Abstract
Deposition of amyloid β-protein (Aβ), a hallmark of Alzheimer’s disease, occurs to some extent in the brains of most elderly individuals. We sought to learn when Aβ deposition begins and how deposition is affected by apolipoprotein E allele ε4, a strong risk factor for late-onset Alzheimer’s disease. Using an improved extraction protocol and specific enzyme-linked immunosorbent assay, we quantified the levels of Aβ40 and Aβ42 in the insoluble fractions of brains from 105 autopsy cases, aged 22 to 81 years at death, who showed no signs of dementia. Aβ40 and Aβ42 were detected in the insoluble fractions from all of the brains examined; low levels were even found in the brains of patients as young as 20 to 30 years of age. The incidence of significant Aβ accumulation increased age-dependently, with Aβ42 levels beginning to rise steeply in some patients in their late 40’s, accompanied by much smaller increases in Aβ40 levels. The presence of the apolipoprotein E ε4 allele was found to significantly enhance the accumulation of Aβ42 and, to a lesser extent, that of Aβ40. These findings strongly suggest that the presence of ε4 allele results in an earlier onset of Aβ42 accumulation in the brain.
Apolipoprotein E (apoE), a 34-kd protein component of plasma lipoproteins, plays a central role in the transport of lipids, especially cholesterol, to various tissues. 1 In humans, three isoforms (apoE2, E3, and E4) are encoded by three alleles (ε2, ε3, and ε4) at a single apoE gene locus on the long arm of chromosome 19. ApoE3 is the most common and basic form, whereas apoE2 and apoE4 are relatively minor variants that differ from apoE3 by a single amino acid substitution. Genetic linkage studies suggest that the ε4 allele is a strong risk factor for late-onset Alzheimer’s disease (AD), and is associated with an earlier age of onset. 2,3 The potential role of the ε4 allele in amyloid β-protein (Aβ) deposition has thus been of particular interest, given the critical involvement of Aβ in the pathogenesis of AD.
Most cells, including brain cells, secrete substantial amounts of Aβ40 (the major Aβ species, ending at Val40) and Aβ42 (a longer, minor species ending at Ala42) into the extracellular space at a ratio of ∼10 to 1. 4 During aging, insoluble amorphous and/or fibrillar deposits of Aβ gradually develop in the extracellular space of most brains. This Aβ deposition is initiated by accumulation of Aβ42, 5,6 which is much more amyloidogenic than Aβ40. Indeed, mutations within amyloid precursor protein (APP) and presenilin 1 and 2, three genes known to be causatively involved in the development of familial AD, all induce increased secretion of Aβ42. 7 Such increased secretion is reasonably postulated to result in significant Aβ42 deposition at a much earlier stage of life, eventually leading to early-onset familial AD. 4
In contrast, the role of the ε4 allele in Aβ deposition remains an enigma. Most puzzling are the findings that the ε4 allele is associated with increased numbers of Aβ40-positive, but not Aβ42-positive, plaques in sporadic AD brains. 8,9 These findings are substantiated by enzyme-linked immunosorbent assay (ELISA) showing that ε4 allele-positive, sporadic AD brains are characterized by increased levels of Aβ40 but not Aβ42. 10 This raises the possibility that the ε4 allele is not involved in Aβ42 deposition, and suggests that other potential targets of the allele should be explored. On the other hand, given that Aβ42 is by far the predominant species in the majority of senile plaques, this hypothesis runs contrary to recent observations that ε4 carriers have a greater number of plaques than noncarriers among unselected autopsy cases. 11-13 We therefore sought to clarify the role of the ε4 allele in the initial phase of Aβ40 and Aβ42 accumulation in the human brain using an improved extraction protocol and a sensitive ELISA.
Materials and Methods
Patients and Tissue Preparation
The present study was based in part on autopsy cases (n = 85; 62 men, 23 women) from the Gunma Cancer Center (Ohta, Gunma, Japan); all of the patients had malignant neoplasms and the ages at death ranged from 25 to 81 years (one at 20 to 29 years, nine at 40 to 49 years, 21 at 50 to 59 years, 25 at 60 to 69 years, 29 at 70 to 81 years; postmortem delay, 1 to 13 hours). The Tokyo Medical Examiner’s Office (Otsuka, Tokyo, Japan) 6 was a second source of autopsy cases (n = 20; 16 men, four women), among whom the age at death ranged from 22 to 49 years (four at 20 to 29 years, five at 30 to 39 years, and 11 at 40 to 49 years; postmortem delay, 2 to 24 hours). Cortical blocks were obtained from the prefrontal cortex in each case (Brodmann areas 9 to 11) and stored at −80°C until use. In addition, blocks from adjacent sites and/or from the same locations on the contralateral side were fixed in 10% buffered formalin and processed for histological and immunocytochemical examination.
