Abstract
Trypsinogen is a serine proteinase produced mainly by the pancreas, but it has recently been found to be expressed also in several cancers such as ovarian and colon cancer and in vascular endothelial cells. In this study, we found that trypsinogen-1 and -2 are present at high concentrations (median levels, 0.4 and 0.5 mg/L, respectively) in human seminal fluid and purified them to homogeneity by immunoaffinity and anion exchange chromatography. Purified trypsinogen isoenzymes displayed a Mr of 25 to 28 kd in sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. Most of the trypsinogen-1 purified from seminal fluid was enzymatically active whereas trypsinogen-2 occurred as the proform, which could be activated by enteropeptidase in vitro. Immunohistochemically, trypsinogen protein was detected in the human prostate, urethra, utriculus, ejaculatory duct, seminal vesicles, deferent duct, epididymal glands, and testis. Expression of trypsinogen mRNA in the same organs was demonstrated by in situ hybridization. Trypsinogen mRNA was also detected in the prostate and seminal vesicles by reverse transcriptase-polymerase chain reaction and Northern blotting. Isolated trypsin was shown to activate the proenzyme form of prostate-specific antigen. These results suggest that trypsinogen isoenzymes found in seminal fluid are produced locally in the male genital tract and that they may play a physiological role in the semen.
Trypsin is an arginine- and lysine-restricted serine proteinase. The pancreatic trypsins are secreted as proenzymes into pancreatic fluid and are activated by enteropeptidase in the intestine, where they activate other digestive enzymes. Four trypsinogen genes are expressed in humans. Cationic trypsinogen (trypsinogen-1), 1 anionic trypsinogen (trypsinogen-2), 1 and mesotrypsinogen (trypsinogen-3) 2 are expressed in the pancreas. However, trypsinogens are also expressed outside the gastrointestinal tract. We have previously purified trypsinogen-1 and -2 from mucinous ovarian tumor cyst fluid. 3 Trypsinogen-1 and -2 are also expressed by several tumors 4-6 and cancer cell lines 7 as well as by endothelial cells 8 and epithelial cells of various normal tissues. 9 Trypsinogen-4 is expressed in the brain. 10
Human semen consists primarily of the secretions of the sex accessory tissues which include the prostate, seminal vesicles, epididymis, vas deferens, ampullae, Cowper’s gland, and glands of Littre. These organs produce several proteolytic enzymes such as human kallikrein-2 (hK2) 11 and prostate-specific antigen (PSA). 12 PSA is a chymotrypsin-like serine proteinase, 13 whereas hK2 has trypsin-like enzymatic activity. 14 Recent studies have shown that a recombinant proform of PSA is activated by bovine trypsin and recombinant hK2 in vitro, 15,16 but the physiological activators of proPSA and prohK2 are not known.
In this study, we have purified and characterized two trypsinogen isoenzymes from human seminal fluid. Furthermore, we have examined their expression and localization in various tissues of the male genital tract.
Materials and Methods
Samples
Fresh tissue specimens of human prostate, seminal vesicles, vas deferens, epididymis, and testis obtained by surgery were frozen by immersion in liquid nitrogen and stored at −80°C until analyzed. For immunohistochemistry and in situ hybridization studies, tissues were fixed within 30 minutes after removal in Bouin’s fixative (for 4 to 18 hours) or 4% buffered paraformaldehyde (overnight). The specimens were obtained from patients undergoing transurethral resection of the prostate or transvesical prostatectomy because of benign enlargement of the prostate, cystoprostatectomy because of invasive cancer of the urinary bladder, or radical prostatectomy or orchidectomy as treatment for prostate cancer. For control purposes, normal pancreatic tissue was obtained at surgery from two patients undergoing resection of small pancreatic tumors. All tissues were histopathologically normal according to hematoxylin and eosin staining. The Helsinki Declaration regarding the use of human tissues was followed.
Human semen was collected and allowed to liquefy at room temperature, after which the sperm was removed by low-speed centrifugation (600 × g, 10 minutes, room temperature). The resulting seminal fluid was further clarified by high-speed centrifugation (35,000 × g, 20 minutes, 4°C) and stored at −20°C until analyzed.
Antibodies and Immunoaffinity Media
Monoclonal antibodies (mAb) to PSA (6C11) (Leinonen J, Stenman UH, unpublished data), trypsinogen-1 (3E8 and 6D11), 17 and trypsinogen-2 (14F10 and 14D4) 17 were produced by standard procedures. A polyclonal antibody to α1-antichymotrypsin (ACT) was from DAKO A/S (Glostrup, Denmark). For immunoaffinity chromatography, mAbs 14F10, 3E8, and 6C11 were immobilized separately on CNBr-activated Sepharose 4B (Amersham Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer’s instructions. For immunoassays, mAbs 14F10 and 6D11, and the polyclonal antibody to ACT were labeled with an Eu3+ chelate as described. 18 A polyclonal antiserum against trypsinogen was produced as described 3 and a peroxidase-conjugated swine anti-rabbit IgG was from DAKO A/S. Characteristics of the primary antibodies used in immunohistochemistry are given in Table 1 ▶ . Biotinylated secondary antibodies, anti-rabbit and anti-mouse IgGs were included in the DAKO ChemMate kit (code K5003; BioTek Solutions, Carpenteria, CA) and anti-sheep IgG (code AB360) was from Binding-Site (Birmingham, UK).
Table 1.
Primary Antibodies Used in Immunohistochemistry
| Code | Source | Immunogen | Working concentration | Antigen retrieval (microwave/protease) | Reference |
|---|---|---|---|---|---|
| mAb 1482 | Mouse | Purified human pancreatic trypsin | 4 μg/ml | Both | Chemicon International Inc., Temecula, CA |
| 14D4 | Mouse | Trypsinogen-2 | 5 μg/ml | Both | Itkonen et al, 199017 |
| 14F10 | Mouse | Trypsinogen-2 | 2.5 μg/ml | Both | Itkonen et al, 199017 |
| 8F7 | Mouse | Trypsinogen-2 | 7.5 μg/ml | Both | Itkonen et al, 199017 |
| 3E8 | Mouse ascites | Trypsinogen-1 | 20 μg/ml | Both | Itkonen et al, 199017 |
| 6D11 | Mouse | Trypsinogen-1 | 2 μg/ml | Both | Itkonen et al, 199017 |
| 7401 | Sheep serum | Trypsin-1 | Diluted 1:400 | Protease only | Borgström et al, 197625 |
| Fahat | Sheep serum | Trypsinogen-2 | Diluted 1:100 | Protease only | Kimland et al, 198926 |
| 8336 | Rabbit | Trypsinogen-2 | 25 μg/ml | Both | Kimland et al, 198926 |
| X0931 | Mouse | Nonimmune | Diluted 1:200 | Both | DAKO A/S Glostrup, Denmark |
| X0936 | Rabbit | Nonimmune | Diluted 1:8000 | Both | DAKO A/S Glostrup, Denmark |
Immunoassays
The concentration of PSA was determined by the Delfia EQM PSA kit (Wallac, Turku, Finland) which recognizes free PSA and total PSA equally. The complex between PSA and ACT (PSA-ACT), was determined as described. 19,20 Trypsinogen-1 and -2 were determined by specific time-resolved immunofluorometric assays. 17 The detection limit was 0.01 μg/L for PSA, 0.2 μg/L for PSA-ACT, 19,20 and 0.1 and 0.3 μg/L for trypsinogen-1 and -2, respectively. 17 The inter- and intra-assay coefficients of variation were both 2 to 4% for the assay of PSA, 5 to 10% and 8 to 13% for PSA-ACT, 19,20 10 to 15% for trypsinogen-1, and 10 to 12% for trypsinogen-2. 17
