Abstract
Ewing’s sarcoma is a primitive highly malignant tumor of bone and soft tissues usually metastasizing to bone, bone marrow, and lung. Growth factor receptors and their ligands may be involved in its growth and dissemination. We analyzed the expression of c-kit and its ligand stem cell factor (SCF) in a panel of six Ewing’s sarcoma cell lines. All cell lines exhibited substantial levels of surface c-kit expression, and five of six displayed transmembrane SCF on the cell surface. Expression of c-kit was down-modulated in all lines by exposure to exogenous SCF. The SCF treatment was able to confer to cells a growth advantage in vitro, due both to an increase in cell proliferation and to a reduction in the apoptotic rate. When used in the lower compartment of a migration chamber, SCF acted as a strong chemoattractant for Ewing’s sarcoma cells. The pretreatment of cells with SCF reduced their chemotactic response to SCF. In athymic nude mice, Ewing’s sarcoma cells injected intravenously metastasized to the lung and to a variety of extrapulmonary sites, including bone and bone marrow. Metastatic sites resembled those observed in Ewing’s sarcoma patients and corresponded to SCF-rich microenvironments. The in vitro pretreatment of cells with SCF strongly reduced the metastatic ability of Ewing’s sarcoma cells, both to the lung and to extrapulmonary sites. This could be dependent on the down-modulation of c-kit expression observed in SCF-pretreated cells, leading to a reduced sensitivity to the chemotactic and proliferative actions of SCF. Our results indicate that the response to SCF mediated by c-kit may be involved in growth, migration, and metastatic ability of Ewing’s sarcoma cells.
Ewing’s sarcoma is a primitive malignant tumor of bone and soft tissues preferentially arising in children and young adults. It shows an extremely aggressive behavior and rapidly disseminates to bones, bone marrow, and lungs. 1-3 Although the histogenesis of Ewing’s sarcoma is still controversial, 4,5 recent molecular and cellular studies have helped in defining common genetic features, 6,7 antigenic profiles, 8-10 and receptor patterns. 2,11,12
Tumor growth, invasion, and metastasis can be influenced by several different interactions with the organ microenvironment both at the site of origin and in distal organs. 13-15 Autocrine and paracrine stimulation of growth factor receptors by their ligands may play a crucial role in malignancy. In Ewing’s sarcoma the loop based on insulin-like growth factor-I receptor has been shown to be constantly present and to strongly affect tumor growth both in vitro and in vivo. 16
Among the many growth factor/receptor systems, triggering of the c-kit receptor by its ligand stem cell factor (SCF) exerts multiple effects both in development and adult life supporting survival, proliferation, migration, and homing of hematopoietic cells, mast cells, melanocyte precursors, and primordial germ cells. 17
SCF can exist in two forms deriving from two alternatively spliced mRNAs that give rise either to a transmembrane protein or to a soluble factor, depending on the presence of exon 6, which codes for a protease recognition site. 18 Membrane-bound SCF has biological activities distinct from those of the soluble form. Transmembrane SCF enhances stability of the receptor c-kit, avoids its rapid down-regulation and sustains longer c-kit kinase activity, appearing much more potent than its soluble counterpart. The difference in activity between the soluble and transmembrane forms was attributed to a different kinetic of internalization and degradation of the receptor/ligand complex. 19 Moreover, membrane-bound SCF has been shown to mediate cell-cell adhesion through interaction with its receptor c-kit. 20,21 Finally, the presence of a cytoplasmic domain (36 amino acids) in transmembrane SCF suggested that it could transduce signals, either by itself or in association with other molecules. 18,22
Expression of c-kit and/or of its ligand SCF has been documented in several tumor systems, including small cell lung carcinoma, 23 gastrointestinal stromal tumor, 24 melanoma, 25 neuroblastoma, 26-28 rhabdomyosarcoma, 29 and Ewing’s sarcoma. 12 c-kit expression alone was not predictive of SCF responsiveness, and autocrine stimulation of growth has been shown to occur only in few cases. 23,26-30
In this article, we analyzed the expression of the c-kit/SCF system in Ewing’s sarcoma cells and studied its involvement in proliferation, apoptosis, migration, and metastasis.
Materials and Methods
Cell Lines
The Ewing’s sarcoma cell lines SK-ES and RD-ES, and the Askin’s tumor cell line SK-N-MC were obtained from the American Type Culture Collection (Rockville, MD). The Ewing’s sarcoma cell lines TC-71 and 6647 were kindly provided by T. J. Triche (Children’s Hospital, Los Angeles, CA). The primitive neuroectodermal tumor cell line LAP-35 was previously established at Istituti Ortopedici Rizzoli (Bologna, Italy). The human megakaryoblastic cell line M-07e 31 (kindly provided by Dr. L. Pegoraro, University of Turin, Turin, Italy) was used as positive control for c-kit expression.
Cells were routinely cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS). Cells were maintained at 37°C in a humidified 5% CO2 atmosphere. All media constituents were purchased from Life Technologies (Milan, Italy).
SCF Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was isolated from cells cultured in IMDM plus 10% FBS. Extraction of RNA and cDNA synthesis were performed as reported previously. 9 RT-PCR conditions for human SCF 32 are: forward primer 5′-ATTCAAGAGCCCAGAACCCA-3′, reverse primer 5′-CTGTTACCAGCCAATGTACG-3′, annealing temperature 60°C. The expected size of the specific SCF amplification products was 494 bp for the soluble isoform including exon 6 coding for the proteolytic cleavage site, and 409 bp for the transmembrane isoform not containing exon 6. RT-PCR for glyceraldehyde-3-phosphate-dehydrogenase gene (Clontech, Palo Alto, CA) was also performed to demonstrate mRNA integrity.
Detection of c-kit and Transmembrane SCF Expression
c-kit and SCF membrane expression was evaluated by indirect immunofluorescence and cytofluorometric analysis. Briefly, cells from subconfluent cultures grown in IMDM plus 1% FBS for 48 hours were harvested by treatment with 5 mmol/L ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid in Ca2+/Mg2+-free phosphate-buffered saline (PBS), washed with 1% bovine serum albumin (BSA), and incubated with the anti-c-kit monoclonal antibody YB5.B8 (Pharmingen, San Diego, CA) or anti-human SCF monoclonal antibody (Genzyme, Cambridge, MA). After washing with PBS-BSA, cells were incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin antiserum (Kierkegaard & Perry Laboratories, Gaithersburg, MD). Cells were again washed and resuspended in PBS containing 1 μg/ml ethidium bromide to exclude dead cells from analysis. Cell surface fluorescence was then evaluated with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Detection of Soluble SCF
To detect secretion of SCF, cells were seeded in 25-cm 2 flasks in IMDM plus 10% FBS. Culture medium was collected 96 and 120 hours later. Soluble SCF was determined by the enzyme-linked immunosorbent assay test Quantikine (R&D System, Minneapolis, MN).