None of the cases whose brains were analyzed for this study showed any signs of dementia; cases of AD or dementia from other causes were excluded. AD was diagnosed based on both clinical and neuropathological criteria; all cases met the A2 criteria as defined by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, 14 and were classified type C as defined by the Consortium to Establish a Registry for Alzheimer’s Disease. 15
Antibodies and Authentic Aβ
The monoclonal antibodies against Aβ used were BAN50 (raised against Aβ1-16), BNT77 (raised against Aβ11-28), BA27 (raised against Aβ1-40; specific for Aβ40), and BC05 (raised against Aβ35-43; specific for Aβ42). 16,17 4G8 (specific for Aβ17-24) and 6E10 (raised against Aβ1-17) were obtained from Senetek PLC (Maryland Heights, MO).
Synthetic Aβ1-40 and Aβ1-42 were purchased from Bachem (Torrance, CA). Two species of p3 (Aβ17-40 and Aβ17-42) were from AnaSpec (San Jose, CA). Aβ3(pE)−42 and Aβ11(pE)−42 (pE: pyroglutamate) were kindly provided by Dr. T. C. Saido (RIKEN Brain Science Institute, Saitama, Japan).
ELISA
After carefully dissecting out attached leptomeninges and vessels, each brain tissue sample (∼120 mg) was homogenized in 4 volumes of Tris-saline (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl) containing 1 mmol/L EGTA, 0.5 mmol/L diisopropyl fluorophosphate, 0.5 mmol/L phenylmethylsulfonyl fluoride, 1 μg/ml Nα-p-tosyl-l-lysine chloromethyl ketone, 1 μg/ml antipain, 0.1 μg/ml pepstatin, and 1 μg/ml leupeptin. The homogenate was centrifuged at 540,000 × g for 20 minutes in a TLX ultracentrifuge (Beckman, Palo Alto, CA), after which the pellet was washed with the same buffer, resuspended either by homogenization in 50 volumes of 70% formic acid 18 or by brief sonication in 10 volumes of 6 mol/L guanidine-HCl in 50 mmol/L Tris-HCl, pH 7.6, 19 and centrifuged once again at 265,000 × g for 20 minutes. The formic acid supernatant was neutralized with NaOH and Trizma base, 18 whereas the guanidine-HCl supernatant was diluted at 1:12 to reduce the concentration of guanidine-HCl to 0.5 mol/L. Both supernatants were then subjected to two-site ELISA as previously described: 6 BNT77 was coated onto a microtiter plate as the capture antibody, whereas BA27 or BC05 was used as the detection antibody after conjugation with horseradish peroxidase.
BC05 is known to have a low affinity for Aβ43 (1/5 to 1/10 that for Aβ42), but because Aβ43 was virtually undetectable in extracts probed with BC65, a monoclonal anti-Aβ43 antibody, 18 BC05-based Aβ values were considered to reflect Aβ42 exclusively. On the other hand, because BC05 weakly cross-reacts with APP (1/300 to 1/500 that for Aβ42), in cases where the Aβ42 levels were less than ∼5 pmol/g tissue, it was necessary to correct for APP binding to accurately assess Aβ42 levels. 20 As the APP concentrations in the guanidine-HCl extracts ranged from 8 to 15 nmol/L, the corrected Aβ42 levels in these cases were ∼1/5 of those presented in Figure 1 ▶ .
Figure 1.
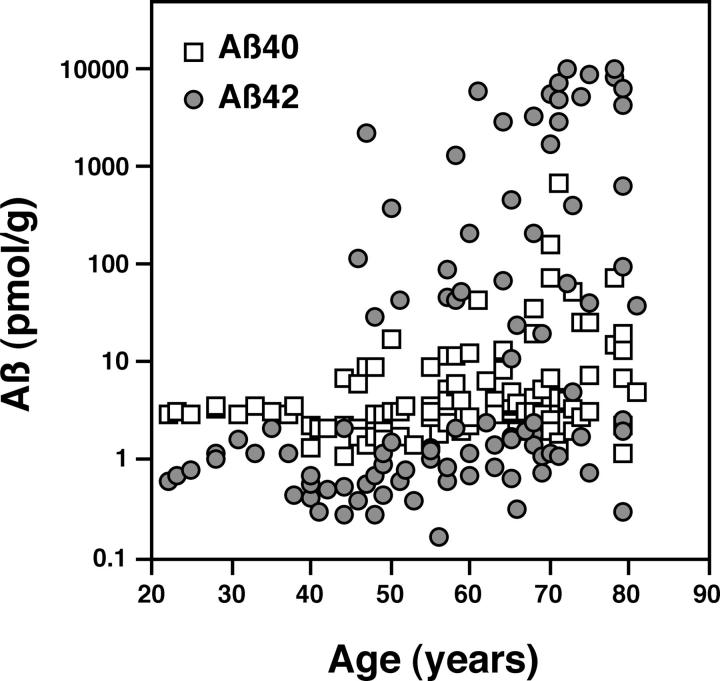
Levels of Aβ in the insoluble fractions of human brains of various age. Aβ40 (open squares) and Aβ42 (shaded circles) were extracted from the insoluble fractions of 105 normal brains using a guanidine-HCl extraction protocol and quantified by ELISA. The values obtained are plotted as function of age at the time of the patient’s death. Both Aβ40 and Aβ42 were detected in all brains tested, even in those from patients as young as 20 to 30 years of age. The incidence of significant accumulation of Aβ increased age-dependently. Note that y axis is a log scale, and thus levels of Aβ42 increased much more steeply than those of Aβ40.
