Abstract
The study of the pathogenesis of islet amyloidosis and its relationship to the development and progression of type 2 diabetes mellitus has been hampered by the lack of an experimentally inducible animal model. The domestic cat, by virtue of the fact that it is one of the few species that spontaneously develop a form of diabetes mellitus that closely resembles human type 2 diabetes, including the formation of amyloid deposits derived from islet amyloid polypeptide (IAPP), was considered to be an excellent candidate species in which to attempt to develop a nontransgenic animal model for this disease process. To develop the model, 8 healthy domestic cats were given a 50% pancreatectomy, which was followed by treatment with growth hormone and dexamethasone. Once a stable diabetic state was established, cats were randomly assigned to groups treated with either glipizide or insulin at doses appropriate to control hyperglycemia. Cats were maintained on this treatment regimen for 18 months and then euthanized. Based on light microscopic examination of Congo red-stained sections of pancreas, all cats were negative for the presence of islet amyloid at the time of pancreatectomy. At the end of the study all 4 glipizide-treated cats had islet amyloid deposits, whereas only 1 of 4 insulin-treated cats had detectable amyloid. In addition, the glipizide treated cats had threefold higher basal and fivefold higher glucose-stimulated plasma IAPP concentrations than insulin-treated cats, suggesting an association between elevated IAPP secretion and islet amyloidosis. Blood-glycosylated hemoglobin concentrations were not significantly different between the two treatment groups. This study documents for the first time an inducible model of islet amyloidosis in a nontransgenic animal.
Islet amyloidosis is the most common and consistent morphological feature of the pancreatic islets of humans, cats, and macaques with type 2 diabetes mellitus (DM). 1-7 Amyloid deposits can be found in the pancreatic islets of over 90% of human patients with type 2 DM, in 80% of spontaneously diabetic cats, and in 100% of spontaneously diabetic cynomolgus macaques. 4,7,8 Islet amyloid deposits in humans, as well as in cats and macaques, are derived from islet amyloid polypeptide (IAPP) or amylin. 9,10 IAPP is a normal secretory product of the islet β cells and is co-secreted with insulin. 11-13 The mechanisms underlying the transformation of IAPP, a normal secretory product of the β cells, into amyloid fibrils are largely unknown. Of potentially great importance to the pathogenesis of type 2 DM, is the fact that islet amyloid deposits in humans and in the feline and macaque animal models of type 2 DM are associated with significant loss of islet β cells. The mechanisms underlying this partial β cell loss have thus far not been determined, however, many lines of evidence now implicate the generation of fibrillar aggregates of IAPP in this process. Recent in vitro studies with human IAPP suggest a potential role for IAPP fibrils in the induction of apoptosis in several cell types including islet cells. 14-18 Furthermore, several recent studies involving mice, which are transgenic for human IAPP, support a significant role for IAPP fibrillogenesis in the pathogenesis of type 2 DM. 19-22 Therefore, gaining an understanding of the means by which pathological aggregates of IAPP may induce β cell dysfunction and/or β cell death and loss, and of the mechanisms underlying IAPP fibrillogenesis, will have important implications in the prevention and treatment of type 2 DM.
The study of the pathogenesis of IAPP-derived islet amyloidosis has been hampered by the lack of animal models whereby the various factors in islet amyloidogenesis can be studied sequentially. Therefore, the goal of the present study was to develop an inducible model of islet amyloidosis in a species (feline) that can also develop spontaneous IAPP-derived islet amyloidosis. Although transgenic mouse models involving overexpression of human IAPP are also useful for these purposes, they have the disadvantage of having β cells that are further removed from being physiologically normal due to the presence of several transgenes, and are complicated by the concurrent synthesis and secretion of murine IAPP. The present study used, in sequence, partial pancreatectomy, induction of insulin resistance with corticosteroid and growth hormone treatment, and stimulation of IAPP/insulin secretion after induction of diabetes with glipizide, a sulfonylurea drug, to successfully induce islet amyloidosis in domestic cats.
Research Design and Methods
Animals and Protocol
Eight castrated male purpose-bred domestic shorthair cats weighing 4.22 to 5.6 kg (4.7 ± 0.6 kg; mean ± SD) were used in this study. The cats ranged in age from 24 to 29 months at the beginning of the study. All cats had normal complete blood counts, blood biochemical analyses, and urinalyses before study. The cats were housed individually in stainless steel cages. Commercially available cat food was fed twice daily (35 kcal/kg b.i.d.) and water was available ad libitum. A partial pancreatectomy, which entailed removal of the splenic lobe, was performed under general anesthesia in all cats. Sections of pancreas were saved for light and electron microscopic examination (see below).
After a 2-month recovery period, diabetes was induced by daily subcutaneous administration of 1 mg bovine growth hormone (a donation of Monsanto, St. Louis, MO) and oral administration of 1.5 mg dexamethasone. All cats were diabetics after 4 months on this treatment regimen. Stable diabetes was indicated by the fact that the cats remained hyperglycemic after discontinuation of the growth hormone and dexamethasone treatment. The cats had a 3-week period during which they were hyperglycemic but were not yet treated with antihyperglycemic agents. Four of the cats then received Humulin N (Eli Lilly Corp., Indianapolis, IN) subcutaneously twice daily. The average insulin dose given twice daily was 1.5 units/kg. Four cats received 5 mg glipizide (Glucotrol, Pfizer) 2 to 3 times daily. The cats were treated for 18 months with either glipizide or insulin. They were then euthanized with an overdose of pentobarbital. A complete necropsy was performed, and sections of pancreas were fixed for light microscopic examination (see below).
