Abstract
Mice with targeted mutation of chemokine receptor 1 (CCR1) were used to assess the contribution of CCR1 agonists to local, regional, and systemic inflammatory-related events during experimental pulmonary granuloma formation. Models of Th1 (type-1) and Th2 (type-2) cell-mediated lung granulomas were induced in wild-type (CCR+/+) and knockout (CCR1−/−) mice by embolizing Sepharose beads coupled to the purified protein derivative of Mycobacterium bovis or soluble antigens derived from Schistosoma mansoni eggs. Morphometric analysis indicated that granuloma sizes were unchanged in CCR1−/− mice, but flow cytometric analyses of dispersed granulomas revealed that natural killer cell recruitment to type-1 lesions was abrogated by 60%. Analysis of cytokine production by draining lymph node cultures showed altered expression in CCR1−/− mice characterized by reduced interleukin-2 and interferon-γ in the type-1 response, and enhanced interleukin-5 and interleukin-13 in the type-2 response. Peripheral blood leukocytosis was also enhanced in the type-1 but not the type-2 response. These findings suggest that CCR1 agonists contribute to multiple immunoinflammatory events in the type-1 granulomatous response with natural killer cell accumulation being particularly sensitive to CCR1 disruption. Although functional efficacy of granulomas may be altered, chemokine redundancy and cytokine reserve seem to make the bulk of the exudative response resistant to CCR1 disruption.
Chemokines are thought to play an important role in cellular trafficking and physiology, but the in vivo analysis of chemokine function has been impeded by the apparent high degree of redundancy among the numerous chemokines. 1-5 The molecular and biological characterization of G protein-coupled chemokine receptors has allowed the development of mice with targeted receptor knockout as an alternative analytic approach to study chemokine function. Among chemokine receptors, chemokine receptor 1 (CCR1) is expressed by a broad spectrum of leukocytes and is known to bind a number of chemokine ligands such as MIP-1α, MIP-3, MIP-5, RANTES, MCP-3, MIP-1γ, and mC10. 3,6 Previous studies using CCR1 knockout (CCR1−/−) mice have reported abrogated inflammation, altered hematopoiesis, and delayed cardiac graft rejection. 7-9 It has also been reported that CCR1−/− mice display exacerbated inflammation and associated enhancement of selected parameters of Th1 immunity in a murine model of nephrotoxic nephritis. 10 The latter findings suggested that Th1 responses may be favored in CCR1−/− knockout mice, but other reports indicate that agonists of CCR1 promote Th1 responses. 11,12 The in vitro studies of Lukacs and colleagues 13 demonstrated that MIP-1α, a CCR1 ligand, had different effects on Th1 and Th2 cytokines during the primary inductive phase versus the memory/secondary response. Thus, conflicting results might arise depending on the type of response and the stage at which it is examined.
As yet, there is no systematic study of the effect of CCR1 knockout on secondary Th1 and Th2 responses. In an attempt of clarify the role of CCR1 in these responses, we examined the effect of CCR1 knockout on defined models of polarized type-1 and type-2 pulmonary granulomatous inflammation elicited by antigens derived from Mycobacteria bovis and ova of the helminthic parasite, Schistosoma mansoni. 14,15 The findings indicate that although CCR1 knockout had no effect on gross lesion size in either the type-1 or type-2 response, there was evidence that CCR1 regulated levels of circulating leukocytes and was required for recruitment of lymphoid subpopulations. Specifically, natural killer (NK) cells were reduced in CCR1−/− mice with type-1 lesions. In addition, their draining lymph nodes displayed partial impairment of Th1 cytokines similar to that described in CCR2−/− mice. 16 Conversely, there was enhancement of Th2 cytokines during the type-2 response. Lymph node changes could not be attributed to altered proportions of lymphoid populations; therefore the effect was likely because of functional changes. These findings would suggest that CCR1 has nonredundant functions under particular circumstances, such as in responses that depend on NK cells. In addition, CCR1 likely shares redundant functions with other chemokine receptors that promote the secretion of interferon (IFN)-γ.
Materials and Methods
Animals
CCR1 knockout mice on 129Sv X B6 background were generated from 129Sv strain embryonic stem cells using targeting vectors as previously described. 7 Control animals consisted of age-matched nonmutant 129Sv X B6 F2 mice. Mice were maintained in isolator cages under specific pathogen-free conditions and provided with food and water ad libitum.
Sensitization and Granuloma Induction
Types 1 and 2, secondary antigen-bead granulomas were generated as previously described. 17 Briefly, mice were sensitized by subcutaneous injection of 20 μg M. bovis-purified protein derivative (PPD; Department of Agriculture, Veterinary Division, Ames, IA) incorporated into 0.25 ml of complete Freund’s adjuvant (product number F-5881; Sigma, St. Louis, MO) or 3,000 S. mansoni eggs suspended in 0.5 ml of phosphate-buffered saline (PBS). Fourteen to 16 days later PPD and schistosome egg-sensitized mice were respectively challenged by tail vein with 6,000 Sepharose 4B beads (in 0.5 ml PBS) covalently coupled to PPD or to soluble schistosome egg antigens (SEA) obtained from the World Health Organization, Geneva, Switzerland.
Granuloma Dispersal and Draining Lymph Node Culture
Groups of mice were killed at 1, 2, 4, and 8 days of granuloma formation. After perfusion with cold RPMI, lungs excluding trachea and major bronchi were excised. The right upper lung of each mouse was snap-frozen in liquid N2 for mRNA isolation. The left lower lobe was postinflated and formalin-fixed. The remaining lung lobes were placed in cold RPMI medium then granulomas were isolated and dispersed as previously described. 14,18 For differential counting, duplicate cytospin preparations were prepared from the remaining dispersed granuloma cells and stained with Wright’s stain. Blood was also collected from each animal and total white cell count and differential performed.
Mediastinal lymph nodes were collected at the time of lung harvest and teased into single cell suspension. After washing, the cells (5 × 106/ml) were cultured for 24 hours in RPMI-1640 medium (JRH Biosciences, Lenexa, KS) containing 10% fetal bovine serum (Intergen, Purchase, NY), 10 mmol/L glutamine, and 100 mg/ml streptomycin and 100 U/ml penicillin (RPMI-fetal bovine serum) in the presence or absence of 5 μg/ml of PPD or SEA. Supernates were collected by centrifugation at 1,000 × g for 10 minutes and stored at −45°C.