Western Blotting
The insoluble pellet in Tris-saline was delipidated with chloroform/methanol (2:1) and then with chloroform/methanol/water (1:2:0.8). The residue was then extracted with formic acid, and the extract was centrifuged. An aliquot of the supernatant was then dried using a Speed Vac (Savant Instruments, Farmingdale, NY) and solubilized with Laemmli sample buffer containing 4 mol/L of urea. The resultant samples were electrophoresed on 16.5% Tris/tricine gels, and the separated proteins were transferred onto nitrocellulose membranes. The blot was then placed in boiled phosphate-buffered saline to enhance the sensitivity, 20 and then incubated with BA27, BC05, BAN50, or 6E10. The bound antibodies were detected using either enhanced chemiluminescence or enhanced chemiluminescence plus (Amersham Pharmacia Biotech, Buckinghamshire, UK). Antigen levels in the enhanced chemiluminescence bands of interest were quantified using a model GS-700 imaging densitometer with Molecular Analyst Software (Bio-Rad Laboratories, Hercules, CA). 20,21
Immunocytochemistry
Immunocytochemical examination for senile plaques was performed using 4G8 or Aβ polyclonal antibodies as previously described. 6 Detailed description of the immunocytochemical data will be published elsewhere (Yamaguchi H et al, submitted). Aβ levels in the insoluble fractions were logarithmically related to the density of the senile plaques, as previously reported. 6 With a single exception, senile plaques were clearly observed in brains containing more than ∼100 pmol of Aβ42/g tissue. There was no apparent correlation between amyloid angiopathies (seen mostly in the leptomeningeal vessels) and levels of either Aβ40 or Aβ42 in the present autopsy series, which is indicative of the successful removal of leptomeninges during dissection of the cortex.
ApoE Genotyping
The apoE genotype was determined by polymerase chain reaction as described previously. 22 The frequencies of the ε2, ε3, and ε4 alleles among the patients were 3.3%, 82.4%, and 14.3%, respectively. These figures are similar to those reported for the populations of Europe and North America, 2 and the frequency of the ε4 allele is somewhat higher than that in the general Japanese population (∼9%). 23,24
Statistical Analysis
Statistical analyses were performed using Microsoft Excel 2000 (Microsoft, Redmond, WA), StatView 4.5 (SAS Institute Inc., Cary, NC), and SPSS statistics programs (SPSS Inc., Chicago, IL). Aβ levels >5 pmol/g tissue were considered to reflect significant accumulation, because Aβ levels in the majority of patients less than 40 years of age were below this threshold. After subdividing the patients into seven age groups (20 to 29 years, 30 to 39 years, 40 to 49 years, 50 to 59 years, 60 to 69 years, 70 to 79 years, and >80 years), the incidence of significant Aβ accumulation was assessed as a function of age using the Cochran-Armitage trend test. Respective levels of the monomeric and dimeric forms of Aβ were determined by quantitative Western blotting. For each protocol the correlation was evaluated by linear regression analysis. The median ages of the ε4 carriers and noncarriers were compared using the Mann-Whitney U test, and the prevalence of significant accumulation of Aβ in the two groups was compared using Fisher’s exact test. Because of the small number of patients homozygous for ε4 (two patients), the gene dosage effect of the ε4 allele was not investigated. Multiple linear regression analysis was performed using Aβ level as the dependent variable, and the age at death and the presence of the ε4 allele as independent variables. Values of P < 0.05 were considered significant.
Results
Validation of the Guanidine-HCl Extraction Protocol
We previously showed that Aβ could be extracted from the insoluble fraction of nontransfected SH-SY5Y neuroblastoma cells using a guanidine-HCl protocol. 20 We also used guanidine-HCl, rather than formic acid, to extract Aβ from the insoluble fraction of brains in the present study, because the formic acid protocol requires neutralization with a large volume of alkaline solution, resulting in marked dilution of the protein and a large decrease in the sensitivity of our ELISA.
To test the validity of the protocol, Aβ was extracted from the insoluble fractions of many nondemented human brains using either formic acid or guanidine-HCl, and Aβ levels in the two groups of extracts were compared. We found that there was a good correlation between Aβ levels determined by the two protocols among patients with significant accumulations of Aβ [linear regression: y = 0.771x −0.184, r 2 = 0.522, P = 0.0001 for Aβ40; y = 1.178x −0.576, r 2 = 0.796, P < 0.0001 for Aβ42, where x = log (Aβ levels obtained by the formic acid protocol) and y = log (Aβ levels obtained by the guanidine-HCl protocol)]. Interestingly, although formic acid had been believed to be the most effective agent for extracting deposits of fibrillar Aβ, guanidine-HCl proved even more effective at extracting low levels of Aβ and, importantly, yielded highly reproducible results. As a result, we were able to accurately quantify Aβ in the insoluble fractions from all of the brains examined, including those from younger patients, despite the fact that the Aβ levels in >60% of the brains were below the detection limit of the formic acid protocol.