In Vitro Cell Growth
To study the effects of exogenous SCF, 20,000 cells/cm 2 were seeded in 25-cm 2 flasks in IMDM plus 1% FBS with or without 10 to 100 ng/ml recombinant human SCF (PeproTech, Rocky Hill, NJ). Every 24 hours cultures were harvested with trypsin-ethylenediaminetetraacetic acid (Life Technologies) without discharging cells grown in suspension. The number of viable cells was determined by trypan blue dye exclusion. SCF containing medium was renewed after 72 hours of culture.
BrdUrd Labeling Index
The 6647 cell line, which showed the highest c-kit expression, was used to analyze the proportion of cells in S phase after treatment with exogenous SCF. Cells were seeded at a concentration of 20,000/cm 2 in 25-cm 2 flasks in IMDM plus 1% FCS with or without 10 ng/ml SCF (PeproTech). After 48 hours, cell cultures were incubated with 10 μmol/L BrdUrd (Sigma, St. Louis, MO) for 3 hours in a 5% CO2 atmosphere at 37°C. Harvested cells were fixed in 70% ethanol for 30 minutes and DNA was denaturated with 2 N HCl for 30 minutes at room temperature. After washing with 0.1 mol/L Na2B4O7 (pH 8.5) cells were processed for indirect immunofluorescence with the mouse anti-BrdUrd antibody (Euro-Diagnostics, Milan, Italy) and then analyzed by FACScan flow cytometer.
Apoptosis
Control or SCF-treated cultures were harvested without discharging cells in the supernatants. After staining with Hoechst 33342 (Merck, Milan, Italy) cells were morphologically examined at high-power magnification in a Leica DM microscope. Cells with three or more chromatin fragments or with condensed nucleus were considered apoptotic. 9 At least 500 elements were evaluated for each sample.
Migration Assay
Migration assay was made using Transwell chambers (Costar, Cambridge, MA) with 8-μm pore size, polyvinylpyrrolidone-free polycarbonate filters. IMDM plus 1% FBS alone or supplemented with 0.1 to 10 ng/ml SCF was put in the lower compartment, after which 5 × 10 5 cells resuspended in IMDM plus 1% FBS were seeded in the upper compartment and incubated for 5 hours or overnight at 37°C in a 5% CO2 atmosphere. Cells that migrated through the filter to reach the lower chamber were counted at the inverted microscope. A checkerboard analysis was also performed. 33
Mice and in Vivo Studies
Athymic Crl:nu/nu(CD-1)BR female mice (4 to 6 weeks old) were purchased from Charles River (Calco, Italy). To assess experimental metastatic ability, nude mice were pretreated intravenously with 0.4 ml of a 1:30 dilution of anti-asialo GM1 antiserum (Wako, Dusseldorf, Germany). 34 Twenty-four hours later, 2 × 10 6 cells pretreated or not with 10 ng/ml of SCF for 48 hours were injected into a lateral tail vein. All animals were killed 31 days after cell injection. Lungs (stained with black India ink to better outline metastasis) were fixed in Fekete’s solution. All of the other organs: liver, kidneys, adrenals, ovaries, uterus, lymph nodes, bones, skeletal muscles, and brain were carefully inspected for metastasis and fixed in 10% phosphate-buffered formalin. In a second set of experiments mice, either injected with control or with SCF-pretreated cells, were individually sacrificed, for ethical reasons, when symptoms of metastatic growth became evident: time at sacrifice was recorded as survival time. Care of mice and experimental protocols were in accordance with the European Community and Italian guidelines.
Histology and Immunohistochemistry
Bones suspected to have metastases were fixed in 10% phosphate-buffered formalin and decalcified in a solution containing 10% ethylenediaminetetraacetic acid, pH 7.4, before embedding into paraffin. Morphological assessment was performed on 5-μm sections after staining with hematoxylin and eosin. Furthermore, the immunohistochemical detection of p30/32MIC2 (CD99) antigen, 8 by means of the avidin-biotin-peroxidase reaction with O13 monoclonal antibody (1:50; Signet, Dedham, MA), was also used to confirm the presence of Ewing’s sarcoma cells.
Results
c-kit Expression by Ewing’s Sarcoma
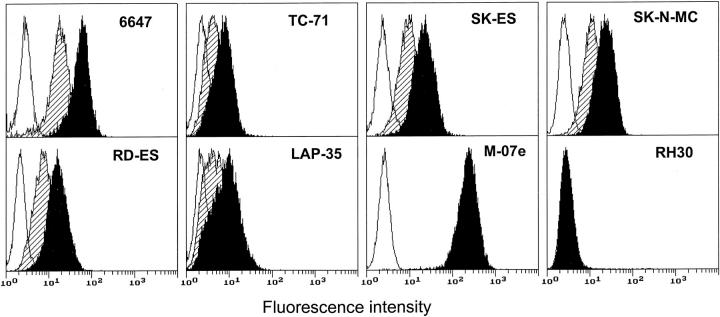
By means of indirect immunofluorescence and cytofluorometric analysis we studied the expression of c-kit protein on a panel of six Ewing’s sarcoma cell lines. All showed specific expression of the c-kit protein as indicated by the cytofluorometric profile of the cells stained with the anti-c-kit antibody that did not overlap the profile of the cells stained with the secondary antibody alone (Figure 1) ▶ . The level of c-kit expression of the positive control M-07e (a megakaryoblastic cell line which is one of the strongest c-kit expressors known) and of the negative control RH30 (a rhabdomyosarcoma cell line) are reported for comparison. In our panel of Ewing’s sarcoma cell lines the highest expression was shown by the 6647 cell line (mean fluorescence intensity in arbitrary units 52.5 ± 3.4, n = 11), and the lowest by the TC-71 cell line (mean fluorescence intensity 10.0 ± 0.6, n = 3), but in general c-kit showed a homogeneous pattern of expression among the Ewing’s sarcoma cell lines analyzed. Treatment with exogenous SCF at 10 ng/ml for 48 hours induced down-regulation of the c-kit protein expression in all of the Ewing’s sarcoma cell lines (Figure 1) ▶ .