Purification of Trypsinogen-1 and -2 from Human Seminal Fluid
Human seminal fluid (200 ml) was sequentially precipitated with ammonium sulfate at 30 and 70% saturation for 20 minutes at 4°C. The precipitate formed at 70% saturation was collected by centrifugation (5,000 × g, 20 minutes, 4°C), and dissolved in 50 ml of 50 mmol/L Tris-HCl, pH 7.4, containing 8 mmol/L NaN3, and 10 mmol/L benzamidine (buffer A). The resulting solution was clarified by centrifugation (35,000 × g, 20 minutes, 4°C), and applied to anti-trypsinogen-1 and -2 affinity columns (10 ml) connected in series. The columns were washed with buffer A containing 1 mmol/L NaCl and 0.1% (v/v) Triton X-100 until the absorbance at 280 nm in the flow-through fraction was <0.001. The two columns were separated and the bound proteins were eluted with 0.1% (v/v) trifluoroacetatic acid. Two-ml fractions were collected and immediately neutralized by adding 0.2 ml of 1 mol/L Tris-HCl, pH 8.0. Fractions containing trypsinogen-1 or -2 immunoreactivity were pooled and dialyzed against 10 mmol/L Tris-HCl, pH 8.0, containing 8 mmol/L NaN3 and 2 mmol/L benzamidine (buffer B), and applied to an anion exchange Resource Q column (6 ml) (Amersham Pharmacia Biotech) equilibrated with buffer B containing 0.5 mol/L NaCl (buffer C). The column was washed with five bed volumes of the same buffer. Bound proteins were eluted with a linear gradient consisting of 60 ml of buffer B and 60 ml of buffer C. Flow rate was 2 ml/min and fractions of 1 ml were collected. Trypsinogen in the fractions was detected by immunofluorometric assays. The fractions in each peak were pooled and dialyzed against 10 mmol/L Tris-HCl, pH 8.0, containing 8 mmol/L NaN3 before measuring the enzyme activity of trypsin.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blotting
For Western blotting, 1 μg of trypsinogen-1 and -2 purified by immunoaffinity chromatography from seminal fluid were separated on a 2-mm thick 3 to 16% gradient sodium dodecyl sulfate-polyacrylamide gel under reducing conditions 21 and electrophoretically transferred to an Immobilon-P membrane (Millipore, Bedford, MA). 22 Nonspecific binding was blocked with 1% (w/v) bovine serum albumin (Sigma, St. Louis, MO) in phosphate-buffered saline (PBS) (50 mmol/L sodium phosphate, 150 mmol/L NaCl, pH 7.4) at 4°C (overnight). The membrane was incubated with a polyclonal antibody against trypsinogens 3 (100 μg/ml) for 2 hours at 37°C followed by peroxidase-conjugated swine anti-rabbit IgG (DAKO A/S) diluted 1:1,000 for 4 hours at room temperature. After washing with PBS, the blot was developed with a solution of 0.3 mg/ml 3,3′-diaminobenzidine tetrahydrochloride (Sigma) and 0.08% H2O2 in PBS.
Measurement of the Enzymatic Activity of Trypsin
The enzymatic activity of trypsinogen fractions purified by immunoaffinity and anion exchange chromatography from seminal fluid were measured using a chromogenic peptide substrate N-benzoyl-l-isoleucyl-glutamyl-l-arginine-p-nitroanilide (S-2222) (Kabi, Stockholm, Sweden). Trypsinogen (0.9 nmol/L) and S-2222 (0.5 mmol/L) were incubated in the presence or absence of enteropeptidase (Sigma) (5 ng) in 1 ml of 50 mmol/L Tris-HCl buffer, pH 8.0, containing 10 mmol/L CaCl2 and 1% (v/v) Triton X-100 at room temperature. Substrate hydrolysis was followed for 110 minutes by reading the absorbance at 405 nm on a microplate reader (Labsystems Multiskan Bichromatic; Labsystems, Helsinki, Finland).
Inhibition of trypsin-2 purified from urine of pancreatitis patients 3 (0.7 nmol/L) by zinc was studied using the substrate S-2222 (100 μmol/L) and ZnCl2 concentrations varying from 25 μmol/L to 1 mmol/L in 50 μl of 50 mmol/L Tris-HCl, pH 8.0, containing 20 mmol/L CaCl2 and 0.01% (v/v) Triton X-100. Tumor-associated trypsin inhibitor purified from urine of pancreatitis patients 3 (1.5 nmol/L) was used as a control. Substrate hydrolysis was followed for 20 minutes at 37°C starting 10 minutes after mixing the enzyme with ZnCl2 by reading the absorbance at 405 nm on a microplate reader.
Activation of proPSA by Trypsin
Activation of proPSA by trypsin was studied by using proPSA purified from LNCap cell medium and trypsin-1 and -2 purified from seminal fluid, and further by using recombinant proPSA. 16 ProPSA was purified from LNCap cell medium by immunoaffinity chromatography as described earlier. 23 Intact mature PSA (B isoenzyme) was purified from seminal fluid as described. 24 Purified PSA (10 μg) was incubated with 0.05 μg or 10 μg of trypsinogens purified from seminal fluid (molar ratio ∼1:200 and 1:1, respectively) in the presence or absence of enteropeptidase (5 ng; Sigma) in 100 μl of 50 mmol/L Tris-HCl containing 150 mmol/L NaCl, 8 mmol/L NaN3, 1% bovine serum albumin, pH 7.4, for 1 hour at 37°C. Then 80 μg of α1-antichymotrypsin (Athens Research Technology Inc., Athens, Georgia) (molar ratio to PSA 4:1) in 1 ml of the above-mentioned buffer was added and the incubation continued for 12 hours at room temperature. The concentration of PSA and PSA-ACT in aliquots taken at 1, 4, and 12 hours was determined by specific immunoassays. Formation of PSA-ACT complex was taken as a measure of proPSA activation.
Activation of recombinant proPSA 16 (kindly provided by Dr. Janita Lövgren, University of Turku, Finland) (0.7 μmol/L) by trypsin-2 purified from urine of a pancreatitis patient 3 (40 nmol/L) was studied using a chromogenic peptide substrate 3-carbomethoxy-propionyl-l-arginyl-l-prolyl-l-tyrosine-p-nitroanilide (S-2586) (Kabi) (0.5 mmol/L) in 50 μl of 50 mmol/L Tris-HCl, pH 7.5, containing 150 mmol/L NaCl and 1 mmol/L CaCl2. ProPSA and trypsin incubated alone with the substrate were used as negative controls. Substrate hydrolysis was followed for 4 hours at 37°C by monitoring the absorbance at 405 nm on a microplate reader.
Immunohistochemistry
Immunohistochemistry was performed using a detection kit (DAKO ChemMate Detection Kit Peroxidase/Carbazole, Rabbit/Mouse) and a staining machine (DAKO TechMate 500/1000 Instrument; BioTek Solutions). Briefly, the sections were deparaffinized in xylene, rehydrated, and treated with 0.3% H2O2 in methanol for 30 minutes at room temperature to quench endogenous peroxidase activity. For antigen retrieval, tissue sections were first incubated with sodium citrate (10 mmol/L, pH 6.0) and boiled in a microwave oven at 750 W for 2 × 3 minutes, and then digested with proteinase K (20 μg/ml in 20 mmol/L Tris-HCl, 2 mmol/L CaCl2, pH 7.5) for 25 minutes at 37°C. The sections were incubated with primary antibodies 17,25,26 (Table 1) ▶ for 60 minutes at room temperature, after which they were incubated with either biotinylated secondary antibodies against rabbit or mouse IgG, which were included in the ChemMate kit (code K5003; BioTek), or with an anti-sheep IgG (Binding-Site), diluted 1:200 for 60 minutes at room temperature. The immunoreactivity was visualized using the manufacturer’s protocol for the peroxidase/3-amino-9-ethyl-carbazole reagent in the ChemMate kit. The sections were counterstained with Mayer’s hematoxylin solution. As a negative control, adjacent tissue sections were processed by replacing the primary antibody with nonimmune mouse IgG1 diluted 1:200 (code X0931; DAKO) or nonimmune rabbit IgG diluted 1:8,000 (code X0936; DAKO). Pancreatic tissue was used as a positive control.