Aβ Levels in the Insoluble Fraction Increase during Aging
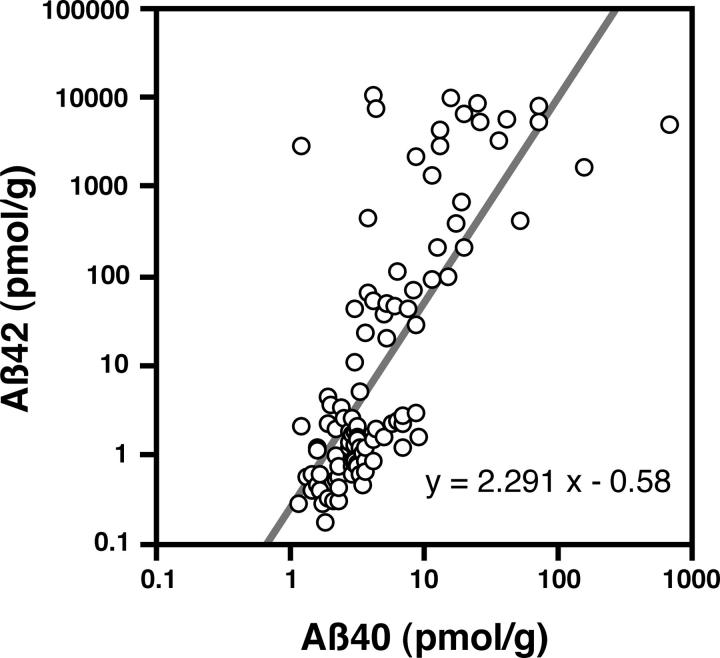
Figure 1 ▶ shows Aβ levels plotted as a function of the age at death. Both Aβ40 and Aβ42 were detected in the insoluble fractions of the brains of 20- to 30-year-old patients, with levels of the former being severalfold (more than 10-fold, if corrections were made; see above and Figure 1 ▶ ) higher than those of the latter [Aβ40, ∼3 pmol/g tissue; Aβ42 (corrected), ∼0.2 to 0.3 pmol/g tissue]. Aβ levels seemed to be stable during the next 20 years, but then began to increase exponentially in some patients, beginning at <50 of age (Figure 1) ▶ . The incidence of significant accumulation of Aβ40 or Aβ42 thus increased with age at death (Cochran-Armitage: chi square = 10.497, P < 0.005 for Aβ40; chi square = 17.863, P < 0.001 for Aβ42), as did the magnitude of the accumulation [linear regression: y = 0.0116x −0.0012, r 2 = 0.125, P = 0.0002 for Aβ40; y = 0.048x −1.835, r 2 = 0.231, P < 0.0001 for Aβ42, where x = age and y = log (Aβ levels)]. In addition, there was a close correlation between the Aβ40 and Aβ42 levels among individuals [linear regression: y = 2.291x −0.58, r 2 = 0.569, P < 0.0001, where x = log (Aβ40 levels) and y = log(Aβ42 levels)] (Figure 2) ▶ , strongly suggesting that they increase in a coordinate manner, although the slope of the age-dependent increases in Aβ42 was much steeper than that for Aβ40 (P < 0.001). As a result, Aβ42 was by far the predominant species in many brains of elderly patients, with levels eventually reaching a plateau in patients older than 70 years of age (Figure 1) ▶ .
Figure 2.
Correlation between levels of insoluble Aβ40 and Aβ42 in brain. Aβ40 and Aβ42 levels among individuals were well correlated, suggesting their coordinate increase.
The presence of Aβ40 and Aβ42 in the insoluble fractions of normal human brains (containing <5 pmol of Aβ40 or Aβ42/g tissue) was confirmed by Western blot analysis (Figure 3) ▶ . Two or three distinct Aβ bands migrating at 3 to 4 kd were labeled with both BA27 and BC05. In terms of mobility, these bands were very similar to those observed in samples from elderly patients exhibiting Aβ deposition, although the signal intensity differed greatly. Only the uppermost band at 4 kd was labeled by BAN50, whereas the upper two bands were labeled with 6E10.
Figure 3.
Representative Western blots of Aβ extracted from the insoluble fractions of normal brains. Samples containing <5 pmol of Aβ40 or Aβ42/g tissue were probed using monoclonal antibodies, BA27 (specific for Aβ40) (A) and BC05 (specific for Aβ42) (B). Two or three distinct Aβ monomer bands at 3 to 4 kd were labeled with both antibodies. A broad band at ∼6 kd represents a sodium dodecyl sulfate-stable Aβ dimer and was observed in most cases. Synthetic Aβ1-40 or Aβ1-42 (10 pg) serving as controls are shown in the left-most lane.