Granuloma Measurement
Granulomas were measured blindly from formalin-inflated lungs that were paraffin-embedded, sectioned, and then stained with hematoxylin and eosin. Granuloma area was measured by computerized morphometry. A minimum of 20 lesions was measured per lung.
Southern Blot Analysis
Genotyping was confirmed by Southern analysis of genomic DNA as previously described, 7 with the exception that the SacI restriction enzyme was used to generate DNA fragments. Characteristic, 5-kb wild-type and 7-kb mutant gene fragments were identified by specific probe hybridization.
Flow Cytometry
Dispersed granulomas and mediastinal lymph node suspensions were subjected to flow cytometry using one- and two-color fluorescent analysis. For the staining of CCR1 and CCR2b chemokine receptors, cells (1 × 106) were fixed and permeabilized with Cytofix/Cytoperm (Pharmingen, San Diego, CA). After the permeabilization step, cells were incubated with a control antibody (goat IgG; Zymed Laboratories, Inc., San Francisco, CA) or polyclonal antibodies against the cytoplasmic tail of murine CCR1 or CCR2b (goat IgG; Santa Cruz Biotechnology Inc., Santa Cruz, CA). After 30 minutes at 4°C, cells were washed and stained in the dark for 30 minutes with fluorescein isothiocyanate-labeled anti-goat IgG (Santa Cruz Biotechnology Inc.). For dual staining of cell surface markers and chemokine receptors, cells were first incubated with phycoerythrin-labeled monoclonal antibodies to CD3, CD4, CD8, CD19, pan-NK (DX5), or NK1.1 (PK136) (Pharmingen), and then stained with antibodies against the cytoplasmic tail of mouse chemokine receptors by the intracellular procedure mentioned above. Stained cells were analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain view, CA). Lymphocyte population was gated using forward and side scatter characteristics. Cells (10,000 to 20,000) were analyzed and data were processed with CellQuest software (Becton Dickinson).
Cytokine Measurement
Interleukin (IL)-2, -5, and -13, MIP-1α, and IFN-γ were measured by enzyme-linked immunosorbent assay using commercially available reagents (Pharmingen and R&D Systems, Minneapolis, MN); sensitivities ranged from 10 pg/ml to 50 pg/ml. Commercially available recombinant murine cytokines served as standards in all assays (Genzyme, Cambridge, Preprotech Inc., Rocky Hill, NJ, and R&D Systems).
Statistics
The Student’s t-test (two-tailed) was used to compare control with experimental groups. Values of P > 0.05 were considered to indicate lack of significance.
Results
CCR1 Knockout Fails to Abrogate Secondary Type-1 and Type-2 Pulmonary Granuloma Formation
Mice with knockout of the CCR1 gene reportedly display impaired granuloma formation in response to primary challenge with S. mansoni eggs. 8 In the present study we tested the capacity of CCR1−/− mice to generate type-1 and type-2 granulomas under anamnestic conditions using Ag bead challenge of presensitized mice. Anamnestic type-1 and type-2 inflammatory responses can be studied in mice by sensitizing with mycobacterial PPD or soluble SEA Ags, followed 2 weeks later by respective intravenous challenge with agarose beads covalently coupled to PPD or SEA. In these models, the antigen-coated beads embolize to the lung where they induce pulmonary granulomas mediated by type-1 and type-2 cytokines. 14,15,19
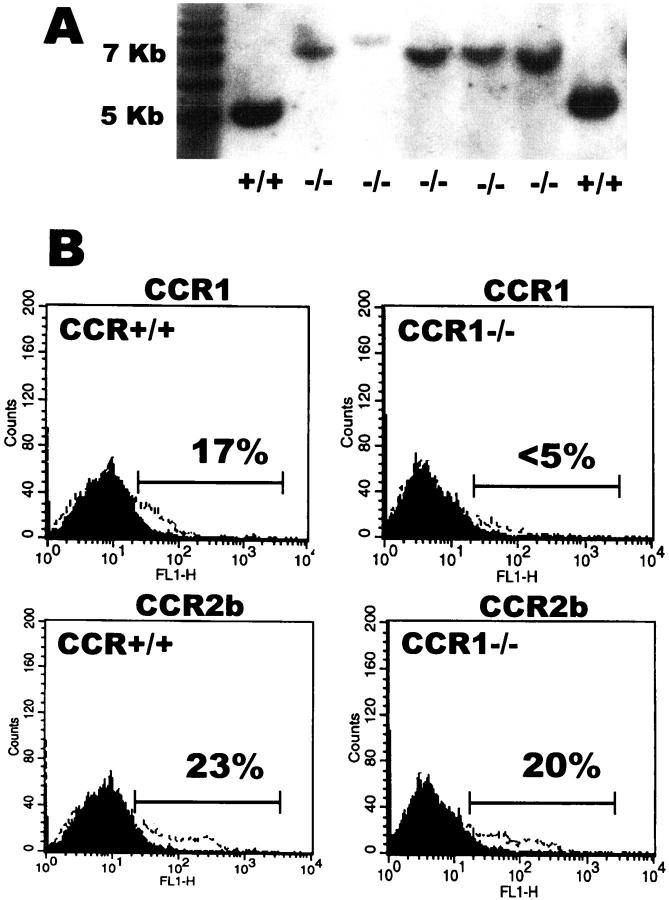
Mice with targeted disruption of the CCR1 gene were generated as previously described. 7 Southern blot analysis and direct flow cytometric detection was used to confirm CCR1 gene disruption. Wild-type mice displayed a normal 5-kb SacI digest fragment whereas knockout mice showed a 7-kb mutant form. In addition, direct staining of splenocytes indicated CCR1 protein expression was essentially ablated, whereas an independent CC chemokine receptor, CCR2b, was preserved (Figure 1) ▶ .
Figure 1.
Targeted mutation of the CCR1 gene results in abrogated CCR1 protein expression. A: Southern blot analysis of CCR1−/− and control wild-type mice. Mutants display an aberrant 7-kb SacI fragment on hybridization with target probe. B: Flow cytometric quantitation of CCR1 and CCR2b receptor expressing splenocytes from wild-type CCR+/+ and mutant CCR1−/− mice. Fluorescent intensity is shown on the abscissa. The dark-shaded areas represent the fluorescence with nonimmune control antibody. The open areas represent receptor-positive cells stained with receptor-specific antibody. A representative study is shown.