Figure 1.
Cytofluorometric analysis of c-kit expression in human Ewing’s sarcoma cells with or without in vitro SCF pretreatment. Open profile represents cells stained with secondary antibody alone; solid profile represents cells stained with the anti-c-kit antibody; shaded profile represents cells cultured in the presence of SCF (10 ng/ml for 48 hours) and stained with the anti-c-kit antibody. The megakaryoblastic cell line M-07e and the rhabdomyosarcoma cell line RH30 were used as positive and negative controls, respectively. In each panel, the ordinate represents the number of cells. Data from an experiment representative of at least two similar experiments are shown.
In fresh Ewing’s tumor specimens expression of c-kit and SCF mRNA was demonstrated by RT-PCR. 12 Our preliminary immunohistochemical analysis of few cases of Ewing’s sarcoma biopsies also revealed at tissue level the presence of c-kit protein (data not shown).
SCF Expression
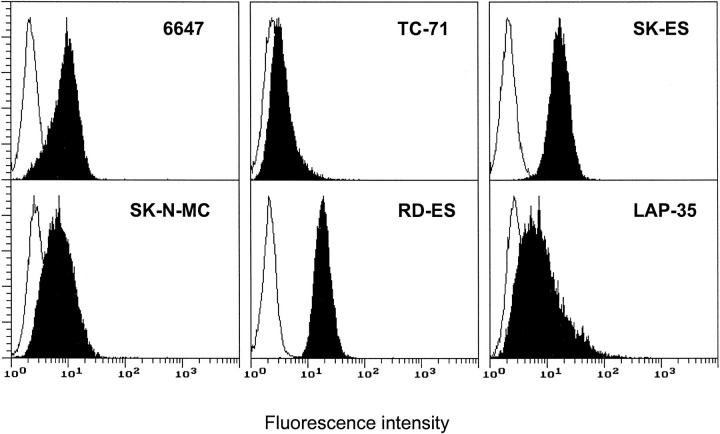
Five of six Ewing’s sarcoma cell lines displayed transmembrane SCF as detected by indirect immunofluorescence and cytofluorometric analysis (Figure 2) ▶ . Expression of membrane-bound SCF was higher in RD-ES and SK-ES cell lines than in 6647, SK-N-MC, and LAP-35 cell lines. The TC-71 cell line was almost negative for transmembrane SCF protein. None of the cell lines showed detectable levels of soluble SCF in supernatants analyzed by enzyme-linked immunosorbent assay. However, all lines showed by RT-PCR the presence of mRNA for both SCF isoforms, respectively, the soluble form (494-bp product) and the transmembrane form (409-bp product) (data not shown).
Figure 2.
Cytofluorometric analysis of transmembrane SCF expression in human Ewing’s sarcoma cells. Open profile represents cells stained with secondary antibody alone; solid profile represents cells stained with the anti-SCF antibody. In each panel, the ordinate represents the number of cells. Data from an experiment representative of at least two similar experiments are shown.
Response of Ewing’s Sarcoma Cells to Exogenous SCF
The interaction of SCF with c-kit has multiple effects on normal and transformed cells of different lineages. We analyzed some biological responses that could be relevant for tumor growth and metastatic spread, namely: 1) stimulation of cell growth as a balance of proliferation and apoptosis, 2) morphological changes, 3) induction of cell motility and migration.
Treatment of the 6647 cell line with 10 ng/ml of SCF induced a significant increase in cell growth at low serum concentration (Figure 3A) ▶ . Higher SCF concentrations (up to 100 ng/ml) did not further increase cell growth (data not shown). The growth advantage became evident after 48 hours of treatment. At the same time an increase in DNA synthesis was observed, with 43% BrdUrd-labeled cells in control culture versus 56% in SCF-treated culture. Cultures treated with SCF also showed a significantly lower proportion of apoptotic cells as compared to control cultures (Figure 3B) ▶ . Therefore, at low serum concentration, the stimulation of cell growth by exogenous SCF was the result of two actions of SCF: induction of cell proliferation and protection from apoptosis. No effect on growth was seen in the other cell lines having a lower expression of c-kit (data not shown).
Figure 3.
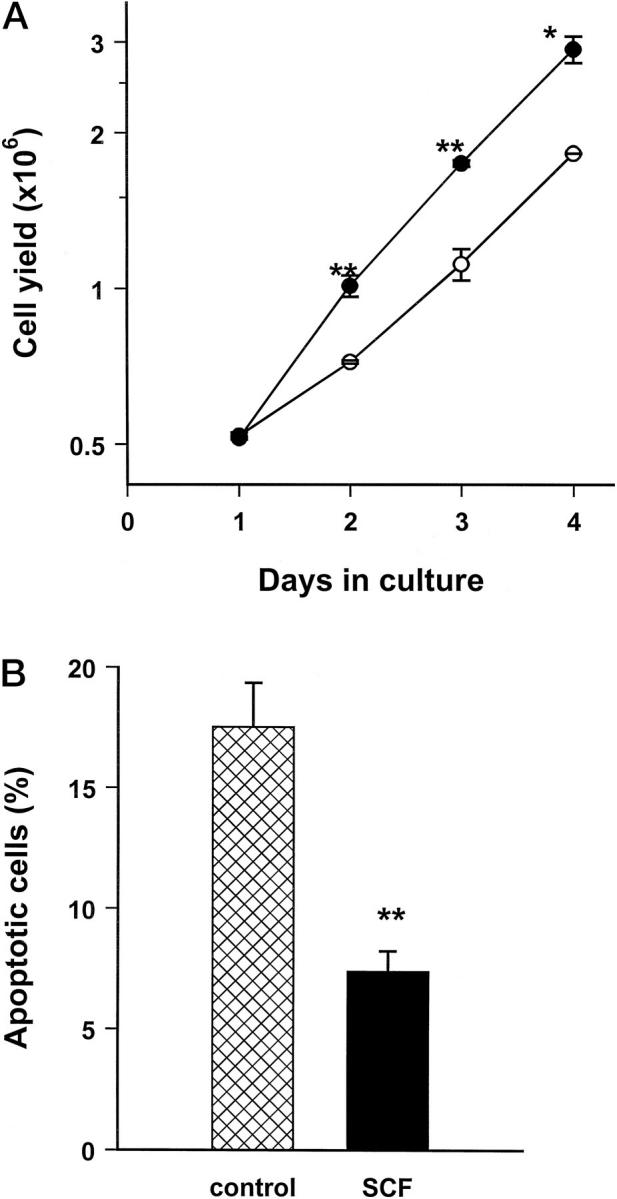
SCF effects on in vitro growth properties of human Ewing’s sarcoma cells. A: In vitro growth of 6647 cells untreated (open symbols) or treated with 10 ng/ml of recombinant human SCF (closed symbols). Each point represents the mean ± SE of three independent experiments. B: Protection from apoptosis by SCF treatment of 6647 cells, as evaluated by morphological analysis of condensed and fragmented nuclei after staining with Hoechst 33342. Each point represents the mean ± SE of three independent experiments. Significance: **, P < 0.01; *, P < 0.05, Student’s t-test.