We were then able to explain the bands based on their immunoreactivities with the various anti-Aβ monoclonal antibodies serving as probes and from their electrophoretic mobilities compared with those of various authentic Aβ species (see Materials and Methods). The 4-kd band likely represents an Aβ monomer beginning at Asp-1, whereas the two lighter bands should represent amino-terminally truncated Aβ species beginning presumably at pyroGlu-3 and pyroGlu-11, respectively, 25,26 which indicates that truncation, a prominent feature of Aβ deposited in senile plaques, also occurs in normal brains. In our hands, p3, an Aβ species beginning at Leu-17, was not detected in the insoluble fractions. In addition, there was a broad band at ∼6 kd representing a sodium dodecyl sulfate-stable Aβ dimer which was labeled with both BA27 and BC05 in most cases (Figure 3) ▶ . The dimer band often consisted of several closely spaced bands that may represent amino-terminally truncated species. The signal intensity of the Aβ dimer occasionally far exceeded that of the monomer. Although this sodium dodecyl sulfate-stable Aβ dimer could not be quantified by ELISA, previous Western blot analyses indicate that it seems to accumulate age-dependently. 21 In fact, the levels of the monomeric and dimeric forms of Aβ were well correlated for both Aβ40 and Aβ42 [y = 0.791x + 0.248, r 2 = 0.662, P < 0.0001 for Aβ40; y = 0.89x + 0.446, r 2 = 0.871, P < 0.0001 for Aβ42, where x = log(Aβ monomer levels) and y = log(Aβ dimer levels)], indicating that the accumulation of the dimer was proportional to that of the monomer.
Effect of the ε4 Allele on Aβ Accumulation
We next examined the effect of ε4 allele on the age-dependent accumulation of Aβ (Figure 4) ▶ . Although there was no significant difference in the ages of ε4 carriers and noncarriers (Mann-Whitney: P = 0.761), the former accumulated significantly greater amounts of both Aβ40 and Aβ42 (Fisher’s exact test: P = 0.0048 for Aβ40; P = 0.0005 for Aβ42).
Figure 4.
Effect of the apoE ε4 allele on levels of insoluble Aβ42 (A) and Aβ40 (B) in normal human brains. The dashed lines indicate the threshold for significant Aβ accumulation (5 pmol/g tissue). Patients were subdivided into seven age groups, and the effect of the ε4 allele was assessed by analyzing Aβ accumulation in the presence (n = 28) (closed symbols) or absence (n = 77) (open symbols) of the allele. The levels of insoluble Aβ42 and Aβ40 were found to be statistically related to the presence of ε4 allele.
To assess the effect of the ε4 allele on Aβ accumulation in more detail, the patients were subdivided into seven age groups (Figure 4) ▶ . In patients <40 years old at death (10 cases), levels of both Aβ40 and Aβ42 were below the 5 pmol/g threshold, irrespective of the apoE genotype. Between the ages of 40 and 49 years, Aβ42 levels in two of seven ε4 carriers (28.6%) were above threshold, whereas they were above threshold in only one of 13 noncarriers (7.7%) (Figure 4A) ▶ . By 50 years of age and older, most of the ε4 carriers (16 of 18 cases, 88.9%) had accumulated significant levels of Aβ42. In contrast, among noncarriers between 50 and 69 years of age, Aβ42 levels remained below 5 pmol/g in most cases (28 of 36 cases, 77.8%), and even at 70 or more years of age, Aβ42 levels in half of the noncarriers remained below threshold (10 of 21 cases, 47.6%). Multiple regression analysis showed that Aβ42 levels correlated with the apoE genotype (slope 0.815, t = 3.021, P = 0.0032) as well as with age at death (slope 0.049, t = 5.892, P < 0.0001) (r = 0.529, P < 0.0001). Thus, the ε4 allele apparently enhances age-dependent accumulation of Aβ42.
The effect of the ε4 allele on Aβ40 levels was less remarkable, although there was a similar tendency toward Aβ40 accumulation (Figure 4B) ▶ . Between the ages of 40 and 59 years, significant accumulation of Aβ40 was detected in five of 11 ε4 carriers (45.5%), which was approximately twice the frequency seen among noncarriers (seven of 30 cases; 23.3%). By 60 years of age and older, 11 of 14 ε4 carriers (78.6%) showed significant accumulation of Aβ40, whereas only three of 19 noncarriers (15.8%) between the ages of 60 and 69 years and 10 of 21 at 70 years and older (47.6%) did so. Thus, Aβ40 levels were also related to the apoE genotype (slope 0.245, t = 2.551, P = 0.0122 for the presence of ε4; slope 0.0119, t = 4.034, P = 0.0001 for age; r = 0.402, P < 0.0001), indicating that, as with Aβ42, age-dependent accumulation of Aβ40 is enhanced in ε4 carriers. Taken together, out findings strongly suggest that the ε4 allele predisposes the carrier to begin accumulating both Aβ42 and Aβ40 earlier in life than do noncarriers.