Riboprobe Synthesis for in Situ Hybridization
In vitro transcriptions of sense and antisense probes were made by fluorescein-UTP riboprobe synthesis using the RNA color kit (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. As a template, we used a 627-bp long trypsinogen-2 cDNA fragment (corresponding to nucleotides 42 to 688, accession number M27602 27 ), cloned from COLO 205 cells by RT-PCR using the TA Cloning Kit (InVitrogen, San Diego, CA) and the following primers: 5′-TGC TGT TGC TGC CCC CTT TG-3′ (sense) and 5′-GCA CAG CCA TAG CCC CAG GAG-3′ (antisense). The integrity and length of the probes was determined by gel electrophoresis.
In Situ Hybridization
All reagents were purchased from Sigma and Amersham Pharmacia Biotech. Tissue specimens were fixed, paraffin-embedded, sectioned (4 μm), dried for 2 hours at 65°C and mounted on SuperFrost plus slides (Menzel-Gläser) under RNase-free conditions. The sections were deparaffinized in xylene and rehydrated, after which they were first treated with 0.2 mol/L HCl to abolish endogenous enzyme activity, and then digested with proteinase K (20 μg/ml in 20 mmol/L Tris-HCl, 2 mmol/L CaCl2, pH 7.5) for 25 minutes at 37°C. The slides were then incubated in 0.25% acetic anhydride containing 0.1 mol/L triethanolamine and 0.9% NaCl, and then equilibrated in 2× standard saline citrate (SSC, 1× contains 150 mmol/L NaCl and 15 mmol/L sodium citrate, pH 7.0). After prehybridization with 40 μl of hybridization buffer containing 50% (v/v) formamide, 10 mmol/L Tris-HCl, pH 7.6, 1× Denhardt’s solution (bovine serum albumin, polyvinylpyrrolidone and Ficoll, all at 0.2 mg/ml), 2× SSC, and 0.4 μg/ml salmon sperm DNA at 55°C for 1 hour, the slides were hybridized with 40 μl of 250 ng/ml antisense or sense probe in hybridization buffer first for 8 minutes at 85°C and then for 16 hours at 55°C.
After hybridization, the slides were washed in 1× SSC at room temperature (2 × 5 minutes), 0.1× SSC at 60°C (4 × 15 minutes), 1× SSC at room temperature (10 minutes), and then equilibrated in Tris-buffered saline (TBS) (100 mmol/L Tris-HCl, 0.4 mol/L NaCl, pH 7.5). For detection of hybridization signals, tissue sections were first incubated in blocking reagent, 0.5% (w/v) in TBS, for 1 hour at room temperature, rinsed in TBS, and subsequently incubated with anti-fluorescein alkaline phosphatase conjugate (Amersham Pharmacia Biotech) diluted 1:1,000 in TBS containing 0.5% (w/v) bovine serum albumin for 2 hours at room temperature. After washing in TBS, the sections were equilibrated in detection buffer (100 mmol/L Tris-HCl containing 100 mmol/L NaCl, 50 mmol/L MgCl2, pH 9.5) (5 minutes), and developed in detection buffer containing 1 mmol/L levamisol, 0.33 mg/ml nitroblue tetrazolium chloride, and 0.175 mg/ml 5-bromo-4-chloro-3-indolyl phosphate. The color reaction was stopped after 2 to 8 hours by incubating the sections in stop buffer (10 mmol/L Tris-HCl containing 10 mmol/L ethylenediaminetetraacetic acid, 0.9% NaCl, pH 7.5) for 10 minutes. The slides were coverslipped using Faramount mounting medium (DAKO).
Isolation of RNA, Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), and Sequencing of the PCR Product
Total RNA was extracted from prostate tissue and seminal vesicle according to the method of Chomczynski and Sacchi. 28 The oligonucleotide primers used for trypsinogen were constructed on the basis of the published sequence for trypsinogen-2 27 so that they also recognized trypsinogen-1 transcripts: 5′-CAT GAA TCT ACT CCT GAT CC-3′ (outer sense), 5′-TGT CAT TGT CCA GAG TCC-3′ (outer antisense), and 5′-CCC CTT TGA TGA TGA TGA C-3′ (inner sense) 5′-AAC TGT TCA TTC CCC TCC-3′ (inner antisense). The outer and inner primer pairs produced fragments of 323 and 213 bp, respectively. β-actin primers were prepared on the basis of the published sequence: 29 5′-CCC AGG CAC CAG GGC GTG AT-3′ (sense) and 5′-TCA AAC ATG ATC TGG GTC AT-3′ (antisense). They produced a fragment of 260 bp. Total RNA (1 μg) was transcribed into cDNA using SuperScript II-RT (GibcoBRL, Paisley, UK) according to the manufacturer’s instructions. Contamination of RNA samples with cDNA was excluded by using control reactions without reverse transcriptase. The reverse transcription product (1 μl) was amplified in a 40-μl reaction volume in 1× PCR buffer (10 mmol/L Tris-HCl, 1.5 mmol/L MgCl2, 50 mmol/L KCl, 0.1% Triton X-100, pH 8.8; Finnzymes, Espoo, Finland) containing 0.25 mmol/L of each dNTP, 20 pmol of both outer antisense and sense primers, and 1.6 U of Dynazyme DNA polymerase (Finnzymes) for 30 cycles at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 30 seconds. After the first PCR round, 1 μl of the PCR product was further amplified using the inner primer pair and the same PCR conditions except the annealing temperature which was 53°C. RNA isolated from COLO 205 cells (ATCC, Rockville, MD) was used as a positive control and water as a negative control in all experiments. The PCR products were separated in a 1.5% agarose gel and stained with ethidium bromide. The PCR products were then purified using a kit for DNA Extraction (Amicon, Inc., Beverly, MA) and sequenced using the ABI Prism Dye Terminator Cycle Sequencing Core Kit with AmpliTaq DNA Polymerase and the ABI Prism 310 Genetic Analyser (PE Biosystems, Foster City, CA). The identity of the mRNA was determined by sequencing the PCR products and comparing the resulting sequences with the ones in the EMBL Nucleotide Sequence Database.
Northern Blotting
Nylon membrane blotted with 2 μg of poly A RNA (Clontech Laboratories, Inc., Palo Alto, CA) was used to study the expression of trypsinogen in the prostate and testis. The membrane was hybridized with a 627-bp long 32P-labeled cDNA probe (labeled with the Rediprime DNA-labeling system, Amersham Pharmacia Biotech) corresponding to nucleotides 42 to 688 of the human trypsinogen-2 cDNA sequence 27 (see riboprobe synthesis for in situ hybridization) in ExpressHyb hybridization buffer (Clontech Laboratories) at 68°C for 16 hours. The membrane was washed in several changes of 2× SSC, 0.05% sodium dodecyl sulfate for 15 minutes at room temperature. For autoradiography, the membrane was exposed to Hyperfilm-MP film (Amersham Pharmacia Biotech) for 1 to 2 days. A glycaraldehyde-3-posphate dehydrogenase cDNA probe was used to quantify the amount and quality of loaded polyA RNA.