The adherence of 6647 cells to plastic is poor. Cultures mainly consist of small round cells preferentially growing in spheroidal, tightly adherent cellular aggregates. Treatment with exogenous SCF rapidly induced a flattened morphology, with a stronger adhesion to the plastic surface and larger cellular dimensions (Figure 4) ▶ . When cells were harvested and resuspended, untreated control cells had a mean diameter of 14.4 ± 0.72 μm (n = 3), whereas SCF-treated cells had a mean diameter of 17.6 ± 0.29 μm (n = 3; P < 0.05, Student’s t-test).
Figure 4.
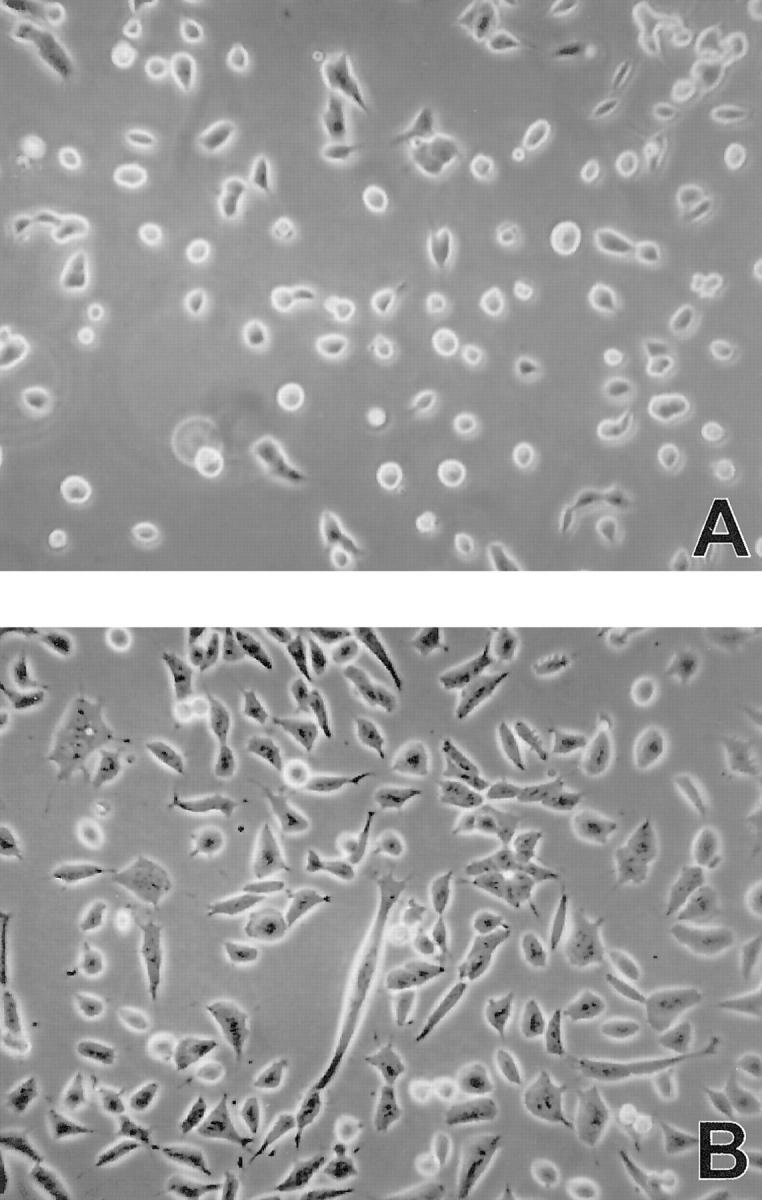
SCF effect on morphology of 6647 cells 48 hours after seeding. A: Untreated control culture. B: Culture treated with 10 ng/ml of SCF. Phase contrast, ×100.
When used as a chemoattractant in the lower compartment of a migration chamber, SCF was able to induce a strong migratory response in three out of six Ewing’s sarcoma cell lines (Figure 5A) ▶ . To discriminate between chemotactic response (migration in a positive gradient of chemoattractant), and induction of random motility, we performed a checkerboard analysis (Figure 5B) ▶ . In the diagram, squares along the diagonal (upper left corner to lower right corner) and above this line represent equivalent or higher concentrations of SCF in the top chamber versus the bottom chamber, and measure induction of random motility. Squares below the diagonal represent a positive gradient of SCF and measure chemotaxis. The checkerboard analysis indicated that SCF induced a chemotactic response in a positive gradient of SCF, in a dose-dependent manner. However, at doses of 1 to 10 ng/ml of SCF in the upper and in the lower chamber a strong response of random motility was also observed.
Figure 5.