Discussion
In the present study, we chose to focus our analysis on Aβ extracted from the insoluble, but not soluble, fraction of brain because: 1) Aβ within senile plaques is recovered exclusively from the insoluble fraction; 2) Aβ levels in the insoluble fraction correlate well with immunocytochemically defined amyloid burden (senile plaque density); 6 3) we cannot exclude the possibility that Aβ in the soluble fraction comes from the insoluble fraction as a consequence of mechanical disruption of amyloid fibrils during homogenization (see Shinkai et al 18 ); and 4) Aβ in the insoluble fraction, but not that in the soluble fraction, is highly resistant to postmortem degradation. According to our preliminary experiments using rat brains, >95% of Aβ42 and ∼90% of Aβ40 in the insoluble fraction remain 24 hours postmortem. In contrast, Aβ levels in the soluble fraction decrease to ∼50% within 4 hours and become negligible within 12 hours postmortem.
Our measurements of Aβ in the insoluble fraction indicate that, contrary to earlier ideas, both Aβ40 and Aβ42 begin to accumulate at almost the same time and continued to do so in a coordinate manner, although the former accumulates at a disproportionately slow rate. The presence of ε4 accelerates the initial accumulation of Aβ42, and to a lesser extent, Aβ40, which likely explains the association between the ε4 allele and the reduction in the age of onset of AD. 27
From our present data it seems reasonable to assume: 1) that once Aβ42 accumulation is triggered, it continues at a similar rate until it reaches the plateau; 2) that the effect of the ε4 allele is to set the point at which Aβ42 begins to accumulate earlier in life; and 3) that there is no apparent ceiling for Aβ40 accumulation (see Walker et al 13 ). The first assumption is consistent with an earlier immunocytochemical observation that the number of senile plaques does not continuously increase during the progression of AD, but seems to level off. 28 This suggests a dynamic balance between Aβ deposition and resolution, a hypothesis that was recently validated by the discovery of the remarkable effect of Aβ42 immunization on Aβ deposition in PDAPP transgenic mice. 29
The present data also explain why ε4 carriers have a greater number of senile plaques than noncarriers among unselected autopsy cases, 11-13 and if we postulate that AD is the final consequence of long-term, progressive Aβ deposition, they may explain the aforementioned contradictory observations. 8-10 Among older individuals, in whom Aβ42 accumulation has reached a plateau, there would be no differences in Aβ42 deposition between ε4-positive and ε4-negative AD brains; the only difference would be the amount of Aβ40 deposition (see Figure 4 ▶ ). 8-10 This may simply mean that the ε4-carrying AD patients might have begun accumulating Aβ earlier and thus accumulated more Aβ40 than AD patients not carrying the ε4 allele.
Secreted soluble Aβ is widely believed to be the source of Aβ deposits in the brain. Aβ42 levels in plasma are reported to be elevated in FAD pedigrees carrying mutant APP or presenilins, 7 but no such observation has been made in ε4 carriers. This means that although mutations of the three causative genes and the presence of ε4 allele may all induce accumulation of Aβ to begin decade(s) earlier than it otherwise would, their respective mechanisms may differ.
Even the brains from young patients contain insoluble Aβ, the levels of which are a sensitive indicator of the earliest stage of Aβ accumulation. It is therefore possible that these particular insoluble Aβ40 and Aβ42 species are involved in very earliest stage of Aβ accumulation. Insoluble Aβ, as defined here, consists of Aβ species from both intracellular and extracellular compartments: the aggregated and/or fibrillar Aβ in the extracellular space are by far the predominant insoluble species in brains with abundant senile plaques, whereas in brains containing minimal amounts of insoluble Aβ42 and no senile plaques, an increase in Aβ42 levels is associated with the low-density membrane compartments often referred to as detergent-insoluble, glycolipid-enriched membrane domains (DIGs) (unpublished observations). 30 Approximately half of the intracellular insoluble Aβ in a human neuroblastoma cell line, 20 and presumably in rat brain, 31 is localized to this compartment. Thus, one possible pathway leading to Aβ accumulation can be traced back to the DIGs within the cells: an abnormal increase in the levels of Aβ42 within DIGs may be induced by slowed membrane trafficking associated with aging. It is of note that recent reports 32,33 suggest that nonfibrillar Aβ42 accumulates intraneuronally in the brains of aged patients not showing signs of dementia, as well as in those with AD and Down’s syndrome patients. Moreover, because caveolae and rafts, representative structures of DIGs, are present mostly in the plasma membrane, 30 excess Aβ42 accumulating on the plasma membrane would be expected to be shed into the extracellular space and act as a seed for Aβ polymerization. This notion is consistent with immunohistochemical and immunoelectron microscopic observations showing Aβ to be situated along the plasma membrane in diffuse plaques. 34-36
Because apoE plays a major role in the metabolism of cholesterol, 1 which is a major constituent of DIGs, 30 the effect of the ε4 allele on Aβ accumulation may reflect alteration of the lipid composition of DIGs. For instance, a significant decrease in the cholesterol levels is observed in the brains of ε4 knock-in mice. 37 Thus, altered cholesterol metabolism may significantly influence the membrane microenvironment surrounding Aβ and/or APP, leading to aberrant turnover of Aβ. The observation that cholesterol depletion completely inhibits the generation of Aβ suggests this possibility. 38
Acknowledgments
We thank Ms. J. Saishoji and Ms. N. Naoi for tissue preparation, and Ms. M. Anzai for the manuscript preparation.