Results
Determination and Purification of Trypsinogens from Seminal Fluid
The concentration of trypsinogen-1 in 24 samples studied ranged from 0.12 to 1.6 mg/L (median, 0.4 mg/L), and that of trypsinogen-2 from 0.02 to 1.4 mg/ml (median, 0.5 mg/L). A pool of 200 ml of seminal fluid containing ∼70 μg of trypsinogen-1 and 100 μg of trypsinogen-2 was used as starting material for purification of trypsinogens. Precipitation with ammonium sulfate resulted in a 2.6-fold purification (Table 2) ▶ . When the precipitate was reconstituted and applied to the immunoaffinity columns, 90% of trypsinogen immunoreactivity was retained. After disconnection of the columns, bound trypsinogen-1 and -2 were separately eluted with 0.1% trichloroacetatic acid, neutralized, and further fractionated by anion exchange chromatography on a Resource Q column. Three peaks with trypsinogen-1 immunoreactivity, designated 1A to 1C and four peaks with trypsinogen-2 immunoreactivity, designated 2A to 2D, were obtained (Figure 1, A and B) ▶ . The major peak with trypsinogen-1 immunoreactivity (1C) represented ∼80% of the trypsinogen-1 immunoreactivity recovered and 26% of that in the starting material. The major trypsinogen-2 peak (2C) represented 50% of the trypsinogen-2 recovered and 30% of that in the starting material (Table 2) ▶ .
Table 2.
Recovery of Trypsinogens during the Purification
| Total protein (mg) | Trypsinogen-1 | Trypsinogen-2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein (μg) | Purity (%) | Purification (fold) | Recovery (%) | Protein (μg) | Purity (%) | Purification (fold) | Recovery (%) | ||
| Seminal fluid | 9720 | 70 | 0.001 | 1.0 | 100 | 100 | 0.001 | 1.0 | 100 |
| Centrifugation | 7070 | 65 | 0.001 | 1.3 | 93 | 99 | 0.001 | 1.4 | 99 |
| Precipitation | 3350 | 59 | 0.002 | 2.5 | 84 | 96 | 0.003 | 2.8 | 96 |
| Immunoaffinity chromatography | Nd | 30 | Nd | Nd | 43 | 60 | Nd | Nd | 60 |
| Anion exhange chromatography* | Nd | 18 | Nd | Nd | 26 | 30 | Nd | Nd | 30 |
The total protein concentration was determined by measuring the absorbance at 280 nm. The concentrations of trypsinogen-1 and -2 were determined by immunofluorometric assays.
Nd, not determined
*Only the major peaks, trypsinogen-1C and trypsinogen-2D (Figure 1) ▶ , were considered.
Figure 1.
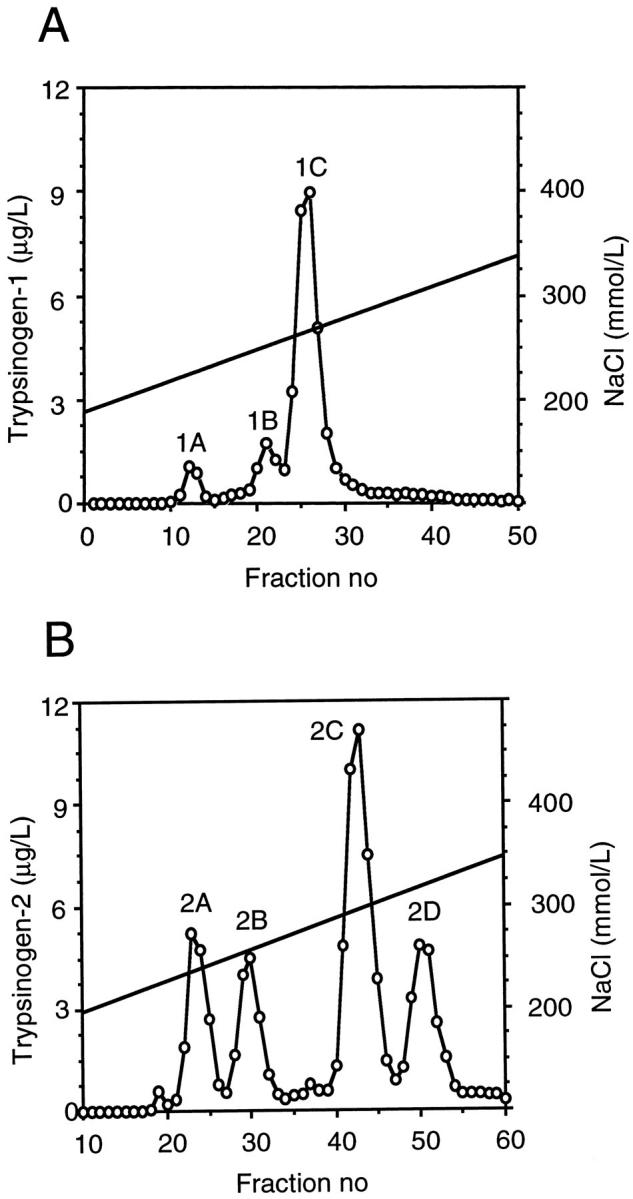
Anion exchange chromatography fractionation of trypsinogen-1 and -2 purified by immunoaffinity chromatography from seminal fluid. Trypsinogen-1 (A) and trypsinogen-2 (B) peaks were determined by specific immunofluorometric assays.
Characterization of Trypsinogens Purified from Seminal Fluid
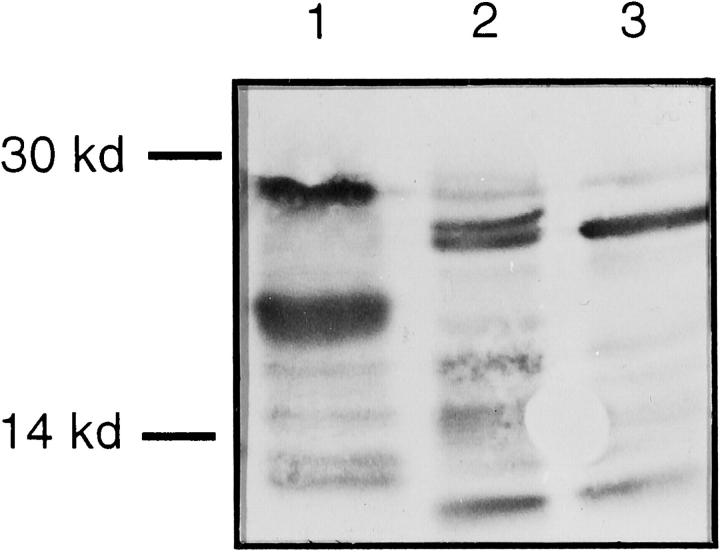
Trypsinogen-1 and -2 purified by immunoaffinity chromatography were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with a polyclonal trypsin antibody. Trypsinogens appeared as two major bands of 25 to 28 kd and several minor fragments of smaller size (Figure 2) ▶ .
Figure 2.
Western blotting of trypsinogen-1 and -2 purified from seminal fluid by immunoaffinity chromatography and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions. Trypsinogen-2 purified from urine of pancreatitis patient (positive control, lane 1), trypsinogen-2 purified from seminal fluid (lane 2), and trypsinogen-1 purified from seminal fluid (lane 3). Peroxidase staining.
After separation by anion exchange chromatography, the major trypsinogen-1 peak (1C) showed strong enzymatic activity against the peptide substrate (Figure 3A) ▶ . The two minor peaks, 1A and 1B, had no enzymatic activity and activity was not induced by enteropeptidase. The four peaks of trypsinogen-2 showed no enzymatic activity, but after incubation with enteropeptidase, the major trypsinogen-2 fraction (2C) was activated (Figure 3B) ▶ .
Figure 3.