Blood glucose concentrations were measured weekly during the diabetes induction after an overnight fast. In addition, before and 2 months after pancreatectomy, every 4 weeks during diabetes induction, and every 8 weeks during the treatment with insulin or glipizide, glycosylated hemoglobin (gHb) concentrations were measured and intravenous glucose tolerance tests (IVGTT) were performed as described. 23,24 Treatment with either insulin or glipizide was withheld on the evening before the glucose tolerance tests were performed. The IVGTT were performed as follows: 50% dextrose (w/v) was injected into a cephalic vein at 1 g/kg body weight. Blood samples for glucose determinations were taken at 5, 10, 15, 30, 45, 60, 90, and 120 minutes. At times 0 and 15, blood samples were also taken for the measurement of IAPP. Because of the large amount of blood that was necessary for the IAPP measurements, only these two time points were chosen for analysis. Blood samples for glucose determinations were kept at room temperature for 30 minutes and then centrifuged for 10 minutes at 500 × g (Beckman GPR, Palo Alto, CA). The serum was then collected and frozen at −20°C until assayed. Glucose measurements were performed using a colorimetric glucose oxidase method (glucose trinder kit; Sigma, St. Louis, MO). Serum insulin concentrations were measured as described using a charcoal method. 23 The intra-assay coefficient of variation (CV) was 2.7%; the interassay CV was 3.9%.
Light Microscopy
Pieces of pancreas to be examined by light microscopy were fixed in 10% neutral buffered formalin for 6 hours and then transferred to 70% ethanol solution until processed. Tissues were then routinely dehydrated and paraffin-embedded, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E) and Congo red. Immunohistochemistry was performed on a Dako Autostainer (Dako, Carpenteria, CA) using rabbit anti-human IAPP (1:1000, Peninsula Laboratories, Belmont, CA), and a labeled streptavidin kit (LSAB2 Detection Kit with AEC chromogen, Dako). Positive control tissue (normal cat pancreas) exhibited strong IAPP immunoreactivity in islet β cells, whereas omission of the primary antibody eliminated all staining. Tissue sections were examined by brightfield microscopy and, in addition, Congo red-stained sections were examined with cross-polarized light for the presence of green birefringence, typical of amyloid. The severity of islet amyloidosis was semiquantitatively scored in each pancreas on a 0 to 4 scale: 0 = no lesion, 1 = minimal (<10% of islets affected and amyloid comprising <10% of islet), 2 = mild (10–24% of islets affected and amyloid comprising <25% of islet), 3 = moderate (25–49% of islets affected and amyloid comprising up to 50% of islet), 4 = severe (>50% of islets affected and amyloid comprising >50% of most islets). To further assess the degree of islet amyloidosis, all islets in each of 3 sections of pancreas from each cat were counted in the Congo red-stained sections along with the number of islets in which there was detectable islet amyloid (IA). Results were expressed as percentage of islets with IA.
IAPP Radioimmunoassay
Samples were collected into chilled tubes containing EDTA and trasylol (400 kIU) and were then centrifuged for 10 minutes at 500 × g (Beckman GPR). Plasma IAPP concentrations were measured essentially as described with modifications for feline IAPP. 25 Plasma was extracted using Sep-Pak C18 cartridges (Waters, Marlborough, MA) with solvent flow controlled by a Harvard pump (Harvard Apparatus, South Natick, MA). Cartridges were activated with 5 ml of 100% acetonitrile/0.1% TFA and then washed with 10 ml of H2O/0.1% TFA. Three milliliters of plasma were then loaded onto each column, followed by a wash with 10 ml of H2O/0.1% TFA. Columns were then eluted with 3 ml of 70% acetonitrile/0.1% TFA and plasma extracts lyophilized. Radioimmunoassays were performed at 4°C in polypropylene tubes. A feline IAPP standard curve (1–100 fmol/tube, cat. no. 7290, Peninsula Laboratories, Belmont, CA) was prepared in Sorenson’s assay buffer (0.6 mol/L phosphate, pH 7.4). Lyophilized plasma extracts were reconstituted in 700 μl radioimmunoassay buffer. For the assay, duplicate 300 μl samples were incubated at 4°C for 7 days with 100 μl anti-human IAPP antiserum (cat. no. RAS7321, Peninsula Laboratories) and 100 μl 125I-labeled IAPP (Amersham). After incubation, separation of free and bound peptide was done by addition of 100 μl of bovine γ globulin (10 mg/ml in water, Sigma) and 1 ml 20% polyethylene glycol (Sigma) to samples followed by centrifugation at 2500 rpm for 30 minutes at 4°C. The supernatant was aspirated and 125I radioactivity of the pellet assayed on a γ counter (Compugamma, LKB Wallac, Gaithersburg, MD). Tracer recovery after extraction was 77%. Minimum detectable peptide was 2.5 fmol/tube. Intra-assay CV was 8.3%; interassay CV was 16%. The CV for parallelism (3 concentrations) was 10.1%.
The data were analyzed using Data Desk software for Macintosh computers. The data are expressed as means ± SD, unless stated otherwise. The significance of differences of means between groups was assessed by Student’s t-test for paired samples. A P value <0.05 was considered statistically significant.
