences in colon MMP expression (Figure 2E) ▶ were not as marked as with the serine proteinases. There was a trend for pro- (P = 0.01) and active (P = 0.14) MMP-9 and active MMP-2 (P = 0.01) to decrease proximal to distal in the colitic colons. There was no apparent regional variation in the levels of proMMP-2 (P = 0.6).
Some colitic samples were generally more proteolytically active than others, and hence there was a correspondence between levels of different protease species in these samples. Correlations ranged from r = 0.285 (not significant) for activated MMP-9 and the 30-kd serine protease, to r = 0.871 (P < 0.001) for pancreatic trypsin and the 45-kd serine protease. A very good correspondence was seen between MMP-2 activation and total serine protease levels (r = 0.824, P < 0.001), and more particularly with pancreatic trypsin (r = 0.817, P < 0.001). This suggests a relationship between serine protease penetration into gut tissue and resident MMP activation.
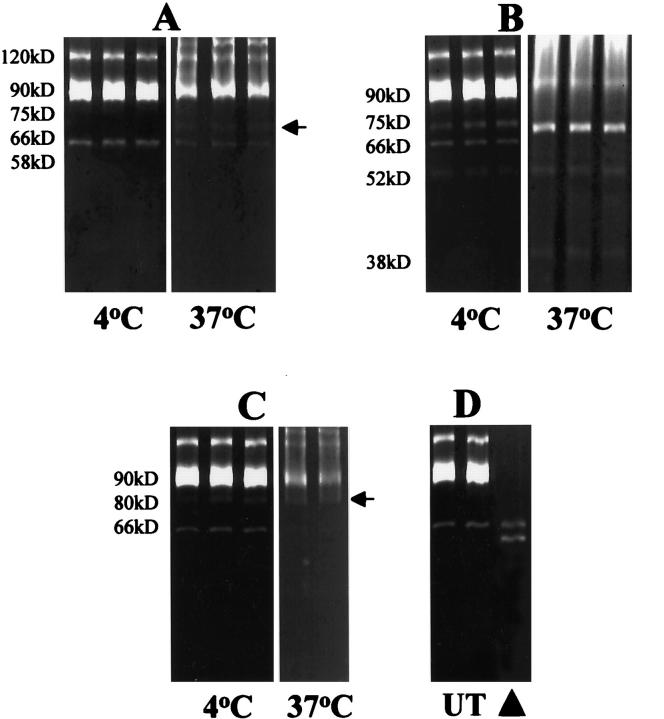
MMPs Are Activated by Fecal Proteases, but not during Extraction
Experiments were performed to investigate the potential for gut lumen-derived proteases to activate tissue MMPs. Firstly, under conditions prevailing during extraction, the potential influence of fecal contamination on the apparent tissue levels of activated MMPs was determined. Secondly, the potential for luminal proteases to activate MMPs within the tissue was evaluated, by incubating under conditions appropriate for proteolysis.
Mouse gut luminal contents were titrated, and monitored by casein zymography, to equalize protease levels present during incubations to those identified in colitic tissues. Human acute wound fluid, containing proMMP-9 and proMMP-2, but no activated gelatinases 22 was incubated for 16 hours at 4°C, in accordance with the extraction procedure, or at 37°C in proteolysis buffer. Control incubations were performed using fecal extract alone, wound fluid alone, or wound fluid with lumen contents and soybean trypsin inhibitor and Pefabloc to inactivate serine proteases. Trypsin and chymotrypsin were each titrated to estimate levels found in colitic tissue, as for the fecal extracts, and appropriate levels incubated with wound fluids, as above. All experiments were performed in triplicate.
Under extraction conditions, no activation of wound fluid MMP-9 or MMP-2 was seen with the fecal extracts (Figure 3A) ▶ . However, at 37°C, under conditions appropriate for proteolysis, degradative processing of proMMP-9 and proMMP-2 was apparent, in particular, generating a Mr 75-kd-activated form of MMP-9 seen in colitic tissue (Figure 2A) ▶ . Treatment of wound fluids with trypsin, even under extraction conditions, resulted in processing of MMP-2 and -9 (Figure 3B) ▶ . At 37°C all proMMP-9 was converted to the Mr 75-kd-activated form, and all proMMP-2 was lost, with the appearance of species at Mr 52 and 38 kd. Incubation of the wound fluids with chymotrypsin generated a Mr 80-kd-activated form of MMP-9 which was not seen in the fecal extract incubation, but was seen in colitic tissue, and resulted in processing of proMMP-2 (Figure 3C) ▶ . Wound fluid alone did not generate any activated species either in extraction buffer at 4°C (Figure 3D) ▶ , or under conditions appropriate for proteolysis. No MMPs were identified in the fecal extracts (data not shown).