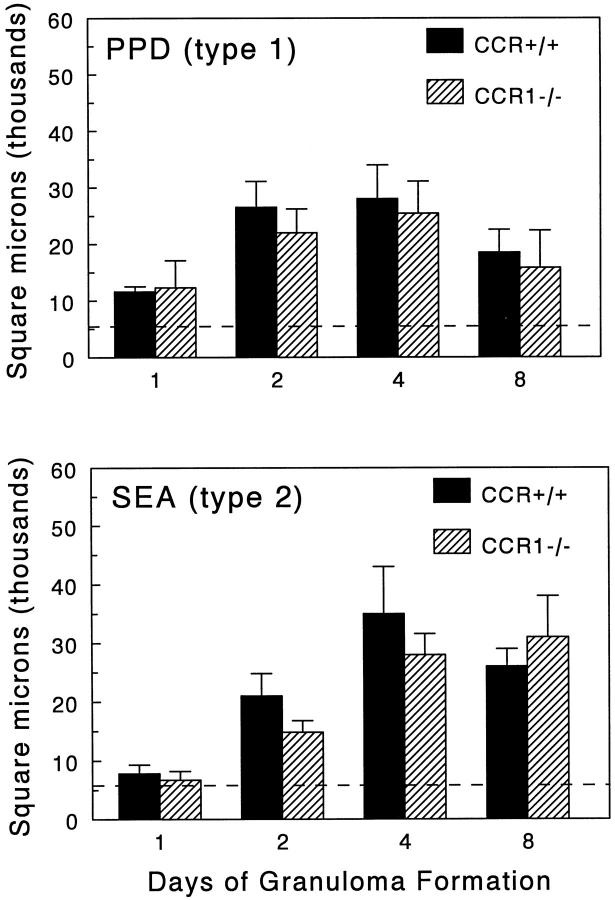
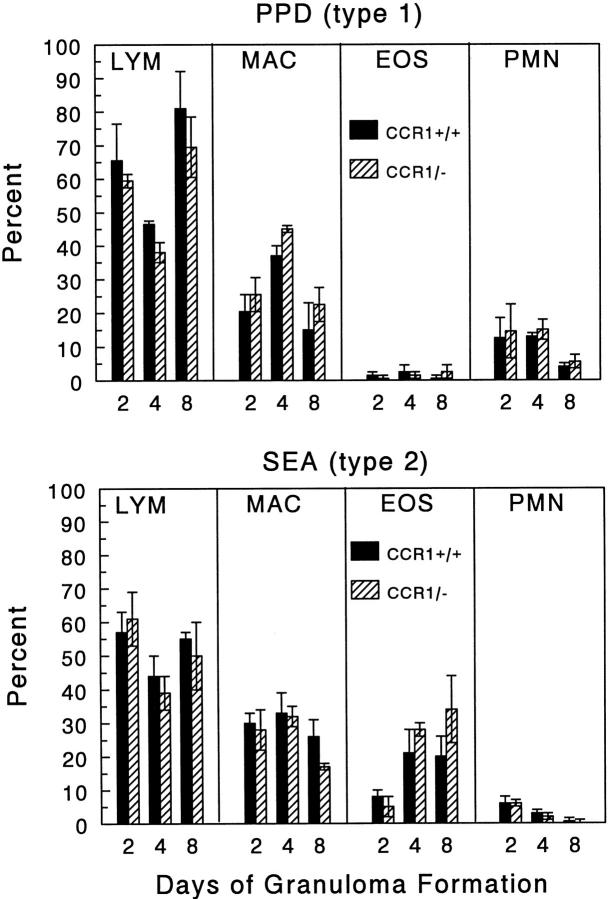
Granulomas were induced in CCR+/+ and CCR1−/− mice and then measured at 1, 2, 4, and 8 days of development. As shown in Figure 2 ▶ , CCR1−/− mice had no significant change in gross lesion dimensions under anamnestic conditions. Histologically, lesions seemed similar in the wild-type and mutant strains (Figure 3) ▶ . Likewise, when differential analysis was performed on dispersed lesions there was no statistically significant difference in leukocyte composition, however it was noted there was a trend to enhanced eosinophils in type-2 SEA bead lesions on day 8 (Figure 4) ▶ .
Figure 2.
Effect of CCR1 knockout on PPD (type-1) and SEA (type-2) bead granuloma formation. Pulmonary granulomas were induced, then sectioned lungs were subjected to computer-assisted morphometric analysis at the indicated time points. Bars are means ± SE of six to eight mice. Mean granuloma size was determined from an N of 20 lesions from each mouse. Solid bars, wild-type CCR+/+ mice; hatched bars, CCR1−/− mice; dashed line, indicates average area occupied by the bead alone.
Figure 3.
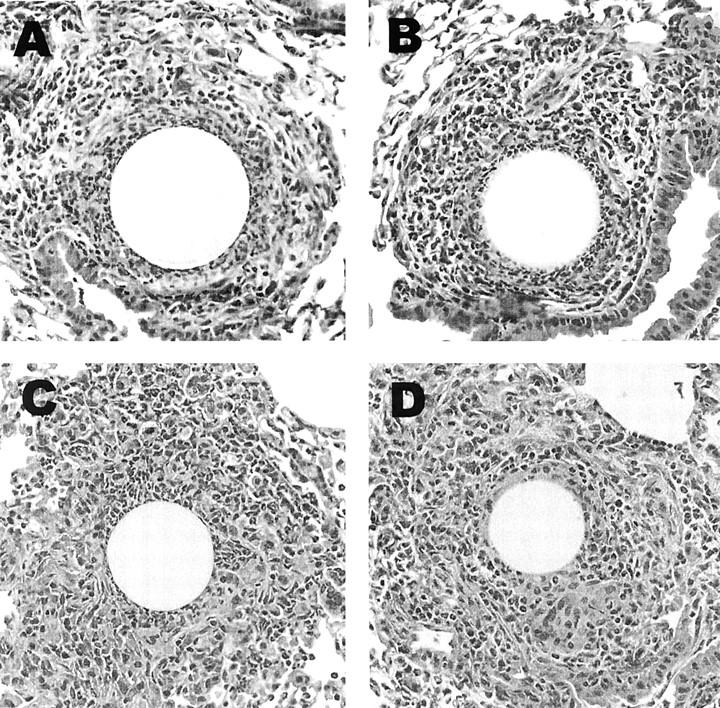
Histological appearance of PPD (type-1) and SEA (type-2) bead granulomas in wild-type CCR +/+ and mutant CCR1−/− mice. A: PPD bead lesion in CCR+/+ mouse. B: PPD bead lesion in CCR1−/− mouse. C: SEA bead lesion in CCR+/+ mouse. D: SEA bead lesion in CCR1−/− mouse. H&E stain. Original magnification, ×400.