Results
Diabetes Induction Period
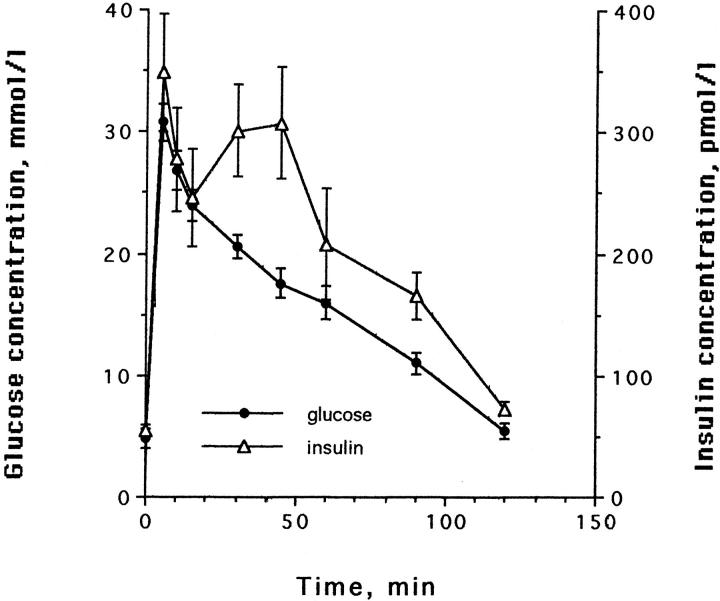
All cats had normal glucose tolerance at the beginning of the study (Figure 1) ▶ . The IAPP concentrations were similar in all cats at the beginning of the study (3.1 ± 0.2 at time 0, and 4.8 ± 1.0 at time 15 of the IVGTT). During the growth hormone and dexamethasone treatment period, glucose intolerance developed, as indicated by changes in the insulin secretion pattern, an increase in fasting blood glucose, increased gHb, and decreased glucose disposal rate during IVGTT (K value). The results of changes in blood glucose, gHb, and K value have been described previously. 19 The insulin release pattern was the first to change. First phase insulin release became delayed and smaller, whereas second phase became more exaggerated (Figure 2, a and b) ▶ . The fasting blood glucose did not become abnormal until first phase insulin secretion had almost completely disappeared and the amount of insulin secreted during the 2-hour test had dropped about fivefold (see Figure 2c ▶ ). The area under curve for insulin in the cats before diabetes induction was 25.6 ± 3.8 nmoles/l*120 minutes, whereas it was 5.0 ± 2.9 nmoles/l*120 minutes when the fasting blood glucose increased above the normal range (ie, >120 mg/dl).
Figure 1.
Plasma glucose and insulin concentrations in 8 cats after i.v. administration of glucose before partial pancreatectomy and treatment with growth hormone and dexamethasone (mean ± SD).
Figure 2.
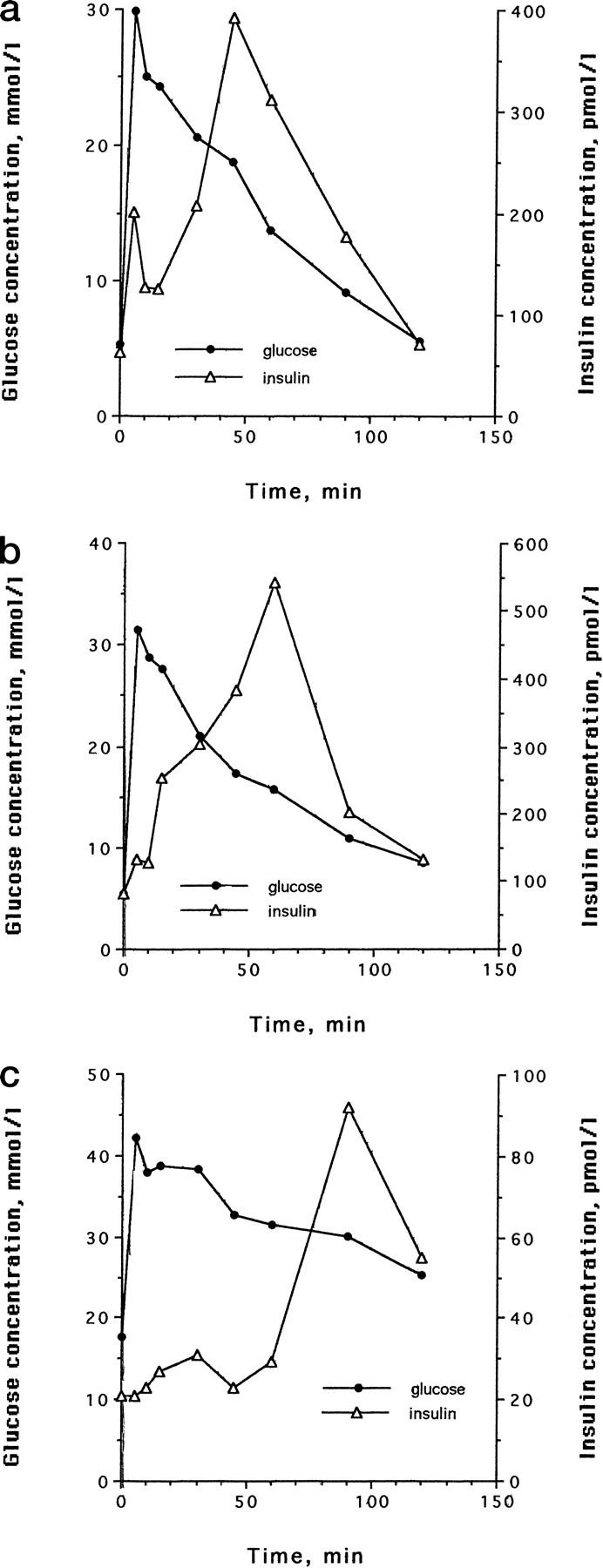
Representative examples of sequential changes in glucose and insulin release in one cat during the diabetes induction with growth hormone and dexamethasone. a: Changes in insulin secretion but normal glucose tolerance after 1 month. b: After 2 months, changes in glucose tolerance are present and the changes in insulin secretion are more marked. c: Insulin secretion has markedly decreased after 3 months. There is now marked glucose intolerance and a marked defect in insulin secretion.