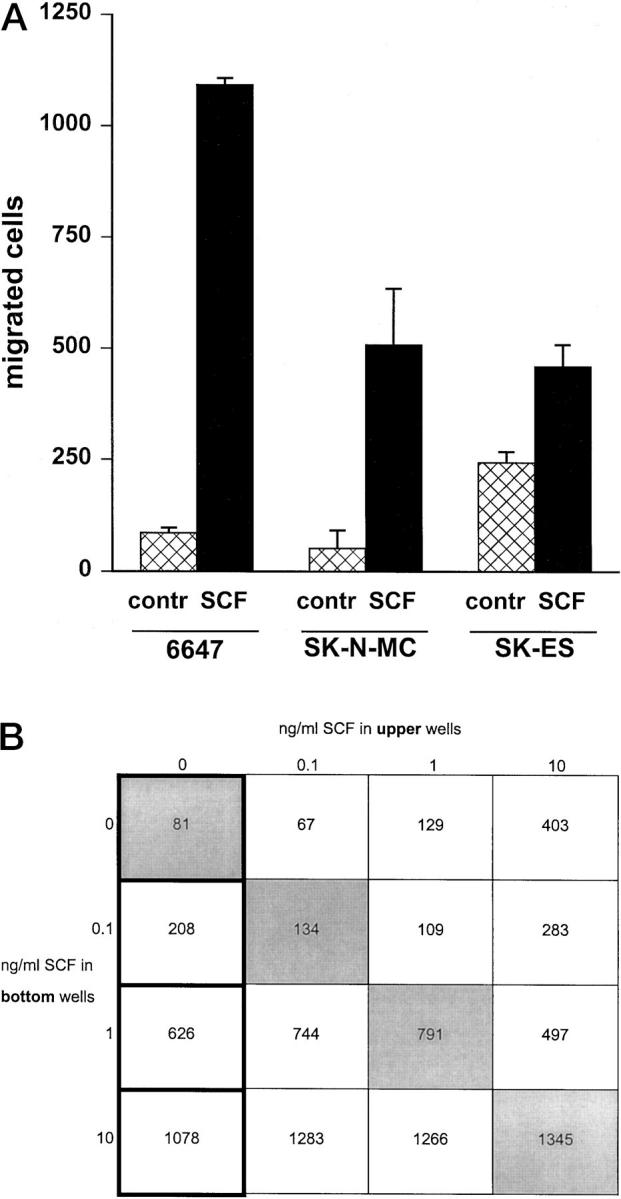
SCF effect on migration ability of human Ewing’s sarcoma cells. A: Migration of 6647, SK-N-MC, and SK-ES cells in response to SCF (10 ng/ml) used as a chemoattractant in the lower compartment of a Transwell chamber. Each bar represents the mean ± SE of three independent experiments. Migration in a positive gradient of SCF was significantly higher than toward control medium (P < 0.05, Student’s t-test). B: Checkerboard analysis of 6647 migration in response to SCF. In each square the number of migrated cells is reported. The first column represents the dose-dependent chemotactic response in a positive gradient of SCF. Squares below the diagonal (upper left corner to lower right corner) represent a positive gradient of SCF and measure chemotactic response. Squares along the diagonal represent equivalent SCF concentrations in upper and lower wells of the migration chamber. Squares above this line represent a reversed gradient of the factor.
Pretreatment of 6647 cells with 10 ng/ml SCF for 48 hours strongly affected their migration ability in response to SCF: pretreated cells showed a 59% inhibition in migration as compared to untreated control cells.
Metastatic Ability
Ewing’s sarcoma shows a high tendency to early metastatic spread with a specific pattern of tissue distribution to bones, bone marrow, lungs, and lymph nodes.
We studied the experimental metastatic ability of 6647 cells injected intravenously into nude mice. Approximately 1 month after intravenous cell injection, symptoms of metastatic growth such as gait problems or disseminated growing masses became evident in most mice. At autopsy, metastases were found in lungs and in several extrapulmonary sites, such as interscapular brown fat, scapula, vertebral bodies and skull, lymph nodes, kidney and adrenals, ovary, and uterus. Most localizations correspond to well-known sites of SCF production. 17,35 Histological examination of a metastasis in a vertebral body showed the presence of tumor cells replacing the medullary canal with destruction of cortical bone and invasion of vertebral body interspace. Its origin from 6647 Ewing’s sarcoma cells was confirmed by a strong positivity for CD99 antigen, highly expressed in Ewing’s sarcoma cells (data not shown). 8
Human cells are able to bind and respond to murine SCF, 36 therefore metastatic behavior in nude mice of human cells expressing c-kit could be affected by murine SCF in a way similar to what happens in humans. To study the role of c-kit and SCF in Ewing’s sarcoma metastasis, 6647 cells were treated in vitro with human SCF and then injected intravenously into nude mice. SCF pretreatment strongly reduced their metastatic ability both to the lungs and to extrapulmonary sites (Figure 6A) ▶ . SCF-pretreated cells were less aggressive than untreated control cells, leading animals to death for metastatic load in a significantly longer time (Figure 6B) ▶ .
Figure 6.
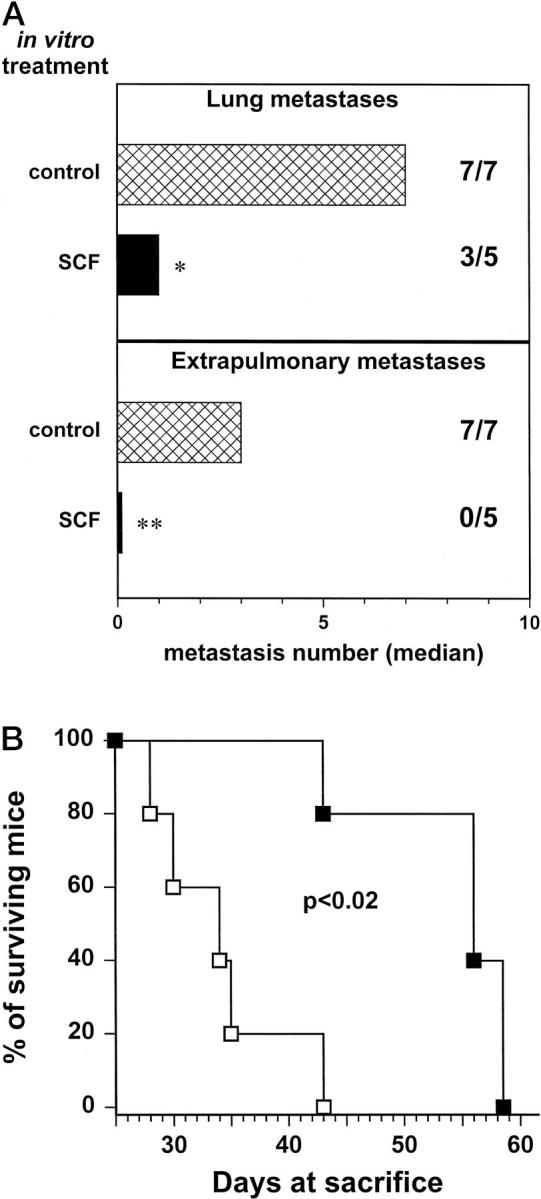
Effect of in vitro pretreatment with SCF on metastatic ability of 6647 cells injected intravenously into nude mice. A: Metastatic ability to the lungs and to extrapulmonary sites. Cross-hatched bars, untreated control cells; solid bars, cells pretreated in vitro with 10 ng/ml of SCF for 48 hours. Metastases were evaluated 31 days after cell injection. Bars represent the median number of metastases; figures reports the number of mice with metastases/total number of mice. Significance: **, P < 0.01; *, P < 0.05 (Wilcoxon’s rank sum test). B: Survival of mice after intravenous injection of untreated cells (open symbols) or cells pretreated in vitro with SCF (closed symbols). Statistical significance was evaluated by the Mantel-Haenszel test.