Figure 4.
Effect of CCR1 knockout on PPD (type-1) and SEA (type-2) bead granuloma cellular composition. Pulmonary granulomas were isolated and digested at the indicated time points. Wright-stained cytospins were prepared from the single cell suspensions and differential analysis was performed. LYM, lymphocytes; MAC, macrophages and large mononuclear cells; EOS, eosinophils; PMN, polymorphonuclear neutrophils. Bars are means ± SE of six to eight mice. Solid bars, wild-type CCR+/+ mice; hatched bars, CCR1−/− mice.
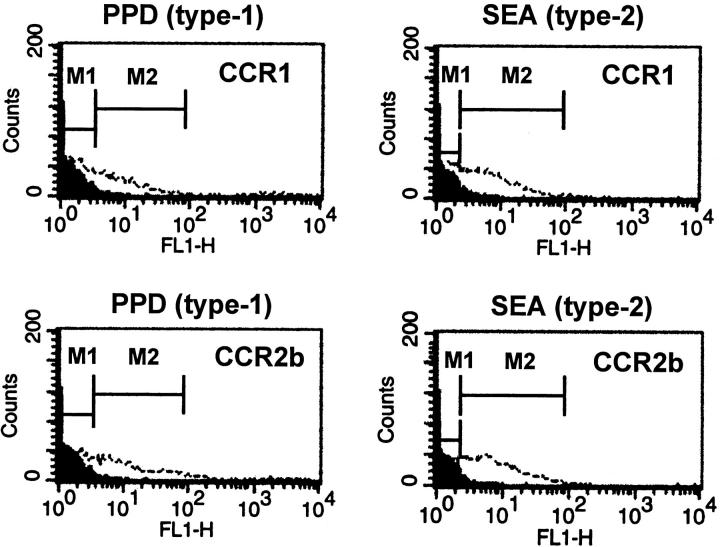
The failure of CCR1 knockout to abrogate inflammation may indicate that recruited granuloma cells do not express CCR1. To test this possibility, we assessed CCR1 and CCR2b expression on dispersed day 4 granuloma cells directly by flow cytometry. In CCR+/+ mice, both CCR1 and CCR2b were detected on granuloma cells in type-1 and type-2 lesions (Figure 5) ▶ . As expected, gating analysis indicated CCR1 was distributed among lymphoid and nonlymphoid cells (data not shown).
Figure 5.
Expression of chemokine receptors by cells from dispersed PPD (type-1) and SEA (type-2) granulomas. Both CCR1 and CCR2b were detected on granuloma cells from type-1 and type-2 lesions. Fluorescent intensity is shown on the abscissa. The dark-shaded areas represent the fluorescence with nonimmune control antibody. The open areas represent receptor-positive cells stained with receptor-specific antibody. The CCR1+ and CCR2b+ cells respectively represented 7 ± 0.6% and 10 ± 0.6% of type-1 lesions, and 9 ± 1.9% and 20 ± 3.5% of type-2 lesions.
CCR1 Knockout Impairs NK Cell Recruitment to Type-1 Pulmonary Granulomas
Leukocyte differential analysis in the Wright-stained cytospin preparation represents only a gross monitor of major leukocyte populations. Because lymphoid subpopulations have been reported to display differential expression of chemokine receptors, 2,4 we analyzed lymphocyte subpopulations in dispersed granuloma cells by one- and two-color flow cytometry to detect potential changes because of CCR1 deficiency. A representative flow cytometric analysis of the proportions of CD4+ (T helper), CD8+ (T cytotoxic), and CD19 (B cells) in day 4 types-1 and -2 granulomas is shown in Figure 6 ▶ . Knockout of CCR1 did not significantly affect these populations.
Figure 6.
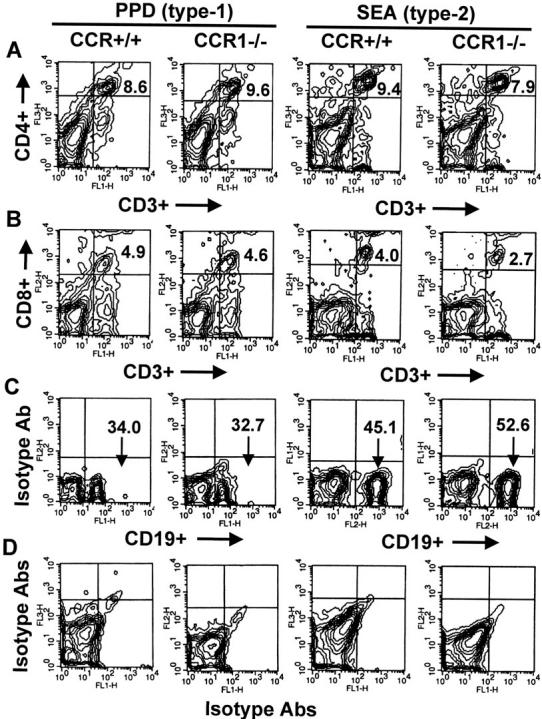
Effect of CCR1 knockout on lymphocyte subpopulation recruitment during PPD (type-1) and SEA (type-2) bead granuloma formation. Day 4-dispersed granuloma cells were stained with fluorescent-labeled antibodies to CD3, CD4, CD8, and CD19, then 20,000 cells were analyzed by two-color flow cytometry. Contour plots of a representative experiment are shown. Data were derived from analysis of gated lymphocytes. Columns compare CCR+/+ and CCR1−/− mice with either type-1 or type-2 lesions. A: CD4+ CD3+ T cells shown in upper right quadrants. B: CD8+ CD3+ T cells shown in upper right quadrants. C: CD19+ B cells shown in lower right quadrant. D: Isotype antibody controls. Similar results were obtained in three separate experiments. In each experiment granulomas were pooled from three to four mice.