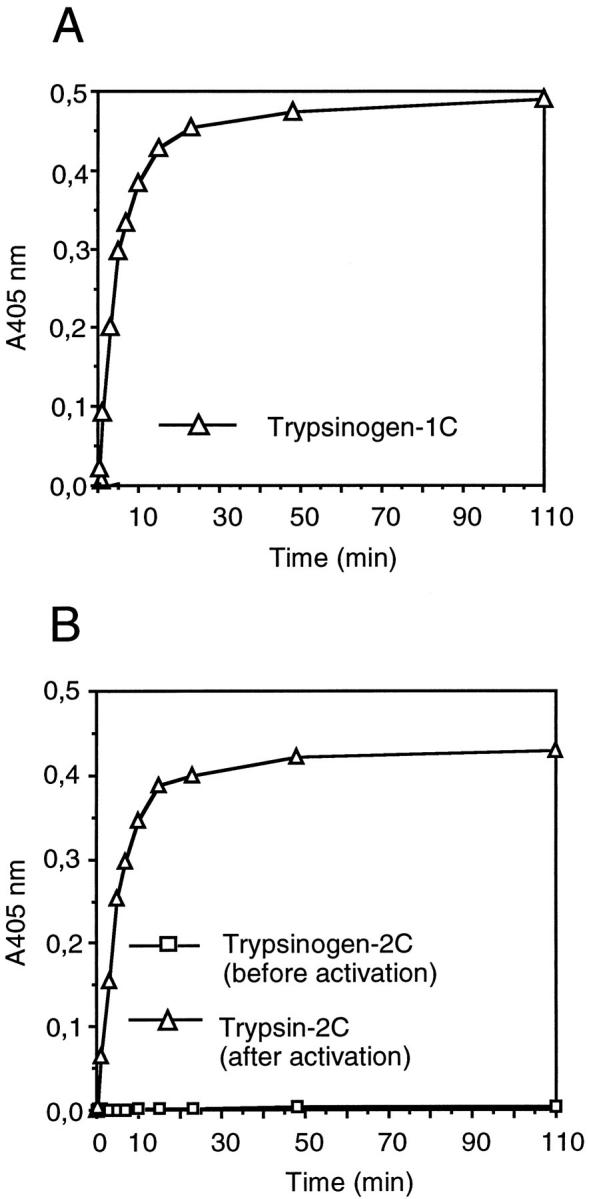
Enzyme activity of trypsinogen-1 and -2 fractionated by anion exchange chromatography from seminal fluid. Enzyme activity of the major trypsinogen-1 peak (1C) (A), and major trypsinogen-2 peak (2C) before and after activation by enteropeptidase (B). Enzyme activity was determined by measuring the cleavage of a chromogenic peptide substrate by reading the absorbance at 405 nm.
Activation of proPSA by Trypsin
The activation of LNCap-proPSA by seminal fluid trypsin was monitored by analyzing the complex formation between PSA and ACT by PSA-ACT immunoassay and recovery of PSA immunoreactivity. When LNCap-proPSA was incubated with ACT for 12 hours at 37°C, ∼25% of the PSA formed a complex with ACT. After incubation of LNCap-PSA with active trypsin-1C at a molar ratio of 1:200 for 1 hour at 37°C, ∼40% of the PSA formed a complex with ACT (Figure 4A) ▶ . The recovery of total PSA immunoreactivity was 90 to 100%. The proenzyme trypsinogen-2C had no effect on the complexation of LNCap-PSA with ACT, but after addition of enteropeptidase, the trypsin-2C formed increased the complexation of LNCap-PSA with ACT from 25 to 37% (Figure 4B) ▶ . When intact mature PSA isolated from seminal fluid was incubated with active trypsin-1C or -2C, there was no further increase in complex formation between PSA and ACT. If PSA was incubated with trypsin-1C at a high molar ratio (1:1), a significant loss (>50%) of PSA immunoreactivity was observed, regardless of whether PSA purified from seminal fluid or from LNCap cell medium was used, indicating degradation of PSA by trypsin.
Figure 4.
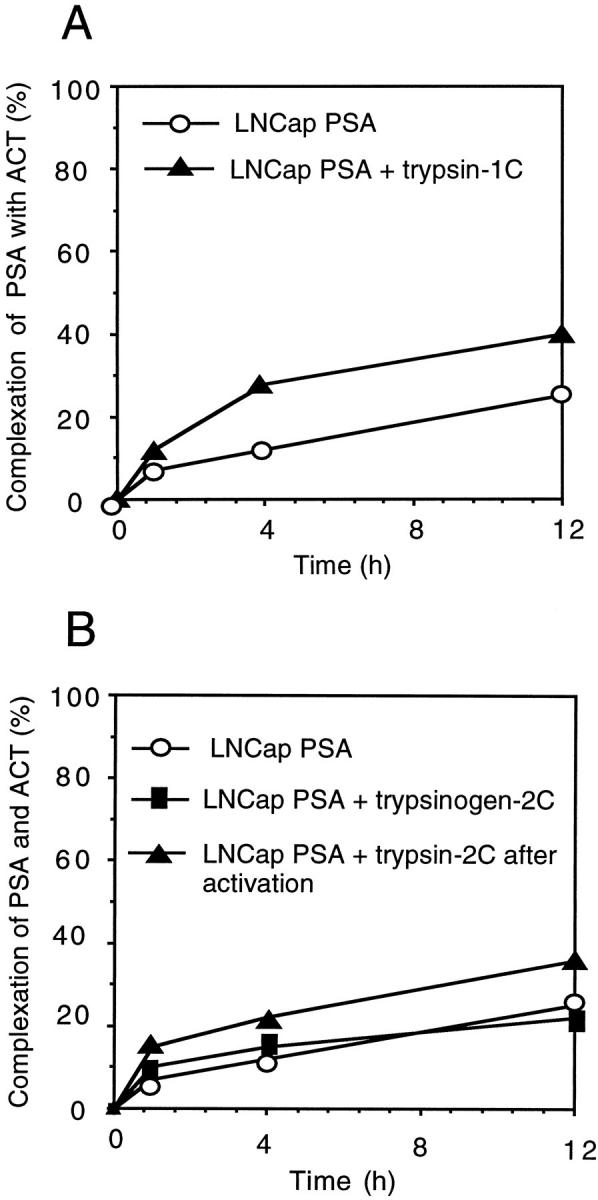
Activation of proPSA produced by prostatic adenocarcinoma (LNCap) cells by trypsin purified from seminal fluid. Activation of proPSA by trypsin-1 (1C) (A), and trypsinogen-2 (2C) with or without preceding activation of trypsinogen by enteropeptidase (B). Activation of proPSA was monitored by analyzing the complex formation between PSA and α1-antichymotrypsin (ACT) by PSA-ACT immunoassay and recovery of PSA immunoreactivity.
Incubation of recombinant proPSA by trypsin-2 yielded active PSA as evidenced by hydrolysis of the chromogenic peptide substrate S-2586. Trypsin-2 or proPSA alone did not cause hydrolysis of the substrate (data not shown).
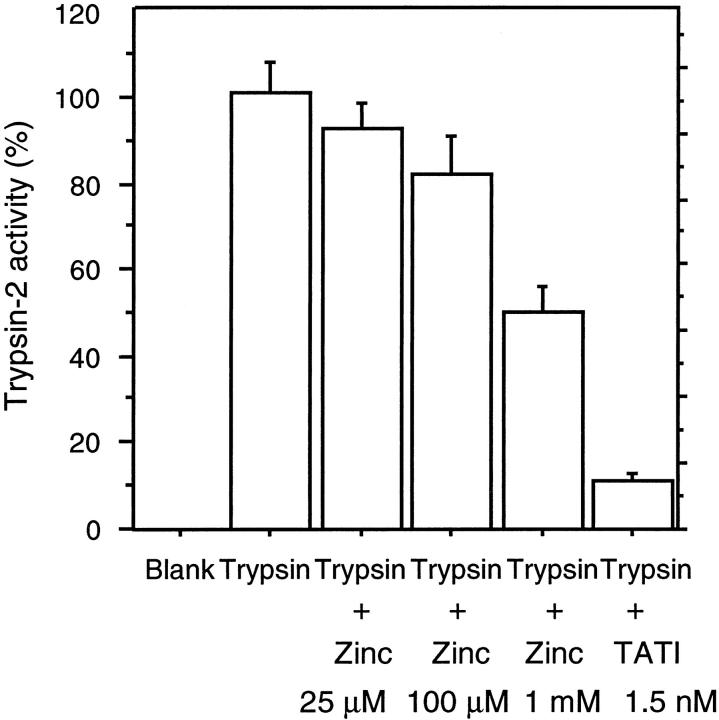
Inhibition of Trypsin-2 Activity by Zinc
Zinc inhibited trypsin-2 activity in a dose-dependent manner. At 0.025, 0.1, and 1 mmol/L Zn2+ concentrations, trypsin-2 activity was inhibited by 7.6%, 18%, and 50%, respectively. In a control experiment 1.5 nmol/L tumor-associated trypsin inhibitor inhibited trypsin-2 activity by 90% (Figure 5) ▶ .