Diabetes Treatment Period
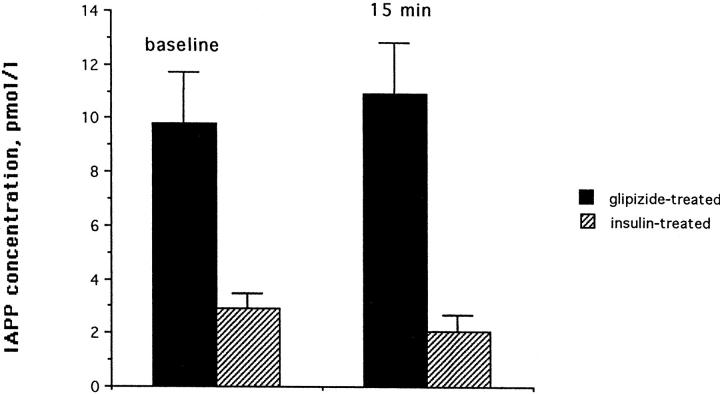
Although the gHb concentrations during the 18 months of diabetes treatment were similar in both groups (2.6 ± 0.2% in the glipizide- and 2.4 ± 0.2 in the insulin-treated group), the IAPP concentrations were significantly higher in the glipizide-treated group than in the insulin-treated group. The average baseline IAPP concentrations (time 0 of the IVGTT) for the glipizide group were 9.8 ± 1.9 pmol/l and were 2.9 ± 0.2 pmol/l for the insulin-treated group; the IAPP concentrations after stimulation (time 15 of the IVGTT) were 10.9 ± 1.9 pmol/l for the glipizide group and 2.1 ± 0.6 pmol/l for the insulin-treated group (P < 0.001 for both). The average IAPP concentration in response to the IVGTT during the insulin and glipizide treatment period is shown in Figure 3 ▶ . The area under curve for insulin in response to the IVGTT was greater for the insulin-treated cats than for the glipizide-treated cats (6.7 ± 4.1 versus 4.4 ± 1.0 nmol/120 minutes, mean ± SD), this was statistically not significant (P = 0.051).
Figure 3.
Comparison of IAPP concentrations of 4 cats treated with glipizide with 4 cats treated with insulin. Baseline concentrations and concentrations 15 minutes after i.v. glucose administration are shown (mean ± SD).
Light Microscopy
Light microscopic examination of pretreatment pancreatic biopsies failed to demonstrate any endocrine or exocrine lesions. No evidence of islet amyloidosis was found on H&E or Congo red-stained sections. Light microscopic examination of post-treatment (necropsy) pancreatic tissues from insulin-treated cats revealed that 1 of 4 cats had detectable IA. The single cat with IA had16% of islets exhibiting deposits (Figure 4) ▶ . All 4 cats in this group had islet cell vacuolar change, which was moderate or severe in 3 cats and minimal in 1 cat.
Figure 4.
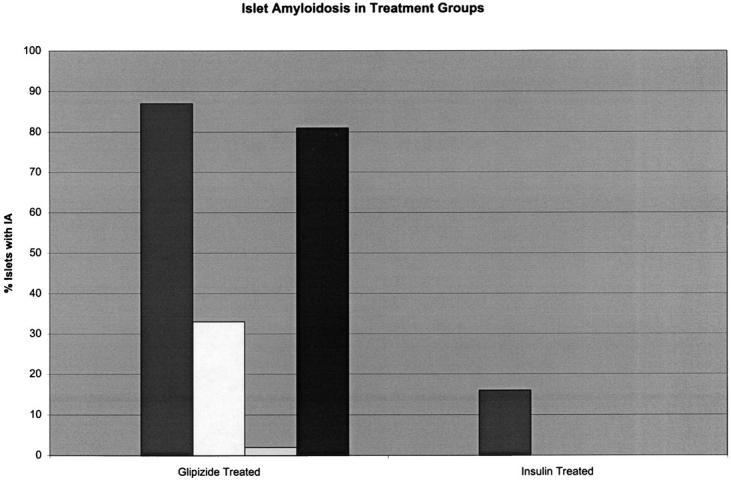
Percentage of islets containing amyloid deposits in glipizide- and insulin-treated cats.