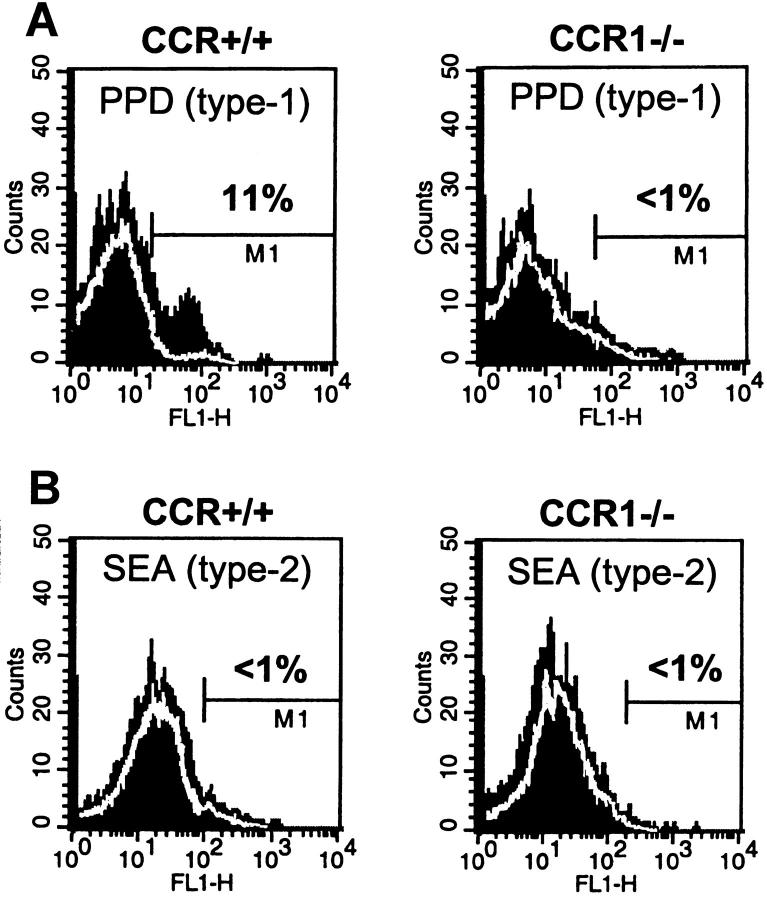
We next examined lymphoid cells expressing NK cell markers. The type-1 and type-2 responses were clearly different with regard to NK populations. Type-1 lesions contained proportionally more pan-NK+ cells than type-2 lesions, (ranging from 10 to 24% of gated lymphocytes in the former and <5% in the latter (Figure 7A and B) ▶ . Moreover, there was a clear effect of CCR1 deficiency with type-1 lesions of CCR1−/− mice displaying significantly decreased levels of NK cells. This finding was repeated using an independent NK1.1-specific antibody and dual staining analysis indicated that 60% of NK1.1+ cells from type-1 lesions bore CCR1 (data not shown). The latter was not surprising because there are several published reports that demonstrate NK cells display strong chemotactic responses to CCR1 ligands. 20,21
Figure 7.
Effect of CCR1 knockout on NK cell recruitment during PPD (type-1) and SEA (type-2) bead granuloma formation. Day 4-dispersed granuloma cells were stained with fluorescent-labeled control isotype or specific antibodies to pan-NK marker. Twenty thousand cells were analyzed by two-color flow cytometry. Histograms of a representative experiment are shown. Data were derived from analysis of gated lymphocytes. Dark-shaded area represents staining with pan-NK-specific antibody. White lines define background staining regions of isotype control antibody. A: Pan-NK+ in PPD (type-1) lesions from CCR+/+ and CCR1−/− mice. B: Pan-NK+ in SEA (type-2) lesions from CCR+/+ and CCR1−/− mice. Relative proportions of NK+ cells are shown. Similar results were obtained in three separate experiments. In each experiment granulomas were pooled from three to four mice.
CCR1 Knockout Abrogates Type-1 Cytokine Profiles in Reactive Draining Lymphoid Tissues
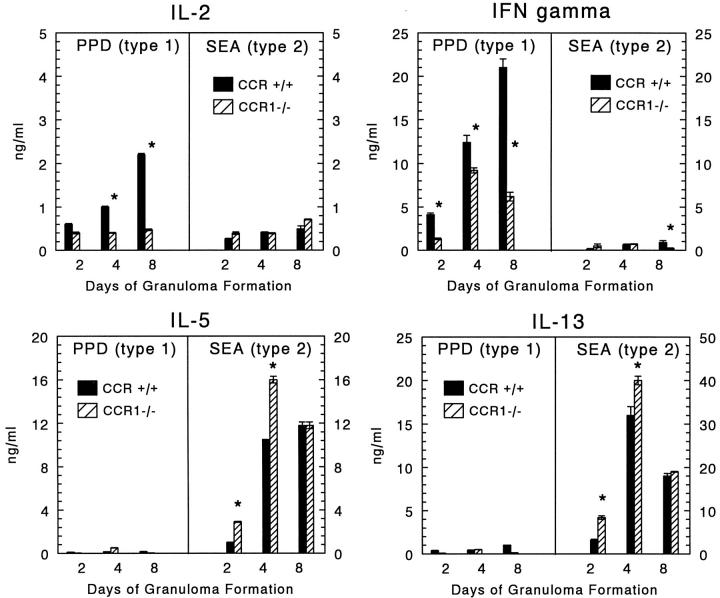
In addition to their chemotactic function, ligands of CCR1 are reported to have a co-stimulatory effect on lymphocyte proliferation and cytokine production. 22 In this regard, we cultured draining mediastinal lymph nodes in the presence of specific Ag then measured the levels of cytokines produced. Two representative Th1-associated cytokines, IFN-γ and IL-2, and two Th2-associated cytokines, IL-5 and IL-13, were measured during the course of granuloma formation and are shown in Figure 8 ▶ . Both CCR+/+ and CCR1−/− mice displayed polarized type-1 and type-2 cytokine profiles. However, IL-2 and IFN-γ levels were reduced in CCR1−/− mice during the type-1 response. There was even some decrease in the already low levels of IFN-γ produced during the type-2 response on day 8. Conversely, there was modest but significant augmentation of Th2-associated cytokines on days 2 and 4 during the type-2 response. These altered cytokine profiles could not be explained by changing proportions of lymphoid populations because flow cytometric analysis of the dispersed nodes from CCR+/+ and CCR1−/− mice revealed comparable percentages of CD3+CD4+, CD3+CD8+, and CD19+ cells. NK cells were consistently <1% of the draining lymph nodes of all mice (data not shown).