Figure 5.
Inhibition of trypsin-2 activity by zinc. Cleavage of a chromogenic peptide substrate by trypsin-2 at indicated zinc and tumor-associated trypsin inhibitor concentrations was determined by measuring the absorbance at 405 nm. The results represent mean values and standard deviations from six parallel reactions.
Immunohistochemistry and in Situ Hybridization
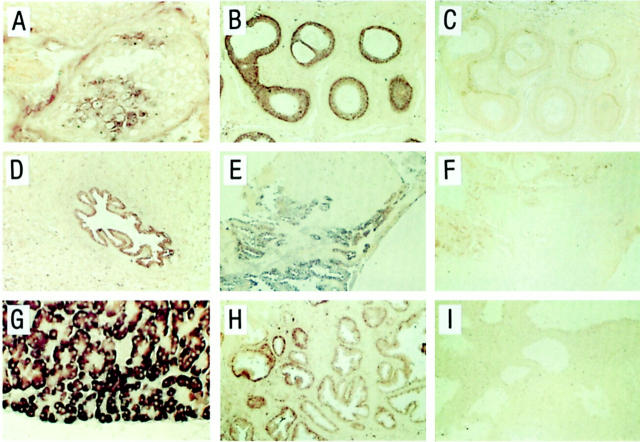
Trypsinogen immunoreactivity was detected in most tissue sections examined, but the number of positive cells and staining intensity varied (Figure 6) ▶ . However, a similar staining pattern was obtained with all antibodies used. In testis, a small number of immunoreactive spermatocytes were found in a few tubules. An intense immunostaining was detected in most epithelial cells of cauda epididymis, but also in a subset of the basal and luminal epithelial cells of the corpus and caput region. A vast majority of the epithelial cells of the vas deferens (close to the epididymis), ampulla vas deferens, seminal vesicles, utriculus prostaticus, and ejaculatory ducts also stained positively for trypsinogen. In the prostatic epithelium, luminal but not basal cells were immunoreactive for trypsinogen. A majority of the luminal cells in prostatic excretory ducts stained positively but in the acini and areas of benign hyperplasia, only few immunoreactive cells were detected. The epithelial cells of the prostatic part of urethra as well as the luminal cells of periurethral glands were immunoreactive.
Figure 6.
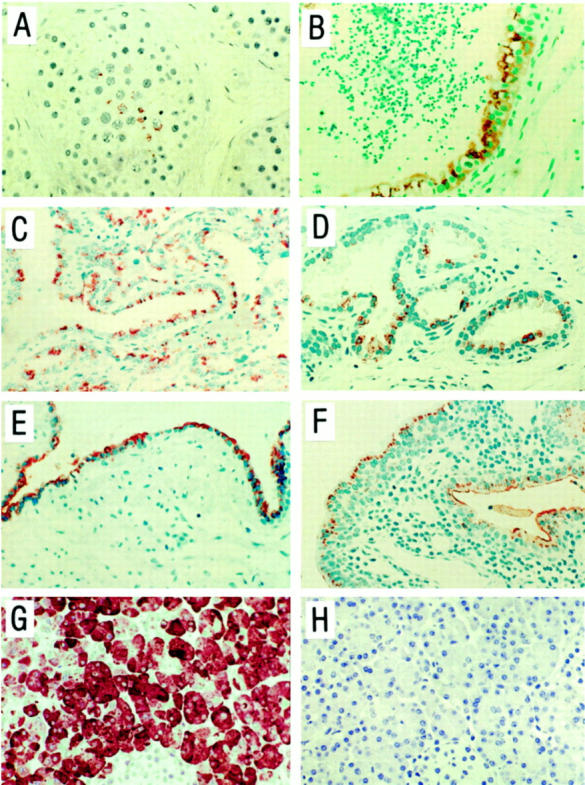
Immunohistochemical analysis for localization of trypsinogen protein in tissues from human male genital tract. Paraffin sections from testis (A), cauda epididymis (B), seminal vesicle (C), prostatic acini (D), prostatic duct (E), prostatic region of urethra and periutheral gland (F), and pancreas (G) were incubated with a monoclonal anti-trypsinogen antibody (mAb 1482), and pancreas (H) with nonimmune IgG1. Immunoperoxidase staining. Original magnifications: ×225 (A, B, G, and H); ×100 (C, E, and F); ×175 (D).
All trypsinogen antibodies generated a strong immunostaining in the exocrine pancreas and no immunostaining was detected in sections of pancreas or tissue sections from the male genital tract when trypsinogen antibodies were replaced by nonimmune IgG.
For in situ hybridization, the fluorescein-labeled antisense and sense probes were hybridized to adjacent tissue sections. In pancreas and in all tissues from the male genital tract that were studied, hybridization signals were generated by the antisense probe, whereas the sense probe did not give rise to any positive signals (Figure 7) ▶ . The intensity of the hybridization signals varied, but the results were in accordance with the immunohistochemical findings (Figure 7) ▶ .
Figure 7.
In situ hybridization analysis for localization of trypsinogen mRNA in tissues from human male genital tract. Paraffin sections from testis (A), cauda epididymis (B and C), vas deferens (D), seminal vesicle (E and F), pancreas (G), and prostate gland (H and I) were hybridized with a 627-base long fluorescein-labeled trypsinogen-2 antisense riboprobe construct (A, B, D, E, G, and H) and the corresponding sense probe (C, F, and I). Anti-fluorescein alkaline phospatase staining. Original magnifications: ×225 (A and G); ×75 (B–F, H, and I).
RT-PCR and Northern Blotting
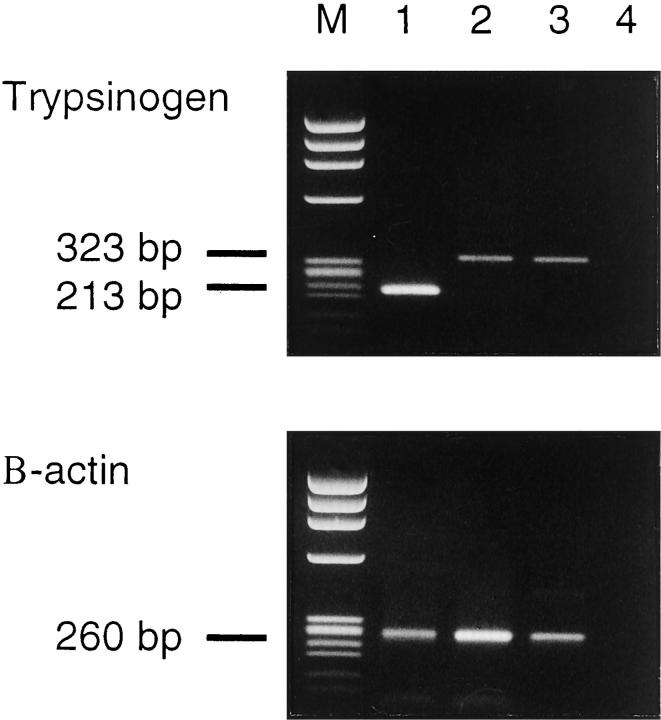
RT-PCR and sequencing of the PCR products showed that trypsinogen-1 and -2 transcripts are expressed in normal prostatic tissue and trypsinogen-1 in seminal vesicles (Figure 8) ▶ . Sequencing of the PCR products revealed the reported cDNA sequences of trypsinogen-1 and -2, respectively. 27 By Northern blotting, trypsinogen transcripts were detected in the prostate, but not in the testis (data not shown).