All cats in the glipizide-treated group (4/4) had IA, which was mild or moderate in 3 cats and minimal in 1 cat (Figure 5) ▶ . The percentage of islets exhibiting IA deposits in this group was as follows: 87, 33, 2, 81 (Figure 4) ▶ . One cat in this group had severe islet vacuolar change while none was found in the other 3 cats. The single cat in the glipizide-treated group that had severe islet vacuolar change also had minimal IA.
Figure 5.
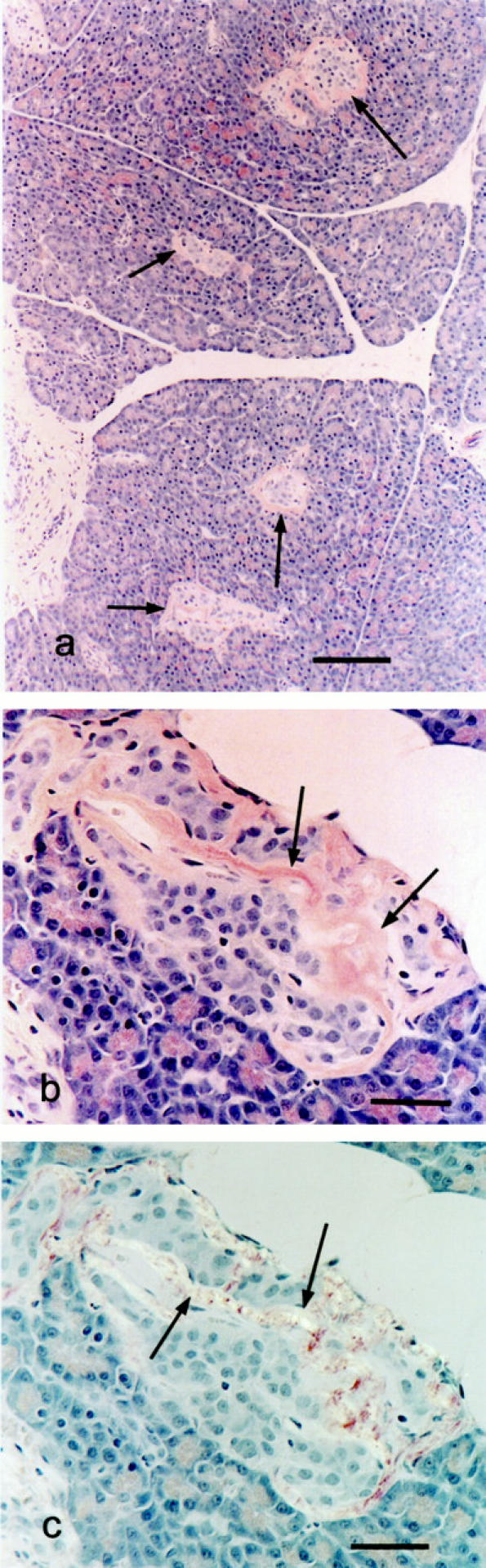
Congo red-stained sections from glipizide-treated cats demonstrating amyloid deposits. a: Low magnification photomicrograph showing 4 islets exhibiting congophilic amyloid deposits (arrows). Bar, 124 μm. b: High magnification photomicrograph showing congophilic peripheral and central amyloid deposits (arrows) in a pancreatic islet. c: Same section with cross-polarized light demonstrating green birefringence of amyloid (arrows). Bars, 44 μm.
Immunohistochemistry for IAPP in pretreatment biopsies in all cases showed normal staining distribution and intensity in pancreatic β cells (Figure 6a) ▶ . Islet amyloid deposits in all cases showed strong immunoreactivity for IAPP (Figure 6b) ▶ . In addition, β cells in islets having IA deposits showed more intense IAPP staining than pretreatment controls and tended to lack the normal polar staining seen in normal β cells. IAPP staining in islets of cats in the insulin-treated group was sparse and tended to occur only in isolated nonvacuolated cells at the margins of the islets (Figure 6c) ▶ .
Figure 6.
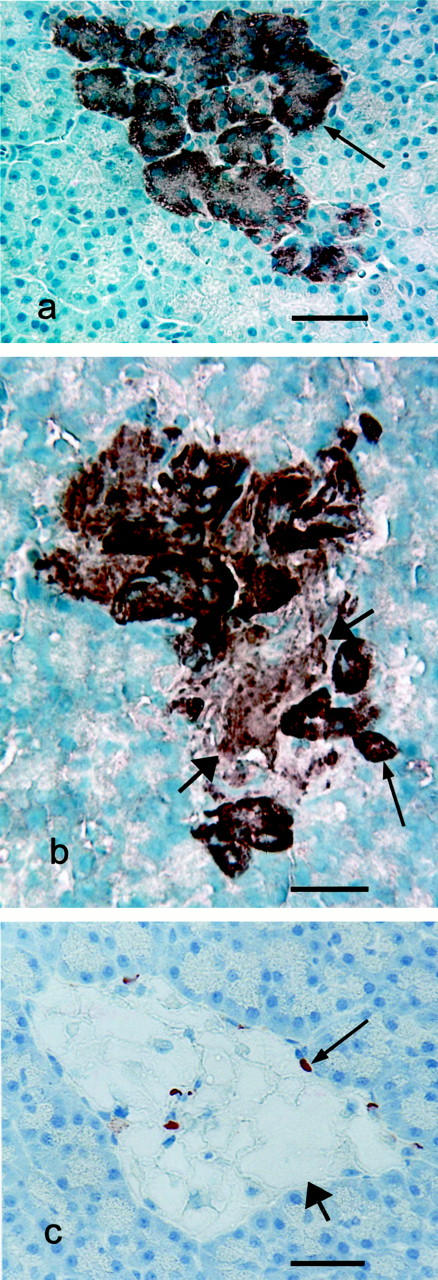
Immunohistochemical staining for islet amyloid polypeptide (IAPP). a: Pancreatic islet from a cat before diabetes induction (normal islet). Note intense staining for IAPP, which is more intense at one pole of the cells (arrow). Bar, 46 μm. b: Pancreatic islet from a glipizide-treated cat showing strong IAPP immunoreactivity in islet amyloid deposit (wide arrows). Note also the diffuse, intense cytoplasmic IAPP staining in the remaining cells (narrow arrows). Bar, 26 μm. c: Pancreatic islet from an insulin-treated cat showing marked vacuolar change in islet cells (wide arrow), most of which show no IAPP immunoreactivity. Note some residual IAPP immunoreactivity in some islet cells (narrow arrow). Bar, 36 μm.