Figure 3.
Incubation of proMMP-2 and proMMP-9, derived from acute wound fluids, with fecal extracts and defined serine proteinases. A: Activated MMP forms were not generated by the action of fecal proteases during extraction conditions (4°C), but activation did occur under near-physiological conditions (37°C), with particular formation of a 75-kd-activated form of MMP-9 (arrow). B: Pancreatic trypsin was able to mediate processing of proMMP-2 and proMMP-9, even at 4°C, with the formation of a characteristic 75-kd active form of MMP-9. C: Pancreatic chymotrypsin was also able to process MMP-2 and MMP-9, with the formation of a characteristic 80-kd active form of MMP-9. D: Wound fluids incubated alone (UT) and MMP-2 standard (arrowhead).
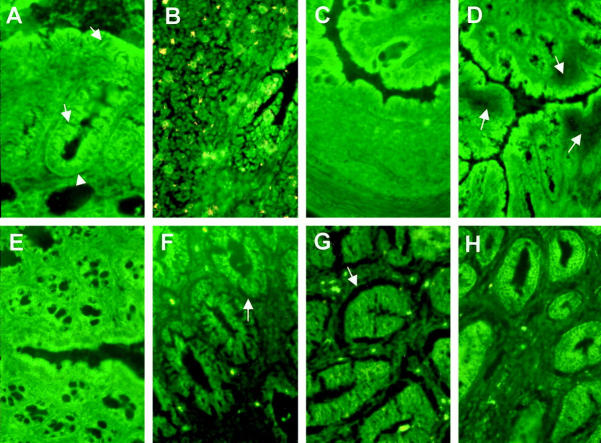
Mucosal and Epithelial Matrix Degradation Was Associated with Infiltrating Leukocytes
In control tissue, low levels of proteinase activity were localized by in situ zymography to normal colonic epithelium of C.B-17+/+, nontransplanted SCID and apparently unaffected colon of transplanted SCID mice. This proteolysis was associated with the luminal epithelium, rather than that of the lower crypt (Figure 4A) ▶ . Proteolysis associated with lymphoid tissue of control colons was observed in mucosal lymphoid follicles of C.B-17+/+ mice (Figure 4B) ▶ , but no proteolysis was localized to the occasional, presumably pre-B cell, lymphoid aggregates of SCID mice (Figure 4C) ▶ . In diseased mice, degradation was particularly marked in areas of epithelium overlying heavily infiltrated regions of mucosa and within the infiltrated lamina propria (Figure 4D) ▶ . In the deeper layers of inflamed mucosa there was evidence for extensive proteolytic activity within the epithelium and as a subepithelial sheath within the lamina propria (Figure 4F) ▶ . Inhibitor studies showed that this was due more to the action of MMPs than of serine proteases (Figure 4, G and H) ▶ , and that neither cysteine nor aspartic proteinases seemed to contribute (not shown). Consecutive sections of the heavily infiltrated regions, treated for in situ zymography and immunohistochemistry, showed that they comprised CD3+ T cells, CD11b+ myeloid cells, and activated macrophages (Figure 5, C–F) ▶ . Immediately subjacent to the epithelial cell basement membrane CD11b+/F4/80− cells, probably neutrophils, were the most abundant cell type (Figure 5D) ▶ .
Figure 4.