Discussion
A complex machinery of receptor expression and growth factor production is supposed to affect tumor malignancy both through autocrine effects and sensitivity to exogenous growth factor provided by the different microenvironments. 15
In this study we have examined the role of c-kit/SCF system in Ewing’s sarcoma, a human tumor that displays a very aggressive behavior with rapid growth and early dissemination. 1 The six Ewing’s sarcoma cell lines invariably expressed surface c-kit. Treatment with exogenous SCF determined down-regulation of c-kit expression in all cell lines, thus suggesting binding between factor and receptor. Down-regulation was probably because of receptor-ligand complex internalization as reported for other cellular systems. 19
The relevance of these observations to Ewing’s sarcoma is sustained by the reported RT-PCR positivity for c-kit and its ligand in almost all Ewing’s sarcoma samples analyzed, 12 and by our preliminary data on c-kit immunostaining at tissue level in Ewing’s sarcoma biopsies.
Although soluble SCF was not detectable in culture supernatants, five of six cell lines expressed transmembrane SCF, thus suggesting the existence of a juxtacrine circuit. Along with the juxtacrine circuit, paracrine stimulation of the c-kit receptor should also be considered. In the human body there are in fact many sources of SCF such as bone marrow stromal cells, endothelial cells, muscle cells, 37 osteoblasts, 38 and neurons, and the concentration of soluble SCF in human peripheral blood is around 3 ng/ml. 39
In the 6647 cell line, having the highest c-kit expression and an intermediate-low level of transmembrane SCF, we showed that, at low serum concentration, treatment with exogenous SCF can induce a significant increase in cell growth. SCF produced such an effect by acting on two fronts: enhancement of proliferation, as shown by the increased proportion of cells in S phase, and protection from apoptosis. In addition, SCF treatment induced a more flattened morphology and larger cellular dimensions, exerting a sort of trophic and adhesion-enhancing action. Similar morphological alterations have been reported for mast cells. 40
SCF has been involved in migration and homing of hematopoietic stem cells, 41 melanoblasts, and primordial germ cells. 17 Induction of motility and migration can be correlated with enhancement of invasion and metastasis. In 6647, SK-N-MC, and SK-ES cell lines SCF had indeed a strong effect on motility and migration. SCF can induce migration in a dose-dependent manner including stimulation of random motility. When considering chemotactic and chemokinetic responses to SCF it should be kept in mind that Ewing’s sarcoma cells express endogenous membrane-bound SCF. To induce migration the soluble SCF added in the lower compartment of a migration chamber must compete with the transmembrane SCF expressed by the cells. On the other hand the addition of soluble SCF to the cells in the upper chamber could favor a chemokinetic response (random motility) by interfering with c-kit/membrane-bound SCF mediated cell-cell interaction. 20,21 The unresponsiveness of some cell lines to exogenous SCF could be because of a level of c-kit expression below the biological threshold, in the case of the TC-71 cell line, as shown for c-kit transfected NIH3T3 fibroblasts, 42 or alternatively to the saturation of the c-kit receptor by the autologous transmembrane SCF in the other cell lines.
The early metastatic dissemination to bones, bone marrow, and lungs is the major determinant of the prognosis of Ewing’s sarcoma patients. The molecular mechanisms responsible for this tissue-specific distribution of metastasis are still poorly understood. Human cells expressing c-kit bind and respond to murine SCF, therefore injection of Ewing’s sarcoma cells into nude mice seems to be a good model to study the role of the c-kit/SCF system in the homing behavior of Ewing’s sarcoma cells. The 6647 cells injected intravenously into athymic nude mice metastasized to the lung and to a variety of extrapulmonary sites closely resembling those observed in Ewing’s sarcoma patients, and corresponding to SCF-rich microenvironments. In vitro pretreatment of 6647 cells with SCF strongly impaired their metastatic ability, significantly reducing lung colonization and extrapulmonary metastatic growth, and consequently increased survival time of animals. Cells pretreated in vitro with human SCF showed down-modulation of c-kit expression and were less sensitive to the chemotactic activity of SCF in vitro. The same mechanism could operate in vivo in response to murine SCF thus leading, at least in the initial phase, to the formation of fewer and slowly growing metastases. A study on hematopoietic progenitor cells showed that exposure to a neutralizing anti-c-kit antibody inhibited their homing to the bone marrow and to the spleen of adult mice. 41 Together with our results, this indicates the importance of the c-kit/SCF interaction in the homing process of normal or neoplastic cells and suggests the possibility of interfering with it by modulation of c-kit expression.
In a study on 52 Ewing’s sarcoma patients it has been reported that 33% of the cases displayed trisomy of chromosome 12 with a higher frequency of trisomy in relapse than in primary tumors. 7 It is interesting to note that SCF gene maps on human chromosome 12q22-q24. It could be speculated that also SCF expression might confer to cells a growth advantage within the tumor, thus leading to positive selection during tumor progression.
It is worth noting that the presence of the c-kit/SCF juxtacrine/paracrine loop in Ewing’s sarcoma extends the list of growth factor loops shared by Ewing’s sarcoma and small-cell lung carcinoma that also includes gastrin-releasing peptide 43,44 and its receptor, and insulin-like growth factor-I and its receptor. 11 It could be hypothesized that this combination of growth factor loops plays a causal role in their early dissemination and aggressive behavior. Moreover, the search of new therapies interfering with autocrine tumor cell growth should take into account the redundancy of autocrine circuits that could compromise the efficacy of therapies targeting a single loop.