Figure 8.
Effect of CCR1 knockout on cytokine production by draining lymph node cultures during PPD (type-1) and SEA (type-2) bead granuloma formation. Bars are means ± SE of triplicate determinations. At each time point lymph node cells of three to four mice were pooled and cultured for 24 hours in the absence or presence of 5 μg/ml-specific Ag (PPD or SEA), then supernates were collected and frozen before assay. Similar results were obtained in three separate experiments. Asterisks indicate P < 0.05.
CCR1 Knockout Enhances Leukocytosis during Type-1 Inflammation
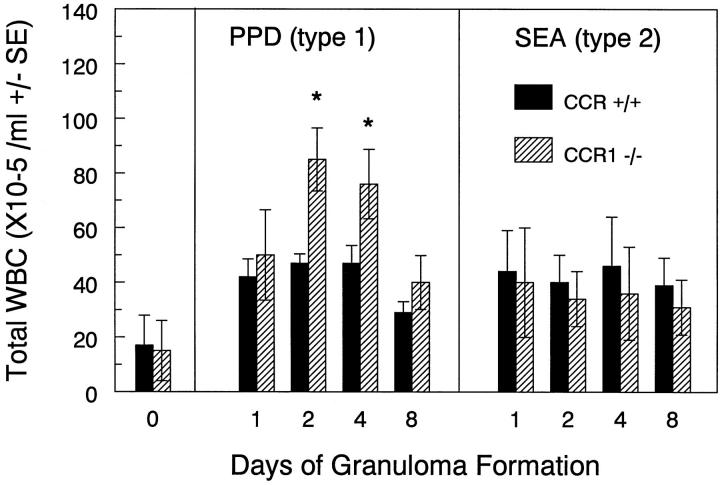
Because CCR1 ligands such as MIP-1α are reported to regulate lymphocyte recirculation and hematopoiesis, 23-25 we monitored blood leukocyte levels and composition. Figure 9 ▶ shows baseline and postchallenge blood leukocyte numbers in control and CCR1−/− mice. Baseline levels were comparable in unchallenged CCR+/+ and CCR1−/− mice. After PPD bead challenge total leukocyte counts achieved higher levels in CCR1−/− mice on days 2 and 4. This enhancement did not occur during the type-2 response to SEA bead challenge, suggesting that blood leukocyte levels were regulated differently in the two responses. When differential leukocyte analysis was performed the increased populations included lymphocytes, neutrophils, and monocytes, indicating that the observed increase in total counts in the type-1 response extended to lymphoid and nonlymphoid populations.
Figure 9.
Effect of CCR1 knockout on peripheral blood leukocyte counts during mycobacterial (type-1) and schistosomal (type-2) Ag bead granuloma formation. Blood was collected at the indicated time points and total leukocyte counts were determined by hemacytometry. Bars are means ± SD of six to eight individual mice. Asterisks indicate P < 0.05.
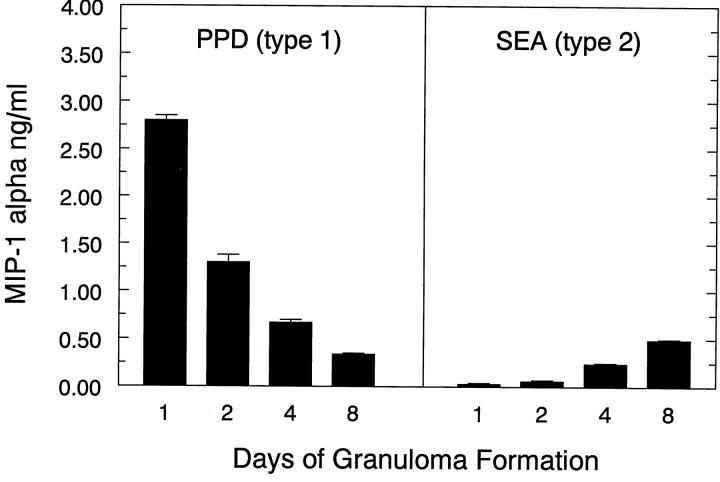
The differential effect of CCR1 knockout on the types-1 and -2 responses was potentially related to different levels of CCR1 agonist production in these models. This was particularly salient as we previously reported greater production of RANTES during the type-1 response. 17 In the present study, we examined levels of MIP-1α in draining lymph node cultures during the course of type-1 and type-2 granuloma formation. As shown in Figure 10 ▶ , MIP-1α was produced to a much greater degree in the PPD (type-1) as compared to the SEA (type-2) lymph node cultures. This was especially true during the early phase (1 to 2 days) of the response. It should be noted that levels of MIP-1α production were similar in CCR1−/− mice (data not shown). Thus, the time course and differential production of MIP-1α correlated well with the greater observed effects of CCR1 deficiency on lymph node events and blood leukocyte levels.
Figure 10.
Production of MIP-1α by draining lymph node cultures during PPD (type-1) and SEA (type-2) bead granuloma formation. Bars are means ± SE of triplicate determinations. Baseline MIP-1α levels (day 0) were 50 pg/ml or less. At each time point lymph node cells of three to four mice were pooled and cultured for 24 hours in the absence or presence of 5 μg/ml-specific Ag (PPD or SEA), then supernates were collected and frozen before assay of the indicated cytokines. Similar results were obtained in three separate experiments.
Discussion
To our knowledge, the present study represents the first comparative analysis of cell-mediated type-1 and type-2 pulmonary granuloma formation in mice with targeted disruption of the CCR1 gene. The product of this gene, CCR1, is a promiscuous receptor binding a number of known chemokines, including MIP-1α, MIP-3, MIP-5, RANTES, MCP-3, MIP-1γ, and mC10. 3,6 Consequently, it is potentially important in leukocyte recruitment events. Previous studies have reported both abrogation and enhancement of inflammatory responses by CCR1 disruption. These varied responses are most likely related to the different experimental models and target organs that were examined. Specifically, abrogation occurred in models of acute lung injury and cardiac allograft rejection, 7,9 whereas exacerbation was reported in a model of nephrotoxic nephritis. 10 In yet another report Gao and colleagues 8 demonstrated impaired primary schistosome egg granuloma formation in CCR1−/− mice, that was associated with enhanced IFN-γ production and reduced IL-4 production. In the present report, using models of highly polarized type-1- and type-2 cell-mediated granuloma formation, CCR1 knockout did not limit the ultimate extent of the inflammatory response. However, we did identify subtle changes indicating that CCR1 was contributing in part to local cellular recruitment, Th1/Th2 cytokine balance, and hematopoiesis.