In situ zymography on casein substrate showing location of protease activities in colonic tissues. A: Nontransplanted SCID mouse colon showing minimal substrate degradation associated with luminal and upper crypt epithelium (arrows), but none with basal crypt epithelium (arrowhead). B: C.B-17 mouse colon, mucosal lymphoid aggregate, showing substrate degradation under epithelium and within the lymphoid tissue. C: Nontransplanted SCID mouse colon, lymphoid aggregate, showing no substrate degradation. D: CD4+ T-cell-transplanted SCID mouse colon, showing extensive areas of substrate degradation under inflammatory infiltrate. E: Nontransplanted SCID mouse colon showing relative lack of substrate degradation, compared with CD4+ T-cell-transplanted SCID mouse colon (F–H). F: No inhibitors, showing substrate degradation particularly under epithelium and under pericryptal lamina propria. G: With soybean trypsin inhibitor to block serine proteases, showing maintenance of substrate degradation. H: With peptidyl hydroxamate to block MMPs, showing reduced proteolysis. Original magnification, ×128 (A–C) and (E–H); ×64 (D). Yellow is autofluorescence.
Figure 5.

Epithelial and stromal proteolytic activity associated with infiltrating cells. Serial sections of a region of inflammatory infiltrate from the colon of a CD4+ T-cell-transplanted SCID mouse (proximal segment, mouse 4). A and B: In situ zymograms, casein substrate, of area of infiltrate shown boxed in C. A: No inhibitors, showing substrate degradation associated with the epithelium (arrow) and the infiltrate (arrowheads). B: With cocktail of inhibitors blocking both serine proteases and MMPs, relative lack of substrate degradation. C–F: Indirect immunohistology showing distribution of CD3+ T cells (C), CD11b myeloid cells (D), F4/80+ myeloid cells (E), activated macrophages, mAb 158.2 (F). Original magnifications, ×128 (A and B); ×64 (C–F).
All mice were evaluated by in situ zymography, and the results shown are representative.
Discussion
In this model of T-cell-mediated colitis, advanced pathology is associated with significantly increased serine protease and matrix metalloproteinase activity within the tissue. This is the first study to indicate a relationship between extracellular matrix degrading proteases in colitic tissue and proteases derived from the gut lumen. Proteolysis resulting from the activities of both metalloproteinases and serine proteinases, was associated with the inflammatory infiltrate and, in areas of intense inflammation, with the epithelium and the subepithelial stroma.
There have been few previous reports of serine proteinases in intestinal inflammation. In human colitis and Dinitrobenzenesulfonic acid-induced colitis in the rat, Hawkins et al 11 showed increases in total serine proteinase activity in diseased colonic tissue similar to those demonstrated here, and identified the same predominant 45- and 23-kd species. Similarly, Baugh et al 7 demonstrated 45-kd and 23-kd gelatinolytic proteases inhibitable by aprotinin, a serine protease inhibitor. Interestingly, the 45-kd species has also been detected in normal human colon, but only after trypsin activation. Our studies in the mouse model show a clear correlation between total serine protease, and trypsin in particular, and MMP activation. Experiments demonstrated that MMP activation was not because of exposure to contaminating luminal serine proteases during the extraction procedure, but did occur when MMPs were exposed to luminal proteases under near physiological conditions. This implies that, at least in advanced colitis, tissue proteases are accessible for activation by luminal proteases through a defective epithelial barrier, even in the absence of frank ulceration, and that activation of tissue proteases by luminal proteases may play a role in pathogenesis. Detection of neutrophil elastase in the mouse model conforms with established characteristics of colitis, and fecal elastase has been proposed as a marker in ulcerative colitis, 32 and a target for therapy. 33 Neutrophil involvement in the model ranged from local foci in the colonic mucosa of mild inflammation, to significant and widespread accumulations, notably in crypt abscesses and the lumen. In a few colitic samples, a 130-kd MMP-9/lipocalin complex, associated with neutrophil infiltrations 31 was evident. This implies that the elastase detected in zymograms in this study reflects tissue neutrophilia, a view corroborated by serial immunohistochemistry and in situ zymography.