Other lesions noted in cats at necropsy included minimal hepatocellular vacuolation, which was due primarily to the presence of glycogen in 2 of the insulin-treated cats, and mild myocardial fibrosis with mild myofiber disarray in 1 of the glipizide-treated cats.
Discussion
Following a protocol that was similar, but with significant modifications, to one used on cats by Lukens and Dohan almost 60 years ago, 26 we were able to develop a model of IAPP-derived islet amyloidosis in a nontransgenic animal. In their classic study, Lukens and Dohan described hyalinization of pancreatic islets in cats made diabetic by treatment with pituitary extracts. However, it was not clear from their study whether the cats truly developed islet amyloidosis, since no Congo red or other amyloid stains were done. Also, because Lukens and Dohan did not biopsy the pancreas at the start of their experiments, it was not certain that their cats developed islet amyloid during the study or if they might have had amyloid deposits at the start of the experiment. In the present study, before the initiation of corticosteroid and growth hormone treatment, all cats were histologically negative for islet amyloid and all cats had normal glucose tolerance. Although pancreatic biopsies were not done at the end of the induction period, it is unlikely that differences in amyloid formation between the glipizide-treated and insulin-treated groups arose in the induction period, since animals were randomly assigned to groups. In addition, there was no significant difference in time to induction of diabetes between the two groups. Suspected amyloid deposits observed in the present study were confirmed to be amyloid by Congo red staining with demonstration of characteristic green birefringence under cross-polarized light. The degree of islet amyloidosis in 3 of 4 glipizide-treated cats based on subjective scoring and percentage of islets with IA was also similar to that found in spontaneous feline diabetes mellitus. 8,27,28 Furthermore, all of the amyloid deposits were positive for IAPP immunoreactivity, consistent with naturally occurring islet amyloid in the cat, human, and macaque. This study is, therefore, the first confirmed instance of experimental induction of islet amyloidosis in a nontransgenic animal.
An interesting finding in this study was the positive association between glipizide treatment and development of islet amyloidosis, or conversely, a negative association between insulin treatment and the development of islet amyloidosis. It appears likely that relatively increased IAPP secretion played a role in this difference since the glipizide-treated cats had a 3× higher basal and 5× higher glucose-stimulated IAPP secretion than insulin-treated cats. These differences in fasting and glucose-stimulated secretion were most likely due to a combination of increased IAPP secretion in the glipizide-treated cats and reduced IAPP secretion in the insulin-treated cats. Similarly, insulin has been shown previously to suppress IAPP secretion in human patients with non-insulin-dependent diabetes mellitus. 29 Our findings are also consistent with data from van Jaarsveld and coworkers, who compared 27 patients treated with oral hypoglycemics to 6 insulin-treated diabetics and 18 nondiabetic patients. The fasting IAPP concentration in the insulin-treated group was approximately 50% of the concentration of the sulfonylurea-treated group. 30 No difference in the fasting IAPP concentration was seen in a recent study between sulfonylurea-treated and diet-treated type 2 diabetic patients, whereas a decrease was seen in insulin-treated patients. Sulfonylurea treatment, however, increased the postprandial IAPP concentration more than either diet alone or insulin, which supports the notion that sulfonylureas have differential effects on IAPP and insulin secretion. 31 Our data also suggested that glipizide preferentially stimulates IAPP secretion, because the glipizide-treated cats had a lower glucose-stimulated insulin secretion. The insulin secretion pattern was abnormal in both groups but in the insulin-treated group, β cells were able to respond to the glucose load much earlier (at 30 minutes) than in the glipizide-treated group (at 90 minutes). Differences in the occurrence of islet amyloid in the two groups were not due to differences in regulation of blood glucose concentrations, since gHb levels in the two groups were not significantly different.