Footnotes
Address reprint requests to Yasuo Ihara, M.D., Department of Neuropathology, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: yihara@m.u-tokyo.ac.jp.
Supported in part by Research Grants for Longevity Sciences from the Ministry of Health and Welfare, Japan, and from the Sasakawa Health Science Foundation, Japan.
References
- 1.Weisgraber KH, Mahley RW: Human apolipoprotein E: the Alzheimer’s disease connection. FASEB J 1996, 10:1485-1494 [DOI] [PubMed] [Google Scholar]
- 2.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD: Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA 1993, 90:1977-1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261:921-923 [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ: The cell biology of β-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol 1998, 8:447-453 [DOI] [PubMed] [Google Scholar]
- 5.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y: Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron 1994, 13:45-53 [DOI] [PubMed] [Google Scholar]
- 6.Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, Namekata K, Okeda R, Ihara Y: Quantitation of amyloid β-protein (Aβ) in the cortex during aging and in Alzheimer’s disease. Am J Pathol 1998, 152:1633-1640 [PMC free article] [PubMed] [Google Scholar]
- 7.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S: Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 1996, 2:864-870 [DOI] [PubMed] [Google Scholar]
- 8.Gearing M, Mori H, Mirra SS: Aβ-peptide length and apolipoprotein E genotype in Alzheimer’s disease. Ann Neurol 1997, 39:395-399 [DOI] [PubMed] [Google Scholar]
- 9.Mann DM, Iwatsubo T, Pickering-Brown SM, Owen F, Saido TC, Perry RH: Preferential deposition of amyloid β protein (Aβ) in the form Aβ40 in Alzheimer’s disease is associated with a gene dosage effect of the apolipoprotein E E4 allele. Neurosci Lett 1997, 221:81-84 [DOI] [PubMed] [Google Scholar]
- 10.Ishii K, Tamaoka A, Mizusawa H, Shoji S, Ohtake T, Fraser PE, Takahashi H, Tsuji S, Gearing M, Mizutani T, Yamada S, Kato M, St George-Hyslop PH, Mirra SS, Mori H: Aβ1-40 but not Aβ1-42 levels in cortex correlate with apolipoprotein E ε4 allele dosage in sporadic Alzheimer’s disease. Brain Res 1997, 748:250-252 [DOI] [PubMed] [Google Scholar]
- 11.Arai T, Ikeda K, Akiyama H, Haga C, Usami M, Sahara N, Iritani S, Mori H: A high incidence of apolipoprotein E ε4 allele in middle-aged non-demented subjects with cerebral amyloid β protein deposits. Acta Neuropathol (Berl) 1999, 97:82-84 [DOI] [PubMed] [Google Scholar]
- 12.Ohm TG, Scharnagl H, Marz W, Bohl J: Apolipoprotein E isoforms and the development of low and high Braak stages of Alzheimer’s disease-related lesions. Acta Neuropathol (Berl) 1999, 98:273-280 [DOI] [PubMed] [Google Scholar]
- 13.Walker LC, Pahnke J, Madauss M, Vogelgesang S, Pahnke A, Herbst EW, Stausske D, Walther R, Kessler C, Warzok RW: Apolipoprotein E4 promotes the early deposition of Aβ42 and then Aβ40 in the elderly. Acta Neuropathol (Berl) 2000, 100:36-42 [DOI] [PubMed] [Google Scholar]
- 14.Tierney MC, Fisher H, Lewis AJ: The NINCDS-ADRDA Work group criteria for the clinical diagnosis of probable Alzheimer’s disease: clinicopathological study of 57 cases. Neurology 1988, 38:356-364 [DOI] [PubMed] [Google Scholar]
- 15.Mirra SS, Heyman A, McKee D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L, : participating CERAD neuropathologists: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 42:1681-1688 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki N, Cheung TT, Cai X-D, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG: An increased percentage of long amyloid β-protein secreted by familial amyloid β-protein precursor (βAPP717) mutants. Science 1994, 264:1336-1340 [DOI] [PubMed] [Google Scholar]
- 17.Asami-Odaka A, Ishibashi Y, Kikuchi T, Kitada C, Suzuki N: Long amyloid β-protein secreted from wild-type human neuroblastoma IMR-32 cells. Biochemistry 1995, 34:10272-10278 [DOI] [PubMed] [Google Scholar]
- 18.Shinkai Y, Yoshimura M, Morishima-Kawashima M, Ito Y, Shimada H, Yanagisawa K, Ihara Y: Amyloid β-protein deposition in the leptomeninges and cerebral cortex. Ann Neurol 1997, 42:899-908 [DOI] [PubMed] [Google Scholar]