In colitic tissue specimens, elevated serine proteinase expression was associated with processing of MMPs, with the appearance of activated forms of MMP-9 (80 and 75 kd) and MMP-2 (58 and 45 kd), and ultimately their complete degradation. Although the overall levels of MMP-2 did not alter as a correlate of disease, the proportion identified in the active form increased considerably and significantly. MMP activation was clearly correlated with serine proteinase activity, comparable to the widespread MMP activation seen in chronic dermal ulcers, also associated with extracellular serine proteinase. 24 Considering the central role plasmin has in the physiological cascade of events leading to MMP activation, 34 this was deemed a candidate for the identity of the uncharacterized serine protease in our studies. However, we were unable to confirm the presence of plasmin in the inflamed tissue. Increased MMP-9 and unchanged MMP-2 expression corroborated the findings of a previous immunohistochemical study, 6 which was unable to distinguish pro- and activated forms. The recent work of Baugh et al 7 clearly shows that MMP-9 is a major factor in human intestinal inflammation, and was assumed to be a product of neutrophilia. Our results also show spatial correlations between MMP-9 activation, neutrophil accumulation, and tissue damage, although low levels of the lipocalin complex implies that other cell types may be expressing this protease.
MMP-1 (interstitial collagenase) and MMP-3 (stromelysin-1), probably derived from fibroblasts and macrophages, respectively, have been implicated in tissue destruction in Crohn’s disease, 9 although these in situ hybridization studies provide no information on enzyme expression or activation. It has also been shown that MMP-3, probably stimulated by T-cell-derived TNF-α, contributes to tissue degradation 10,35 in a system involving mucosal T cell activation in human fetal explants. This early T-cell-mediated MMP-3 induction has primarily been superseded in our model by inflammation and repair. Also, this explant model does not take account of the extensive myeloid cell infiltration seen in the mouse and, frequently, in human disease, with associated increase in neutrophil elastase and MMP-9, identified in this study and in human disease. 7 It may also be that the initial TNF-α induction of MMP-3 has been superseded by TGF-β induction of MMP-9 in advanced pathology. 21,36 This study clearly shows gelatinolytic activity in pericryptal stroma of diseased tissue in the region of the activated myofibroblast sheath, which displays increased TGF-β receptor expression in the mouse model (work in progress). Proteolytic activity facilitating inflammatory infiltration may lead to more extensive matrix degradation as a result of the inappropriate activation events indicated in this study. Furthermore, proteolysis localized to the epithelium overlying areas of intense infiltration was of particular interest, as it may promote damage to the epithelial barrier. The activity observed resulted from both serine proteinases and MMPs, and was associated with activated macrophages and neutrophils. Macrophages transcribing metalloelastase (MMP-12) have been identified close to shedding mucosal epithelium in ulcerative colitis and Crohn’s disease, 8 and matrilysin (MMP-7) 37 has been noted in IBD epithelium, especially adjacent to crypt abscesses, and associated with basement membrane degradation. 9
The clear and significant bias toward higher regional proteinase activity in proximal colon is somewhat at odds with clinical presentation in human disease. This may result from variable distribution of initiating T cells, inflammatory cell infiltration, luminal antigens, protease inhibitors, or the nature of the epithelial barrier, and needs further investigation. In severe disease regional distinctions were mitigated, with high levels detected throughout the colon (data not shown).
The acute inflammatory state normally represents a transient, co-ordinated proteolytic episode which gives way to phases of remodeling and tissue regeneration. Persistence of inflammation may be due to a loss in co-ordination of degradative and reparative processes, maintenance of pro-inflammatory elements, such as infection or activation of cytokines, or the introduction of elements that promote tissue destruction. Colonic tissue, even under normal circumstances, is a site of active proteolysis associated with digestive processes and continual turnover of the epithelium. It is postulated here that, at some stage during the initial inflammatory episode, other factors shift the balance of turnover in favor of proteolysis resulting in tissue damage, thus preventing regeneration and the resolution of inflammation. These factors may include tissue proteolysis resulting from inappropriate penetration of luminal enzymes, widespread activation of MMPs associated with inflammation, or the excessive potentiation of cytokines. Furthermore, these factors are likely to interact in a cascade of events resulting in positive feedback and progressive deterioration. Thus, what begins as an inflammatory episode becomes a disorder of combined connective tissue and inflammatory pathologies. Importantly, this model will permit dissection of the sequence of events from initial T cell infiltration and proliferation, through infiltration by other cell types, to subsequent tissue damage and fibrosis.
Footnotes
Address reprint requests to Dr. John F. Tarlton, Division of Molecular & Cellular Biology, Department of Clinical Veterinary Science, University of Bristol, Langford House, Langford, Bristol BS40 5DU, United Kingdom. E-mail: john.tarlton@bris.ac.uk.