The above findings support the concept that islet amyloidosis occurs as a result of prolonged stimulation of β cells to secrete (the “overworking” hypothesis). This theory postulates that since insulin resistance increases both insulin and IAPP secretion, prolonging or exaggerating this condition (as in long-term obesity) may promote conditions suitable for IAPP fibrillogenesis. It has been speculated that constant overstimulation of β cells due to insulin resistance may lead to amyloid formation through the depletion of factors necessary for the normal processing and secretion of IAPP. 32 This hypothesis is supported by previous observations that cats with impaired glucose tolerance have increased β-cell IAPP immunoreactivity. 8,33 Likewise, in the current study, the β cells in glipizide-treated cats showed exaggerated IAPP immunoreactivity and loss of normal staining polarity. In addition, the fact that insulinomas (in which there is dysregulation of insulin and IAPP secretion) also contain IAPP-derived amyloid deposits lends further support to this hypothesis. 34
The present study raises the intriguing question of whether the long-term use of drugs that increase IAPP (and insulin) secretion, such as sulfonylureas, may induce or accelerate the development of IAPP-derived islet amyloid deposits. Such effects would be predicted to contribute to the progression of diabetes toward insulin dependence. The fact that each year approximately 5% of human patients taking oral hypoglycemic drugs progress to insulin dependence supports this possibility. 35 The striking degree of islet amyloidosis that developed primarily in the glipizide-treated cats in the present study occurred over the course of 18 months of treatment and indicates the rapidity with which this pathological change may occur. It is also possible, however, that glipizide was not so much a promoter of IAPP-derived islet amyloidosis as insulin treatment was an inhibitor of this process. It may be that pathological processes that lead to islet amyloidosis are initiated by the diabetes induction methods used in this study and that insulin treatment inhibits further development of islet amyloid. The facts that islet amyloidosis was found (albeit in trace amounts) in one of the insulin-treated cats, and that islet amyloidosis may be induced by corticosteroid and growth hormone treatment in mice transgenic for human IAPP, 19 also support this latter possibility. The present experiment was not designed to assess these potential mechanisms and elucidation of the precise pathogenesis will require further study.
Acknowledgments
We thank Dr. P.C. Butler, (Los Angeles, CA) for his help in the establishment of the model.
Footnotes
Address reprint requests to Dr. Margarethe Hoenig, Department of Physiology and Pharmacology, College of Veterinary Medicine, The University of Georgia, Athens, GA 30602. E-mail: mhoenig@calc.vet.uga.edu.
Supported by the Morris Animal Foundation.
References
- 1.Ehrlich JC, Ratner JM: Amyloidosis of the islets of Langerhans, a study of islet hyalin in diabetic and nondiabetic individuals. Am J Pathol 1961, 38:49-59 [PMC free article] [PubMed] [Google Scholar]
- 2.Westermark P: Quantitative studies of amyloid in the islets of Langerhans. Uppsala J Med Sci 1972, 77:91-94 [DOI] [PubMed] [Google Scholar]
- 3.Schneider HM, Storkel S, Will W: Das Amyloid der Langerhansschen Inseln und seine Beziehung zum Diabetes Mellitus. Dtsch Med Wschr 1980, 105:1143-1147 [DOI] [PubMed] [Google Scholar]
- 4.Johnson KH, O’Brien TD, Westermark P: Islet amyloid, islet amyloid polypeptide and diabetes mellitus. New Engl J Med 1989, 321:513-518 [DOI] [PubMed] [Google Scholar]
- 5.Clark A, de Koning EJ, Hattersley AT, Hansen BC, Yajnik CS, Poulton J: Pancreatic pathology in non-insulin dependent diabetes (NIDDM). Diabetes Res Clin Pract 1995, 28:S39-S47 [DOI] [PubMed] [Google Scholar]
- 6.Johnson KH, Hayden DW, O’Brien TD, Westermark P: Animal model of human disease: spontaneous diabetes mellitus-islet amyloid complex in adult cats. Am J Pathol 1986, 125:416-419 [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien TD, Carlson CS, Johnson KH, Cefalu WT, Butler PC, Wagner JD: Spontaneous diabetes mellitus in Cynomolgus monkeys: morphometric analysis of islet amyloid and islet cells. Vet Pathol 1996, 33:479-485 [DOI] [PubMed] [Google Scholar]
- 8.Johnson KH, O’Brien TD, Jordan K, Westermark P: Impaired glucose tolerance is associated with increased islet amyloid polypeptide (IAPP) immunoreactivity in pancreatic beta cells. Am J Pathol 1989, 135:245-250 [PMC free article] [PubMed] [Google Scholar]
- 9.Westermark P, Wernstedt C, Heldin C-H, Wilander E, Hayden DW, O’Brien TD, Johnson KH: Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a novel neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA 1987, 84:3881-3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westermark P, Wernstedt C, O’Brien TD, Hayden DW, Johnson KH: Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats is composed of a novel putative polypeptide hormone. Am J Pathol 1987, 127:414-417 [PMC free article] [PubMed] [Google Scholar]
- 11.Butler PC, Chou J, Carter WB, Wang Y, Bu B, Chang D, Chang J, Rizza RA: Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes 1990, 39:752-756 [DOI] [PubMed] [Google Scholar]