- 19.Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L: Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci USA 1997, 94:1550-1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morishima-Kawashima M, Ihara Y: The presence of amyloid β-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry 1998, 37:15247-15253 [DOI] [PubMed] [Google Scholar]
- 21.Enya M, Morishima-Kawashima M, Yoshimura M, Shinkai Y, Kusui K, Khan K, Games D, Schenk D, Sugihara S, Yamaguchi H, Ihara Y: Appearance of sodium dodecyl sulfate-stable amyloid β-protein (Aβ) dimer in the cortex during aging. Am J Pathol 1999, 154:271-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hixson JE, Vernier DT: Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990, 31:545-548 [PubMed] [Google Scholar]
- 23.Ueki A, Kawano M, Namba Y, Kawakami M, Ikeda K: A high frequency of apolipoprotein E4 isoprotein in Japanese patients with late-onset nonfamilial Alzheimer’s disease. Neurosci Lett 1993, 163:166-168 [DOI] [PubMed] [Google Scholar]
- 24.Yoshizawa T, Yamakawa-Kobayashi K, Komatsuzaki Y, Arinami T, Oguni E, Mizusawa H, Shoji S, Hamaguchi H: Dose-dependent association of apolipoprotein E allele ε4 with late-onset, sporadic Alzheimer’s disease. Ann Neurol 1994, 36:656-659 [DOI] [PubMed] [Google Scholar]
- 25.Harigaya Y, Shoji M, Kawarabayashi T, Kanai M, Nakamura T, Iizuka T, Igeta Y, Saido TC, Sahara N, Mori H, Hirai S: Modified amyloid β protein ending at 42 or 40 with different solubility accumulates in the brain of Alzheimer’s disease. Biochem Biophys Res Commun 1995, 211:1015-1022 [DOI] [PubMed] [Google Scholar]
- 26.Russo C, Saido TC, DeBusk LM, Tabaton M, Gambetti P, Teller JK: Heterogeneity of water-soluble amyloid β-peptide in Alzheimer’s disease and Down’s syndrome brains. FEBS Lett 1997, 409:411-416 [DOI] [PubMed] [Google Scholar]
- 27.Okuizumi K, Onodera O, Tanaka H, Kobayashi H, Tsuji S, Takahashi H, Oyanagi K, Seki K, Tanaka M, Naruse S, Miyatake T, Mizusawa H, Kanazawa I: ApoE-ε4 and early-onset Alzheimer’s. Nat Genet 1994, 7:10-11 [DOI] [PubMed] [Google Scholar]
- 28.Hyman BT, Marzloff K, Arriagada PV: The lack of accumulation of senile plaques or amyloid burden in Alzheimer’s disease suggests a dynamic balance between amyloid deposition and resolution. J Neuropathol Exp Neurol 1993, 52:594-600 [DOI] [PubMed] [Google Scholar]
- 29.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P: Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999, 400:173-177 [DOI] [PubMed] [Google Scholar]
- 30.Simons K, Ikonen E: Functional rafts in cell membranes. Nature 1997, 387:569-572 [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Liyanage U, Bickel PE, Xia W, Lansbury PT, Jr, Kosik KS: A detergent-insoluble membrane compartment contains Aβ in vivo. Nat Med 1998, 4:730-734 [DOI] [PubMed] [Google Scholar]
- 32.Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR: Intraneuronal Aβ42 accumulation in human brain. Am J Pathol 2000, 156:15-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mochizuki A, Tamaoka A, Shimohata A, Komatsuzaki Y, Shoji S: Aβ42-positive non-pyramidal neurons around amyloid plaques in Alzheimer’s disease. Lancet 2000, 355:42-43 [DOI] [PubMed] [Google Scholar]
- 34.Probst A, Langui D, Ipsen S, Robakis N, Ulrich J: Deposition of β/A4 protein along neuronal plasma membranes in diffuse senile plaques. Acta Neuropathol (Berl) 1991, 83:21-29 [DOI] [PubMed] [Google Scholar]
- 35.Pappolla MA, Omar RA, Sambamurti K, Anderson JP, Robakis NK: The genesis of the senile plaque. Further evidence in support of its neuronal origin. Am J Pathol 1992, 141:1151-1159 [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi H, Maat-Scieman MLC, van Duinen SG, Prins FA, Neeskens P, Natt R, Roos RAC: Amyloid β protein (Aβ) starts to deposit as plasma membrane-bound form in diffuse plaques of brains from hereditary cerebral hemorrhage with amyloidosis-Dutch type, Alzheimer disease and nondemented aged subjects. J Neuropathol Exp Neurol, 2000, 59:723–732 [DOI] [PubMed]
- 37.Hamanaka H, Katoh-Fukui Y, Suzuki K, Kobayashi M, Suzuki R, Motegi Y, Nakahara Y, Takeshita A, Kawai M, Ishiguro K, Yokoyama M, Fujita SC: Altered cholesterol metabolism in human apolipoprotein E4 knock-in mice. Hum Mol Genet 2000, 9:353-361 [DOI] [PubMed] [Google Scholar]
- 38.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K: Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 1998, 95:6460-6464 [DOI] [PMC free article] [PubMed] [Google Scholar]