Supported by the European Union Research Contract Numbers BMH4-96-0612, QLGI-1999-00050.
References
- 1.Ludviksson BR, Ehrhardt RO, Fuss IJ, Strober W: Mucosal and thymic dysregulation. Role in human intestinal inflammation. Immunologist 2000, 5/6:202-209 [Google Scholar]
- 2.Fuss IJ, Neurath M, Boirivant M, Klein JS, delaMotte C, Strong SA, Fiocchi C, Strober W: Disparate CD4(+) lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease—Crohn’s disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 1996, 157:1261-1270 [PubMed] [Google Scholar]
- 3.Wahl SM: Transforming growth-factor-β (TGF-β) in inflammation—a cause and a cure. J Clin Immunol 1992, 12:61-74 [DOI] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H: Proteolytic remodeling of extracellular-matrix. Curr Opin Cell Biol 1995, 7:728-735 [DOI] [PubMed] [Google Scholar]
- 5.Seifert WF, Wobbes T, Hendriks T: Divergent patterns of matrix metalloproteinase activity during wound healing in ileum and colon of rats. Gut 1996, 39:114-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA: Distribution of the matrix metalloproteinases stromelysin, gelatinase-A and gelatinase-B, and collagenase in Crohn’s-disease and normal intestine. J Clin Pathol 1994, 47:113-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS: Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 1999, 117:814-822 [DOI] [PubMed] [Google Scholar]
- 8.Vaalamo M, KarjalainenLindsberg ML, Puolakkainen P, Kere J, SaarialhoKere U: Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol 1998, 152:1005-1014 [PMC free article] [PubMed] [Google Scholar]
- 9.SaarialhoKere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, KarjalainenLindsberg ML: Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol 1996, 148:519-526 [PMC free article] [PubMed] [Google Scholar]
- 10.Pender SLF, Tickle SP, Docherty AJP, Howie D, Wathen NC, MacDonald TT: A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol 1997, 158:1582-1590 [PubMed] [Google Scholar]
- 11.Hawkins JV, Emmel EL, Feuer JJ, Nedelman MA, Harvey CJ, Klein HJ, Rozmiarek H, Kennedy AR, Lichtenstein GR, Billings PC: Protease activity in a hapten-induced model of ulcerative colitis in rats. Dig Dis Sci 1997, 42:1969-1980 [DOI] [PubMed] [Google Scholar]
- 12.Rudolphi A, Boll G, Poulsen SS, Claesson MH, Reimann J: Gut-homing CD4(+) T-cell receptor αβ (+) T-cells in the pathogenesis of murine inflammatory bowel-disease. Eur J Immunol 1994, 24:2803-2812 [DOI] [PubMed] [Google Scholar]
- 13.Rudolphi A, Bonhagen K, Reimann J: Polyclonal expansion of adoptively transferred CD4(+)αβ T cells in the colonic lamina propria of scid mice with colitis. Eur J Immunol 1996, 26:1156-1163 [DOI] [PubMed] [Google Scholar]
- 14.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD: CD4+ T-cells that express high-levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice—disease development is prevented by cotransfer of purified CD4+ T-cells. J Exp Med 1993, 178:237-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL: Phenotypically distinct subsets of CD4(+) T-cells induce or protect from chronic intestinal inflammation in C.B-17 scid mice. Int Immunol 1993, 5:1461-1471 [DOI] [PubMed] [Google Scholar]
- 16.Williams AM, Whiting CV, Bonhagen K, Reimann J, Bregenholt S, Claesson MH, Bland PW: Tumour necrosis factor-α (TNF-α) transcription and translation in the CD4(+) T cell-transplanted scid mouse model of colitis. Clin Exp Immunol 1999, 116:415-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald TT, Hutchings P, Choy M-Y, Murch S, Cooke A: Tumour necrosis factor-α and interferon-γ production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 1990, 81:301-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murch SH, Lamkin VA, Savage MO, Walkersmith JA, MacDonald TT: Serum concentrations of tumor-necrosis-factor-α in childhood chronic inflammatory bowel-disease. Gut 1991, 32:913-917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB: Characterization of the activation of latent TGF-β by cocultures of endothelial-cells and pericytes or smooth-muscle cells—a self-regulating system. J Cell Biol 1990, 111:757-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauviel A: Cytokine regulation of metalloproteinase gene-expression. J Cell Biochem 1993, 53:288-295 [DOI] [PubMed] [Google Scholar]