- 12.Kahn SE, D’Alessio DA, Schwarz W, Fujimoto WY, Ensinck JW, Taborski GJ, Porte D: Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells. Diabetes 1990, 39:634-638 [DOI] [PubMed] [Google Scholar]
- 13.O’Brien TD, Westermark P, Johnson KH: Islet amyloid polypeptide (IAPP) and insulin secretion from isolated perfused pancreas of fed, fasted, glucose-treated, and dexamethasone-treated rats. Diabetes 1991, 40:1701-1706 [DOI] [PubMed] [Google Scholar]
- 14.O’Brien TD, Butler PC, Kreutter DK, Kane LA, Eberhardt NL: Intracellular amyloid associated with cytotoxicity in COS-1 cells expressing human islet amyloid polypeptide. Am J Pathol 1995, 147:609-616 [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenzo A, Razzaboni R, Weir GC, Yankner BA: Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 1994, 368:756-760 [DOI] [PubMed] [Google Scholar]
- 16.May PC, Boggs LN, Fuson KS: Neurotoxicity of human amylin in rat primary hippocampal cultures: similarity to Alzheimer’s disease amyloid-β neurotoxicity. J Neurochem 1993, 61:2330-2333 [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP, Goodman Y: Different amyloidogenic peptides share a similar mechanism of neurotoxicity involving reactive oxygen species and calcium. Brain Res 1995, 676:219-224 [DOI] [PubMed] [Google Scholar]
- 18.Dore S, Kar S, Quirion R: Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci USA 1997, 94:4772-4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couce M, Kane LA, O’Brien TD, Charlesworth J, Soeller W, McNeish J, Kreutter D, Roche P, Butler PC: Treatment with growth hormone and dexamethasone in mice transgenic for human islet amyloid polypeptide causes islet amyloidosis and β-cell dysfunction. Diabetes 1996, 45:1094-1101 [DOI] [PubMed] [Google Scholar]
- 20.Jensen J, Soeler WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, Butler PC: Spontaneous diabetes mellitus in transgenic mice expression human islet amyloid polypeptide. Proc Natl Acad Sci USA 1996, 93:7283-7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verchere CB, D’Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, Kahn SE: Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci USA 1996, 93:3492-3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soeller WC, Janson J, Hart SE, Parker JC, Carty MD, Stevenson RW, Kreutter DK, Butler PC: Islet amyloid-associated diabetes in obese Avy/a mice expressing human islet amyloid polypeptide. Diabetes 1998, 47:743-750 [DOI] [PubMed] [Google Scholar]
- 23.Hoenig M, Ferguson DC: Impairment of glucose tolerance in hyperthyroid cats. J Endocrinol 1989, 121:249-251 [DOI] [PubMed] [Google Scholar]
- 24.Hoenig M, Ferguson DC: The diagnostic utility of glycosylated hemoglobin in the cat. Domest Anim Endocrinol 1999, 16:11-17 [DOI] [PubMed] [Google Scholar]
- 25.Valdermarsson S, Leckstrom A, Westermark P, Bergenfelz A: Increased plasma levels of islet amyloid polypeptide in patients with primary hyperparathyroidism. Eur J Endocrinol 1996, 134:320-325 [DOI] [PubMed] [Google Scholar]
- 26.Lukens FDW, Dohan FC: Pituitary-diabetes in the cat: recovery following insulin or dietary treatment. Endocrinology 1942, 30:175-201 [Google Scholar]
- 27.Yano BL, Hayden DW, Johnson KH: Feline insular amyloid: association with diabetes mellitus. Vet Pathol 1981, 18:621-627 [DOI] [PubMed] [Google Scholar]
- 28.O’Brien TD, Hayden DW, Johnson KH, Fletcher TF: Immunohistochemical morphometry of pancreatic endocrine cells in diabetic, normoglycemic glucose-intolerant and normal cats. J Comp Pathol 1986, 96:357-369 [DOI] [PubMed] [Google Scholar]
- 29.Lindstrom T, Leckstrom A, Westermark P, Arnqvist HJ: Effect of insulin treatment on circulating islet amyloid polypeptide in patients with NIDDM. Diabet Med 1997, 14:472-476 [DOI] [PubMed] [Google Scholar]
- 30.van Jaarsveld BC, Hackeng WH, Lips CJ, Erkelens DW: Plasma concentrations of islet amyloid polypeptide after glucagon administration in type 2 diabetic patients and non-diabetic subjects. Diabet Med 1993, 10:327-330 [DOI] [PubMed] [Google Scholar]
- 31.Rachman J, Payne MJ, Levy JC, Barrow BA, Holman RR, Turner RC: Changes in amylin and amylin-like peptide concentrations and b-cell function in response to sulfonylurea or insulin therapy in NIDDM. Diabetes Care 1998, 21:810-816 [DOI] [PubMed] [Google Scholar]
- 32.O’Brien TD, Butler PC, Westermark P, Johnson KH: Islet amyloid polypeptide: a review of its biology and potential roles in the pathogenesis of diabetes mellitus. Vet Pathol 1993, 30:317-332 [DOI] [PubMed] [Google Scholar]
- 33.Ma Z, Westermark GT, Johnson KH, O’Brien TD, Westermark P: Quantitative immunohistochemical analysis of islet amyloid polypeptide (IAPP) in normal, impaired glucose tolerant, and diabetic cats. Amyloid. Int J Exp Clin Invest 1998, 5:255-261 [DOI] [PubMed] [Google Scholar]
- 34.O’Brien TD, Butler AE, Johnson KH, Roche PC, Butler PC: Islet amyloid polypeptide (IAPP) on human insulinomas: evidence for intracellular amyloidogenesis. Diabetes 1994, 43:329-336 [DOI] [PubMed] [Google Scholar]
- 35.Genuth S: Management of the adult onset diabetic with sulfonylurea drug failure. Endocrin Metab Clin North Am 1992, 21:351-370 [PubMed] [Google Scholar]