Footnotes
Address reprint requests to Dr. L. Landuzzi, Dipartimento di Patologia Sperimentale, Sezione di Cancerologia, Viale Filopanti 22, I-40126 Bologna, Italy. E-mail: landuzzi@cancer.unibo.it.
Supported by grants from the Italian Association for Cancer Research, the Italian Ministry for University and Research, the Rizzoli Institute, and the National Research Council. C. R. and A. A. are in receipt of a Ph.D. fellowship from the Italian Ministry for University and Research; I. R. is in receipt of a fellowship from University of Bologna.
References
- 1.Dehner LP: Primitive neuroectodermal tumor and Ewing’s sarcoma. Am J Surg Pathol 1993, 17:1-13 [DOI] [PubMed] [Google Scholar]
- 2.Jurgens HF: Ewing’s sarcoma and peripheral primitive neuroectodermal tumor. Curr Opin Oncol 1994, 6:391-396 [PubMed] [Google Scholar]
- 3.Zoubek A, Ladenstein R, Windhager R, Amann G, Fischmeister G, Kager L, Jugovic D, Ambros PF, Gadner H, Kovar H: Predictive potential of testing for bone marrow involvement in Ewing tumor patients by RT-PCR: a preliminary evaluation. Int J Cancer 1998, 79:56-60 [DOI] [PubMed] [Google Scholar]
- 4.Rettig WJ, Garin-Chesa P, Huvos AG: Ewing’s sarcoma: new approaches to histogenesis and molecular plasticity. Lab Invest 1992, 66:133-137 [PubMed] [Google Scholar]
- 5.Pagani A, Fischer-Colbrie R, Eder U, Pelli A, Llombart-Bosch A, Bussolati G: Neuronal and mesenchymal differentiations in Ewing’s sarcoma cell lines. Morphological, immunophenotypic, molecular biological and cytogenetic evidence. Int J Cancer 1995, 63:738-743 [DOI] [PubMed] [Google Scholar]
- 6.May WA, Gishizky ML, Lessnick SL, Lunsford LB, Lewis BC, Delattre O, Zucman J, Thomas G, Denny CT: Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci USA 1993, 90:5752-5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurici D, Perez-Atayde A, Grier HE, Baldini N, Serra M, Fletcher JA: Frequency and implications of chromosome 8 and 12 gains in Ewing sarcoma. Cancer Genet Cytogenet 1998, 100:106-110 [DOI] [PubMed] [Google Scholar]
- 8.Fellinger EJ, Garin-Chesa P, Triche TJ, Huvos AG, Rettig WJ: Immunohistochemical analysis of Ewing’s sarcoma cells surface antigen p30/32MIC2. Am J Pathol 1991, 139:317-325 [PMC free article] [PubMed] [Google Scholar]
- 9.Lollini P-L, Landuzzi L, Frabetti F, Rossi I, Nicoletti G, Scotlandi K, Serra M, Baldini N, De Giovanni C, Nanni P: Expression of functional CD40 on human osteosarcoma and Ewing’s sarcoma cells. Clin Cancer Res 1998, 4:1843-1849 [PubMed] [Google Scholar]
- 10.Sohn HW, Choi EY, Kim SH, Lee I, Chung DH, Sung UA, Hwang DH, Cho SS, Jun BH, Jang JJ, Chi JG, Park SH: Engagement of CD99 induces apoptosis through a calcineurin-independent pathway in Ewing’s sarcoma cells. Am J Pathol 1998, 153:1937-1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scotlandi K, Benini S, Sarti M, Serra M, Lollini P-L, Maurici D, Picci P, Manara MC, Baldini N: Insulin-like growth factor I receptor-mediated circuit in Ewing’s sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res 1996, 56:4570-4574 [PubMed] [Google Scholar]
- 12.Ricotti E, Fagioli F, Garelli E, Linari C, Crescenzio N, Horenstein AL, Pistamiglio P, Vai S, Berger M, Cordero di Montezemolo L, Madon E, Basso G: c-kit is expressed in soft tissue sarcoma of neuroectodermic origin and its ligand prevents apoptosis of neoplastic cells. Blood 1998, 91:2397-2405 [PubMed] [Google Scholar]
- 13.Kerbel RS: Significance of tumor-host interactions in cancer growth and metastases. Cancer Metastasis Rev 1995, 14:259-262 [DOI] [PubMed] [Google Scholar]
- 14.Stetler-Stevenson WG, Aznavoorian S, Liotta LA: Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993, 9:541-573 [DOI] [PubMed] [Google Scholar]
- 15.Fidler IJ: Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst 1995, 87:1558-1592 [DOI] [PubMed] [Google Scholar]
- 16.Scotlandi K, Benini S, Nanni P, Lollini P-L, Nicoletti G, Landuzzi L, Serra M, Manara MC, Picci P, Baldini N: Blockage of insulin-like growth factor-I receptor inhibits the growth of Ewing’s sarcoma in athymic mice. Cancer Res 1998, 58:4127-4131 [PubMed] [Google Scholar]
- 17.Galli SJ, Zsebo KM, Geissler EN: The kit ligand, stem cell factor. Adv Immunol 1994, 55:1-96 [DOI] [PubMed] [Google Scholar]
- 18.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, March CJ, Boswell HS, Gimpel SD, Cosman D, Williams DE: Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell 1990, 63:235-243 [DOI] [PubMed] [Google Scholar]
- 19.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K: Membrane-bound steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood 1995, 85:641-649 [PubMed] [Google Scholar]
- 20.Adachi S, Ebi Y, Nishikawa S, Hayashi S, Yamazaki M, Kasugai T, Yamamura T, Nomura S, Kitamura Y: Necessity of extracellular domain of W (c-kit) receptors for attachment of murine cultured mast cells to fibroblasts. Blood 1992, 79:650-656 [PubMed] [Google Scholar]
- 21.Avraham H, Scadden DT, Chi S, Broudy VC, Zsebo KM, Groopman JE: Interaction of human bone marrow fibroblasts with megakaryocytes: role of the c-kit ligand. Blood 1992, 80:1679-1684 [PubMed] [Google Scholar]