- 21.Ries C, Petrides PE: Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe Seyler 1995, 376:345-355 [PubMed] [Google Scholar]
- 22.Tarlton JF, Vickery CJ, Leaper DJ, Bailey AJ: Postsurgical wound progression monitored by temporal changes in the expression of matrix metalloproteinase-9. Br J Dermatol 1997, 137:506-516 [DOI] [PubMed] [Google Scholar]
- 23.Paul RG, Tarlton JF, Purslow PP, Sims TJ, Watkins P, Marshall F, Ferguson MJ, Bailey AJ: Biomechanical and biochemical study of a standardized wound healing model. Int J Biochem Cell Biol 1997, 29:211-220 [DOI] [PubMed] [Google Scholar]
- 24.Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD: Prognostic value of markers of collagen remodeling in venous ulcers. Wound Repair Regener 1999, 7:347-355 [DOI] [PubMed] [Google Scholar]
- 25.Bonhagen K, Thoma S, Bland P, Bregenholt S, Rudolphi A, Claesson MH, Reimann J: Cytotoxic reactivity of gut lamina propria CD4(+)αβ T cells in SCID mice, with colitis. Eur J Immunol 1996, 26:3074-3083 [DOI] [PubMed] [Google Scholar]
- 26.Armstrong L, Millar AB: Relative production of tumour necrosis factor α and interleukin 10 in adult respiratory distress syndrome. Thorax 1997, 52:442-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarlton JF, Knight PJ: Comparison of reflectance and transmission densitometry, using document and laser scanners, for quantitation of stained Western blots. Anal Biochem 1996, 237:123-128 [DOI] [PubMed] [Google Scholar]
- 28.Tarlton JF, Knight PJ: Clarification of immunoblots on polyvinylidene difluoride (PVDF) membranes for transmission densitometry. J Immunol Methods 1996, 191:65-69 [DOI] [PubMed] [Google Scholar]
- 29.Galis ZS, Sukhova GK, Libby P: Microscopic localization of active proteases by in-situ zymography—detection of matrix metalloproteinase activity in vascular tissue. FASEB J 1995, 9:974-980 [DOI] [PubMed] [Google Scholar]
- 30.Leach MW, Bean AGD, Mauze S, Coffman RL, Powrie F: Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RB(high) subset of CD4(+) T cells. Am J Pathol 1996, 148:1503-1515 [PMC free article] [PubMed] [Google Scholar]
- 31.Kjeldsen L, Bainton DF, Sengelov H, Borregaard N: Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994, 83:799-807 [PubMed] [Google Scholar]
- 32.Adeyemi EO, Hodgson HJF: Fecal elastase reflects disease activity in active ulcerative colitis. Scand J Gastroenterol 1992, 27:139-142 [DOI] [PubMed] [Google Scholar]
- 33.Ottonello L, Dapino P, Pastorino G, Vitale E, Dallegri F: The drug 5-aminosalicylic acid rescues α(1)-proteinase inhibitor from the neutrophil oxidative inactivation—a possible contribution to its therapeutic action in ulcerative-colitis. Digestion 1992, 51:140-145 [DOI] [PubMed] [Google Scholar]
- 34.Knauper V, Murphy G: Membrane-type matrix metalloproteinases and cell surface-associated activation cascades for matrix metalloproteinases. Parks WC Meecham RP eds. Matrix Metalloproteinases. 1998, :pp 199-218 Academic Press, London [Google Scholar]
- 35.Pender SLF, Lionetti P, Murch SH, Wathan N, MacDonald TT: Proteolytic degradation of intestinal mucosal extracellular matrix after lamina propria T cell activation. Gut 1996, 39:284-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr LD, Miller DB, Matrisian LM: TGFβ-1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell 1990, 61:267-278 [DOI] [PubMed] [Google Scholar]
- 37.Wilson CL, Matrisian LM: Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol 1996, 28:123-136 [DOI] [PubMed] [Google Scholar]