With regard to cellular recruitment, we identified specific impairment of NK cell accumulation in type-1 granulomas, which normally harbor a significant component of these cells. However, the reduction of these cells did not significantly limit the overall extent of the inflammatory response indicating that these cells are not essential to the type-1 granuloma. We presently postulate that the NK cell component represents a vestigial background response that is primarily overshadowed by the activity of Ag-specific Th1 cells. However, in responses in which NK cells provide a greater contribution, the effect of CCR1 knockout may be more profound, possibly explaining some of the conflicting effects of CCR1 knockout. We have identified potential CCR1 agonists produced during lung granuloma formation, such as MIP-1α, RANTES, and MCP-3. 17,26,27 Although these chemokines may have a direct role in CCR1-mediated NK cell recruitment, we cannot rule out indirect effects. For example, CXCR3 ligands can also mediate NK cell chemotaxis 20,21 and CCR1 knockout may impair production of CXCR3 ligands, thereby indirectly impairing NK cell recruitment. Other indirect effects causing reduced NK cell accumulation could include impaired NK proliferation or enhanced apoptosis. 28 Also, our understanding of lymphocyte migration efficiency in vivo is limited. Both NK and Th1 cells are reported to respond to CCR1 and CXCR3 ligands, but if NK cells require greater stimulation for efficient migration then they could be at a disadvantage if a chemokine receptor is compromised.
Our analysis revealed that CCR1 contributed to Th1/Th2 cytokine balance in draining lymph node cultures. In three separate experiments, knockout was associated with impaired Th1 and enhanced Th2 cytokines, which did not compromise the type-1 inflammatory response although we noted a tendency to enhanced eosinophil recruitment in type-2 lesions. Our results differ from studies of Gao and colleagues 8 who reported impaired inflammation, augmented IFN-γ, and reduced Th2 cytokines on primary challenge with S. mansoni eggs. This discrepancy is likely because of different experimental conditions, since that study did not examine the anamnestic secondary response to S. mansoni Ags. Because the granulomatous response to S. mansoni eggs shifts from a Th0/Th1 to a Th2-dominant response, 29 it suggests that the early primary stage is more sensitive to CCR1 deficiency. Likewise, it would relate to the finding of Lukacs and co-workers 26 who reported that the primary response to S. mansoni eggs is more dependent on the CCR1 ligand, MIP-1α, than the secondary response. In any case, our findings are fully consistent with several in vitro studies indicating that CCR1 ligands support Th1 cytokine production. 11-13,30 Furthermore, the observed enhancement of type-2 cytokines could result from alleviation of IFN-γ or chemokine-mediated cross-regulation. 17,31 Taken together, the in vivo and in vitro findings argue that CCR1 ligands tend to promote Th1 cytokine production.
In CCR1−/− mice we observed enhanced blood leukocytosis during type-1 but not type-2 granuloma formation. The difference was likely related to the greater production of CCR1 agonists such as MIP-1α during Th1 responses. 17,32 The elevation itself is consistent with a recent in vitro study of Broxmeyer and colleagues 25 demonstrating that CCR1 agonists inhibit granulocyte-monocyte colony stimulating factor (GM-CSF)-mediated myeloid progenitor cell mobilization. Because granuloma induction is associated with release, 33 CCR1−/− mice might be predicted to have elevated blood leukocyte counts during granuloma formation. Interestingly, we have observed a virtually identical pattern of leukocytosis that occurs in MIP-1α knockout mice. These findings would be consistent with the notion that CCR1 and agonists regulate hematopoietic dynamics. However, an additional explanation for the elevated blood leukocytes might include hampered lymphocyte recirculation causing lymphocytes to accumulate in the circulating pool as lymphocytes were an important component of the increased leukocytes in blood. This could reflect impaired CCR1 agonist-mediated trafficking to secondary lymphoid tissues because the CCR1 agonists MIP-1α and MIP-1β have been shown to be important for T cell emigration to lymph nodes. 23
Finally, a comparison should be made between the effect of CCR1 and CCR2 knockout. The latter primarily impaired early phase monocyte/macrophage and to some extent CD4+ T cell accumulation at sites of granuloma formation, 16,34 indicating different recruitment roles for CCR1 and CCR2 agonists. However in both types of mutants, overall granuloma formation was ultimately established, despite transient or selective defects. Interestingly, CCR2 knockout was similarly associated with impaired IFN-γ production by draining lymph node cultures, fully consistent with in vitro studies demonstrating that both CCR1 and CCR2 agonists can promote IFN-γ production. 13 In addition, in preliminary studies we have noted significantly reduced MIP-1α in lymphoid cultures of CCR2−/− mice suggesting that the production of CCR1 agonists may be dependent on upstream CCR2 ligation.
In summary, although CCR1 disruption failed to significantly limit polarized forms of pulmonary granuloma formation, underlying defects were identified primarily in the type-1 response to mycobacterial Ags, consisting of impaired NK cell recruitment, diminished type-1 cytokine production and altered blood leukocyte dynamics. These findings suggest that chemokine redundancy and cytokine reserve make the anamnestic T cell-mediated inflammatory response resistant to CCR1 disruption. However, CCR1 clearly contributes to local, regional, and systemic immunoinflammatory events.
Acknowledgments
We thank Aron Pollack and Stacey Haller for their expert histological assistance.
Footnotes
Address reprint requests to Stephen W. Chensue, M.D., Ph.D., Pathology and Laboratory Medicine 113, Veterans Affairs Medical Center, 2215 Fuller Rd., Ann Arbor, MI 48105. E-mail: schensue@umich.edu.
Supported by National Institutes of Health-National Institute of Allergy and Infectious Diseases grant AI43460 and in part by National Institutes of Health grants HL52773 and HL56306. Dr. Curtis is a Career Investigator of the American Lung Association of Michigan. Schistosome life stages were supplied through National Institutes of Health-National Institute of Allergy and Infectious Diseases Contract N01-AI-55270.