Figure 8.
RT-PCR analysis for trypsinogen mRNA from prostate (lane 1), seminal vesicle (lane 2), colon adenocarcinoma cell line (positive control, lane 3), and H20 (negative control, lane 4). Sample 1 was amplified with nested primers whereas samples 2 to 4 were amplified using the outer primer pair, producing fragments of 213 bp and 323 bp, respectively. M, molecular weight marker φ × 174/HaeIII. The integrity of RNA was checked by amplifying all samples with β-actin primers producing a fragment of 260 bp.
Discussion
Expression of trypsinogen has recently been observed in several extra-pancreatic tumors and tissues. 4-9 We now show that trypsinogen occurs at high concentrations in human seminal plasma, and that it seems to be derived from the auxiliary sex glands as evidenced by immunostaining of secretory epithelial cells of the prostate, seminal vesicles, testes, epididymis, and vas deferens. In situ hybridization and RT-PCR showed that this was because of local production rather than to uptake. Sequencing of the PCR products showed that the two trypsinogen isoenzymes detected were encoded by the same genes as the corresponding pancreatic ones. 27 Interestingly, most of the trypsinogen-1 isolated from seminal fluid was enzymatically active whereas trypsinogen-2 was either inactivated or occurred as a proenzyme that could be activated in vitro. In addition, nicked forms of both isoenzymes were detected by Western blotting. These were enzymatically inactive and could be separated by ion exchange chromatography from trypsinogen and active trypsin. The nicked forms may be the result of autodigestion or fragmentation by other proteinases either in vivo or in vitro.
Trypsin hydrolyzes peptide bonds at the carboxyl side of arginine and lysine residues, and it efficiently activates various serine proteinases and metalloproteinases (MMPs) involved in digestion, fibrinolysis, and tumor invasion. Trypsin is more potent than plasmin and other serine proteinases in activating the latent forms of many MMPs, including MMP-2, MMP-9, and MMP-7 (matrilysin). 30,31 Trypsin is also able to activate membrane receptors such as the proteinase-activated receptor-2. 32,33 These findings suggest that extra-pancreatic trypsin may be involved in tissue remodeling and tumor invasion. The widespread distribution of trypsinogen in the male genital tract suggests that this proteinase plays a physiological role also in reproduction, and it is tempting to speculate that this function is related to the activation of other proteinases such as PSA and hK2 which are present in seminal plasma at high concentrations. ProPSA has been shown to be activated by bovine trypsin 15 and we could show that trypsin purified from seminal plasma activated proPSA produced by LNCap cells. PSA is one of the most abundant serine proteinases in seminal fluid with an average concentration of ∼1.0 mg/ml 34 The main function of PSA is thought to be the dissolution of the sperm-entrapping gel formed immediately after ejaculation by cleavage of the gel forming proteins semenogelin I and II. 13 Multiple cleavages result in the release of progressively motile sperm. In seminal fluid, no proforms of hK2 and PSA are detected. Most PSA is free and enzymatically active 34 whereas hK2 occurs in complex with activated protein C inhibitor. 11 It is most likely that proPSA and prohK2 are activated by an enzyme with trypsin-like specificity, because the cleavage of the activation peptide occurs at the carboxyl terminal side of an arginine. 16 On the other hand, ∼30 to 35% of the PSA in seminal fluid is partially cleaved. The cleavage sites occur after the basic amino acids Arg-85, Lys-145, and Lys-182. 24,35,36 Isoforms of hK2 with multiple internal cleavages at Arg-101, Arg-145, and Arg-226 have also been observed. 11,16,37 All these are typical cleavage sites for trypsin. They are also potential cleavage sites for hK2, but PSA is not degraded by hK2. 16 Thus trypsin may contribute not only to the activation of prohK2 and proPSA, but also to their degradation in seminal plasma.
Another potential physiological activator of proPSA is hK2. 16 The average concentration of hK2 in seminal fluid is 6 μg/ml, 38 which is approximately six times higher than that of the trypsins. However, zinc, that is present in the prostate at very high concentrations (9 mmol/L), 39 inhibits hK2 more efficiently than trypsin: 100 μmol/L zinc causes a 90% reduction in hK2 activity, 40 but only an 18% reduction in trypsin activity. This difference in inhibition of proteinase activity suggests that trypsin may function as an activator of proPSA under circumstances in which hK2 is inhibited. It is also possible that trypsin is an initiator of a proteinase cascade that leads to the activation of both hK2 and PSA.
Of the male sex glands, only the prostate expresses PSA and hK2. In the prostate the distribution of PSA and hK2 is different from that of trypsinogen. PSA and hK2 are highly expressed in secretory cells of the acini, whereas trypsinogen is predominantly found in the luminal cells of the prostatic excretory ducts. Ampulla vas deferens and seminal vesicles, that also store trypsinogen, empty into ejaculatory ducts passing through the prostate. These findings suggest that trypsin is admixed to the prostatic fluid during ejaculation and that it exerts its activity at this step. The fact that PSA is active also in patients with aplasia of the seminal vesicles and the deferent duct 13 suggests that trypsin produced in the prostate may be sufficient for initiation of a proteinase cascade leading to activation of PSA.
The results of the present study show that trypsinogen is widely produced in the male genital tract. Being a highly potent proteinase, it may play a physiological role in reproduction. This function may comprise both activation and degradation of other proteinases. It remains to be studied whether trypsinogen is also expressed in prostate cancer.
Acknowledgments
We thank Ms. Elise Nilsson, Mrs. Ulla Fält, and Mrs. Christina Möller, Department and Laboratory of Pathology, Malmö University Hospital and Lund University, Sweden, for their skillful technical assistance.
Footnotes
Address reprint requests to Annukka Paju, Department of Clinical Chemistry, Helsinki University Central Hospital, Haartmaninkatu 2, PB 140, FIN-00290 Helsinki, Finland. E-mail: annukka.lukkonen@helsinki.fi.
Supported by the Finnish Cancer Foundation; Sigrid Juselius Foundation; Finnish Academy of Sciences; Helsinki University Central Hospital; University of Helsinki; Swedish Cancer Society Research Fund (Project no. 4292-B99-01XAB); Cancer Research Fund at Malmö University Hospital; Foundation for Urology Research in Malmö; and the Thulefjord Foundation.