- 22.Brannan CI, Bedell MA, Resnick JL, Eppig JJ, Handel MA, Williams DE, Lyman SD, Donovan PJ, Jenkins NA, Copeland NG: Developmental abnormalities in Steel17H mice result from a splicing defect in the steel factor cytoplasmic tail. Genes Dev 1992, 6:1832-1842 [DOI] [PubMed] [Google Scholar]
- 23.Krystal GW, Hines SJ, Organ CP: Autocrine growth of small cell lung cancer mediated by coexpression of c-kit and stem cell factor. Cancer Res 1996, 56:370-376 [PubMed] [Google Scholar]
- 24.Lux ML, Rubin BP, Biase TL, Chen C-J, Maclure T, Demetri G, Xiao S, Singer S, Fletcher CDM, Fletcher JA: KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 2000, 156:791-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner AM, Zsebo KM, Martin F, Jacobsen FW, Bennett LG, Broudy VC: Nonhematopoietic tumor cell lines express stem cell factor and display c-kit receptors. Blood 1992, 80:374-381 [PubMed] [Google Scholar]
- 26.Cohen PS, Chan JP, Lipkunskaya M, Biedler JL, Seeger RC: Expression of stem cell factor and c-kit in human neuroblastoma. Blood 1994, 84:3465-3472 [PubMed] [Google Scholar]
- 27.Beck D, Gross N, Beretta Brognara C, Perruisseau G: Expression of stem cell factor and its receptor by human neuroblastoma cells and tumors. Blood 1995, 8:3132-3138 [PubMed] [Google Scholar]
- 28.Timeus F, Crescenzio N, Valle P, Pistamiglio P, Piglione M, Garelli E, Ricotti E, Rocchi P, Strippoli P, Cordero di Montezemolo L, Madon E, Ramenghi U, Basso G: Stem cell factor suppresses apoptosis in neuroblastoma cell lines. Exp Hematol 1997, 25:1253-1260 [PubMed] [Google Scholar]
- 29.Landuzzi L, Strippoli P, De Giovanni C, Nicoletti G, Rossi I, Tonelli R, Frabetti F, Nanni P, Bagnara GP, Lollini P-L: Production of stem cell factor and expression of c-kit in human rhabdomyosarcoma cells: lack of autocrine growth modulation. Int J Cancer 1998, 78:441-445 [DOI] [PubMed] [Google Scholar]
- 30.Lahm H, Amstad P, Yilmaz A, Borbenyi Z, Wyniger J, Fischer JR, Suardet L, Givel JC, Odartchenko N: Interleukin 4 down-regulates expression of c-kit and autocrine stem cell factor in human colorectal carcinoma cells. Cell Growth Differ 1995, 6:1111-1118 [PubMed] [Google Scholar]
- 31.Avanzi GC, Brizzi MF, Giannotti J, Ciarletta A, Yang YC, Pegoraro L, Clark SC: M-07e human leukemic factor-dependent cell line provides a rapid and sensitive bioassay for the human cytokines GM-CSF and IL-3. J Cell Physiol 1990, 145:458-464 [DOI] [PubMed] [Google Scholar]
- 32.Martin FH, Suggs S, Langley KE, Lu HS, Ting J, Okino KH, Morris CF, McNiece IK, Jacobsen FW, Mendiaz EA, Birkett NC, Smith KA, Johnson MJ, Parker VP, Flores JC, Patel AC, Fisher EF, Erjavec HO, Herrera C, Wypych J, Sachdev RK, Pope JA, Leslie I, Wen D, Lin C-H, Cupples RL, Zsebo KM: Primary structure and functional expression of rat and human stem cell factor DNAs. Cell 1990, 63:203-211 [DOI] [PubMed] [Google Scholar]
- 33.Meininger CJ, Yano H, Rottapel R, Bernstein A, Zsebo KM, Zetter BR: The c-kit receptor ligand functions as a mast cell chemoattractant. Blood 1992, 79:958-963 [PubMed] [Google Scholar]
- 34.Scotlandi K, Serra M, Nicoletti G, Vaccari M, Manara MC, Nini G, Landuzzi L, Colacci A, Bacci G, Bertoni F, Picci P, Campanacci M, Baldini N: Multidrug resistance and malignancy in human osteosarcoma. Cancer Res 1996, 56:2434-2439 [PubMed] [Google Scholar]
- 35.Williams DE, Lyman SD: Steel Factor. Aggarwal BB Gutterman JU eds. Human Cytokines Handbook for Basic and Clinical Research, 1996, vol 2.:pp 316-330 Blackwell Science, Boston, MA, [Google Scholar]
- 36.Lyman SD, Jacobsen SEW: c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 1998, 91:1101-1134 [PubMed] [Google Scholar]
- 37.Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C: Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem 1994, 42:1417-1425 [DOI] [PubMed] [Google Scholar]
- 38.Van ’T Hof RJ, Von Lindern M, Nijweide PJ, Beug H: Stem cell factor stimulates chicken osteoclast activity in vitro. FASEB J 1997, 11:287-293 [DOI] [PubMed] [Google Scholar]
- 39.Langley KE, Bennett LG, Wypych J, Yancik SA, Liu XD, Westcott KR, Chang DG, Smith KA, Zsebo KM: Soluble stem cell factor in human serum. Blood 1993, 81:656-660 [PubMed] [Google Scholar]
- 40.Kim HM, Shin HY, Lee EH: Morphological alterations in rat peritoneal mast cells by stem cell factor. Immunology 1998, 94:242-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broudy VC, Lin NL, Priestley GV, Nocka K, Wolf NS: Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood 1996, 88:75-81 [PubMed] [Google Scholar]
- 42.Caruana G, Cambareri AC, Gonda TJ, Ashman LK: Transformation of NIH3T3 fibroblasts by the c-kit receptor tyrosine kinase: effect of receptor density and ligand-requirement. Oncogene 1998, 16:179-190 [DOI] [PubMed] [Google Scholar]
- 43.Lawlor ER, Lim JF, Tao W, Poremba C, Chow CJ, Kalousek IV, Kovar H, MacDonald TJ, Sorensen PH: The Ewing tumor family of peripheral primitive neuroectodermal tumors expresses human gastrin-releasing peptide. Cancer Res 1998, 58:2469-2476 [PubMed] [Google Scholar]
- 44.Sekido Y, Takahashi T, Ueda R, Takahashi M, Suzuki H, Nishida K, Tsukamoto T, Hida T, Shimokata K, Zsebo KM, Takahashi T: Recombinant human stem cell factor mediates chemotaxis of small-cell lung cancer cell lines aberrantly expressing the c-kit protooncogene. Cancer Res 1993, 53:1709-1714 [PubMed] [Google Scholar]