References
- 1.Baggiolini M: Chemokines and leukocyte traffic. Nature 1998, 392:565-568 [DOI] [PubMed] [Google Scholar]
- 2.Kim CH, Broxmeyer HE: Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol 1999, 65:6-15 [DOI] [PubMed] [Google Scholar]
- 3.Howard OM, Oppenheim JJ, Wang JM: Chemokines as molecular targets for therapeutic intervention. J Clin Immunol 1999, 19:280-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A, Mackay CR: Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today 1998, 19:568-574 [DOI] [PubMed] [Google Scholar]
- 5.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL: The role of CXC chemokines as regulators of angiogenesis. Shock 1995, 4:155-160 [DOI] [PubMed] [Google Scholar]
- 6.Wells TN, Power CA, Proudfoot AE: Definition, function and pathophysiological significance of chemokine receptors. Trends Pharmacol Sci 1998, 19:376-380 [DOI] [PubMed] [Google Scholar]
- 7.Gerard C, Frossard JL, Bhatia M, Saluja A, Gerard NP, Lu B, Steer M: Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest 1997, 100:2022-2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao JL, Wynn TA, Chang Y, Lee EJ, Broxmeyer HE, Cooper S, Tiffany HL, Westphal H, Kwon-Chung J, Murphy PM: Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med 1997, 185:1959-1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao W, Topham PS, King JA, Smiley ST, Csizmadia V, Lu B, Gerard CJ, Hancock WW: Targeting of the chemokine receptor CCR1 suppresses development of acute and chronic cardiac allograft rejection. J Clin Invest 2000, 105:35-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topham PS, Csizmadia V, Soler D, Hines D, Gerard CJ, Salant DJ, Hancock WW: Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis. J Clin Invest 1999, 104:1549-1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA: Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol 1997, 158:4129-4136 [PubMed] [Google Scholar]
- 12.Siveke JT, Hamann A: T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol 1998, 160:550-554 [PubMed] [Google Scholar]
- 13.Lukacs NW, Chensue SW, Karpus WJ, Lincoln P, Keefer C, Strieter RM, Kunkel SL: C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am J Pathol 1997, 150:1861-1868 [PMC free article] [PubMed] [Google Scholar]
- 14.Chensue SW, Warmington K, Ruth J, Lincoln P, Kuo MC, Kunkel SL: Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol 1994, 145:1105-1113 [PMC free article] [PubMed] [Google Scholar]
- 15.Chensue SW, Warmington KS, Ruth JH, Lincoln P, Kunkel SL: Cytokine function during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Local and regional participation of IFN-gamma, IL-10, and TNF. J Immunol 1995, 154:5969-5976 [PubMed] [Google Scholar]
- 16.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF: Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997, 100:2552-2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chensue SW, Warmington KS, Allenspach EJ, Lu B, Gerard C, Kunkel SL, Lukacs NW: Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol 1999, 163:165-173 [PubMed] [Google Scholar]
- 18.Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL: Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol 1996, 157:4602-4608 [PubMed] [Google Scholar]
- 19.Chensue SW, Ruth JH, Warmington K, Lincoln P, Kunkel SL: In vivo regulation of macrophage IL-12 production during type 1 and type 2 cytokine-mediated granuloma formation. J Immunol 1995, 155:3546-3551 [PubMed] [Google Scholar]
- 20.Taub DD, Sayers TJ, Carter CR, Ortaldo JR: Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol 1995, 155:3877-3888 [PubMed] [Google Scholar]
- 21.Maghazachi AA, al-Aoukaty A, Schall TJ: C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J Immunol 1994, 153:4969-4977 [PubMed] [Google Scholar]
- 22.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ: Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol 1996, 156:2095-2103 [PubMed] [Google Scholar]
- 23.Tedla N, Wang HW, McNeil HP, Di Girolamo N, Hampartzoumian T, Wakefield D, Lloyd A: Regulation of T lymphocyte trafficking into lymph nodes during an immune response by the chemokines macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta. J Immunol 1998, 161:5663-5672 [PubMed] [Google Scholar]
- 24.Parker AN, Pragnell IB: Inhibitors of haemopoiesis and their potential clinical relevance. Blood Rev 1995, 9:226-233 [DOI] [PubMed] [Google Scholar]
- 25.Broxmeyer HE, Cooper S, Hangoc G, Gao JL, Murphy PM: Dominant myelopoietic effector functions mediated by chemokine receptor CCR1. J Exp Med 1999, 189:1987-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukacs NW, Kunkel SL, Strieter RM, Warmington K, Chensue SW: The role of macrophage inflammatory protein 1 alpha in Schistosoma mansoni egg-induced granulomatous inflammation. J Exp Med 1993, 177:1551-1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL: Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-gamma and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks. J Immunol 1997, 159:3565-3573 [PubMed] [Google Scholar]
- 28.Maghazachi AA, al-Aoukaty A, Schall TJ: CC chemokines induce the generation of killer cells from CD56+ cells. Eur J Immunol 1996, 26:315-319 [DOI] [PubMed] [Google Scholar]
- 29.Pearce EJ, La Flamme A, Sabin E, Brunet LR: The initiation and function of Th2 responses during infection with Schistosoma mansoni. Adv Exp Med Biol 1998, 452:67-73 [DOI] [PubMed] [Google Scholar]
- 30.Taub DD, Ortaldo JR, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ: Beta chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J Leukoc Biol 1996, 59:81-89 [DOI] [PubMed] [Google Scholar]
- 31.Chensue SW, Warmington KS, Ruth J, Lincoln PM, Kunkel SL: Cross-regulatory role of interferon-gamma (IFN-gamma), IL-4 and IL-10 in schistosome egg granuloma formation: in vivo regulation of Th activity and inflammation. Clin Exp Immunol 1994, 98:395-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrum S, Probst P, Fleischer B, Zipfel PF: Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J Immunol 1996, 157:3598-3604 [PubMed] [Google Scholar]
- 33.Bergeron A, Bonay M, Kambouchner M, Lecossier D, Riquet M, Soler P, Hance A, Tazi A: Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J Immunol 1997, 159:3034-3043 [PubMed] [Google Scholar]
- 34.Warmington KS, Boring L, Ruth JH, Sonstein J, Hogaboam CM, Curtis JL, Kunkel SL, Charo IR, Chensue SW: Effect of C-C chemokine receptor 2 (CCR2) knockout on type-2 (schistosomal antigen-elicited) pulmonary granuloma formation: analysis of cellular recruitment and cytokine responses. Am J Pathol 1999, 154:1407-1416 [DOI] [PMC free article] [PubMed] [Google Scholar]