References
- 1.Guy O, Lombardo D, Bartelt DC, Amic J, Figarella C: Two human trypsinogens. Purification, molecular properties and N terminal sequences. Biochemistry 1978, 17:1669-1675 [DOI] [PubMed] [Google Scholar]
- 2.Tani T, Kawashima I, Mita K, Takiguchi Y: Nucleotide sequence of the human pancreatic trypsinogen III cDNA. Nucleic Acids Res 1990, 18:1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koivunen E, Huhtala ML, Stenman UH: Human ovarian tumor-associated trypsin. Its purification and characterization from mucinous cyst fluid and identification as an activator of pro-urokinase. J Biol Chem 1989, 264:14095-14099 [PubMed] [Google Scholar]
- 4.Koivunen E, Itkonen O, Halila H, Stenman UH: Cyst fluid of ovarian cancer patients contains high concentrations of trypsinogen-2. Cancer Res 1990, 50:2375-2378 [PubMed] [Google Scholar]
- 5.Hirahara F, Miyagi Y, Miyagi E, Yasumitsu H, Koshikawa N, Nagashima Y, Kitamura H, Minaguchi H, Umeda M, Miyazaki K: Trypsinogen expression in human ovarian carcinomas. Int J Cancer 1995, 63:176-181 [DOI] [PubMed] [Google Scholar]
- 6.Terada T, Ohta T, Minato H, Nakanuma Y: Expression of pancreatic trypsinogen/trypsin and cathepsin B in human cholangiocarcinomas and hepatocellular carcinomas. Hum Pathol 1995, 26:746-752 [DOI] [PubMed] [Google Scholar]
- 7.Koivunen E, Saksela O, Itkonen O, Osman S, Huhtala ML, Stenman UH: Human colon carcinoma, fibrosarcoma and leukemia cell lines produce tumor-associated trypsinogen. Int J Cancer 1991, 47:592-596 [DOI] [PubMed] [Google Scholar]
- 8.Koshikawa N, Nagashima Y, Miyagi Y, Mizushima H, Yanoma S, Yasumitsu H, Miyazaki K: Expression of trypsin in vascular endothelial cells. FEBS Lett 1997, 409:442-448 [DOI] [PubMed] [Google Scholar]
- 9.Koshikawa N, Hasegawa S, Nagashima Y, Mitsuhashi K, Tsubota Y, Miyata S, Miyagi Y, Yasumitsu H, Miyazaki K: Expression of trypsin by epithelial cells of various tissues, leukocytes, and neurons in human and mouse. Am J Pathol 1998, 153:937-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegand U, Corbach S, Minn A, Kang J, Müller-Hill B: Cloning of the cDNA encoding human brain trypsinogen and characterization of its product. Gene 1993, 136:167-175 [DOI] [PubMed] [Google Scholar]
- 11.Deperthes D, Chapdelaine P, Tremblay RR, Brunet C, Berton J, Hébert J, Lazure C, Dubé JY: Isolation of prostatic kallikrein hK2, also known as hGK-1, in human seminal plasma. Biochim Biophys Acta 1995, 1245:311-316 [DOI] [PubMed] [Google Scholar]
- 12.Wang MC, Valenzuela LA, Murphy GP, Chu TM: Purification of a human prostate specific antigen. Invest Urol 1979, 17:159-163 [PubMed] [Google Scholar]
- 13.Lilja H: A kallikrein-like serine proteinase in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest 1985, 76:1899-1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements JA: The human kallikrein gene family: a diversity of expression and function. Mol Cell Endocrinol 1994, 99:C1-C6 [DOI] [PubMed] [Google Scholar]
- 15.Takayama TK, Fujikawa K, Davie EW: Characterization of the precursor of prostate-specific antigen. Activation by trypsin and by human glandular kallikrein. J Biol Chem 1997, 272:21582-21588 [DOI] [PubMed] [Google Scholar]
- 16.Lövgren J, Rajakoski K, Karp M, Lundwall A, Lilja H: Activation of the zymogen form of prostate-specific antigen by human glandular kallikrein 2. Biochem Biophys Res Commun 1997, 238:549-555 [DOI] [PubMed] [Google Scholar]
- 17.Itkonen O, Koivunen E, Hurme M, Alfthan H, Schröder T, Stenman UH: Time-resolved immunofluorometric assays for trypsinogen-1 and 2 in serum reveal preferential elevation of trypsinogen-2 in pancreatitis. J Lab Clin Med 1990, 115:712-718 [PubMed] [Google Scholar]
- 18.Stenman UH, Leinonen J, Alfthan H, Rannikko S, Tuhkanen K, Alfthan O: A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res 1991, 51:222-226 [PubMed] [Google Scholar]
- 19.Leinonen J, Zhang WM, Stenman UH: Complex formation between PSA isoenzymes and proteinase inhibitors. J Urol 1996, 155:1099-1103 [PubMed] [Google Scholar]
- 20.Zhang WM, Finne P, Leinonen J, Vesalainen S, Nordling S, Stenman UH: Measurement of the complex between prostate-specific antigen and alpha1-protease inhibitor in serum. Clin Chem 1999, 45:814-821 [PubMed] [Google Scholar]
- 21.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 1979, 76:4350-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corey E, Brown LG, Corey MJ, Buhler KR, Vessella RL: LNCaP produces both putative zymogen and inactive, free form of prostate-specific antigen. Prostate 1998, 35:135-143 [DOI] [PubMed] [Google Scholar]
- 24.Zhang WM, Leinonen J, Kalkkinen N, Dowell B, Stenman UH: Purification and characterization of different molecular forms of prostate-specific antigen in human seminal fluid. Clin Chem 1995, 41:1567-1573 [PubMed] [Google Scholar]
- 25.Borgström A, Ohlsson K: Radioimmunological determination and characterisation of cathodal trypsin-like immunoreactivity in normal human plasma. Scand J Clin Lab Invest 1976, 36:809-814 [DOI] [PubMed] [Google Scholar]
- 26.Kimland M, Russick C, Marks WH, Borgström A: Immunoreactive anionic and cationic trypsin in human serum. Clin Chim Acta 1989, 31:31-46 [DOI] [PubMed] [Google Scholar]
- 27.Emi M, Nakamura Y, Ogawa M, Yamamoto T, Nishide T, Mori T, Matsubara K: Cloning, characterization and nucleotide sequences of two cDNAs encoding human pancreatic trypsinogens. Gene 1986, 41:305-310 [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 29.Ponte P, Ng SY, Engel J, Gunning P, Kedes L: Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res 1984, 12:1687-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorsa T, Salo T, Koivunen E, Tyynelä J, Konttinen YT, Bergmann U, Tuuttila U, Niemi E, Teronen O, Heikkilä P, Tschesche H, Leinonen J, Osman S, Stenman UH: Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem 1997, 272:21067-21074 [DOI] [PubMed] [Google Scholar]
- 31.Imai K, Yokohama Y, Nakanishi I, Ohuchi E, Fujii Y, Nakai N, Okada Y: Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem 1995, 270:6691-6697 [DOI] [PubMed] [Google Scholar]
- 32.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J: Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA 1994, 91:9208-9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyata S, Koshikawa N, Yasumitsu H, Miyazaki K: Trypsin stimulates integrin α5β1-dependent adhesion to fibronectin and proliferation of human gastric carcinoma cells through activation of proteinase-activated receptor-2. J Biol Chem 2000, 275:4592-4598 [DOI] [PubMed] [Google Scholar]
- 34.Christensson A, Lilja H: Complex formation between protein C inhibitor and prostate-specific antigen in vitro and in human semen. Eur J Biochem 1994, 220:45-53 [DOI] [PubMed] [Google Scholar]
- 35.Watt KW, Lee PJ, M’Timkulu T, Chan WP, Loor R: Human prostate-specific antigen: structural and functional similarity with serine proteinases. Proc Natl Acad Sci USA 1986, 83:3166-3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensson A, Laurell CB, Lilja H: Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem 1990, 194:755-763 [DOI] [PubMed] [Google Scholar]
- 37.Lövgren J, Tian S, Lundwall Å, Karp M, Lilja H: Production and activation of recombinant hK2 with propeptide mutations resulting in increased expression levels. Eur J Biochem 1999, 266:1050-1055 [DOI] [PubMed] [Google Scholar]
- 38.Lövgren J, Valtonen-André C, Marsal K, Lilja H, Lundwall Å: Measurement of prostate-specific antigen and human glandular kallikrein 2 in different body fluids. J Androl 1999, 20:348-355 [PubMed] [Google Scholar]
- 39.Kavanagh JP: Sodium, potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. J Reprod Fert 1985, 75:35-41 [DOI] [PubMed] [Google Scholar]
- 40.Lövgren J, Airas K, Lilja H: Enzymatic action of human glandular kallikrein 2 (hK2). Substrate specificity and regulation by Zn2+ and extracellular protease inhibitors. Eur J Biochem 1999, 262:781-789 [DOI] [PubMed] [Google Scholar]