Abstract
Subepithelial and intraepithelial lymphocytes of human adenoids and tonsils were characterized and directly compared to determine the potential contribution of these tissues to mucosal and systemic immune responses. The distribution of T and B cell subsets, cytokine patterns, and antibody (Ab) isotype profiles were similar for adenoids and tonsils. Both tissues contained predominantly B cells (∼65%), approximately 5% macrophages, and 30% CD3+ T cells. The T cells were primarily of the CD4+ subset (∼80%). Tonsillar intraepithelial lymphocytes were also enriched in B cells. The analysis of dispersed cells revealed a higher frequency of cells secreting IgG than IgA and the predominant Ig subclass profiles were IgG1 > IgG3 and IgA1 > IgA2, respectively. In situ analysis also revealed higher numbers of IgG- than IgA-positive cells. These IgG-positive cells were present in the epithelium and in the subepithelial zones of both tonsils and adenoids. Mitogen-triggered T cells from tonsils and adenoids produced both Th1- and Th2-type cytokines, clearly exhibiting their pluripotentiality for support of cell-mediated and Ab responses. Interestingly, antigen-specific T cells produced interferon-γ and lower levels of interleukin-5. These results suggest that adenoids and tonsils of the nasopharyngeal-associated lymphoreticular tissues represent a distinct component of the mucosal-associated lymphoreticular tissues with features of both systemic and mucosal compartments.
Human palatine tonsils and the nasopharyngeal tonsil (adenoid) are the largest components of Waldeyer’s ring and are thought to be functionally related to the nasopharyngeal-associated lymphoreticular tissues (NALT) of rodents and other species. 1 The cellular architecture of adenoids and tonsils including germinal centers in B cell follicles and extrafollicular T-cell-enriched areas, resembles that of lymph nodes, although the lack of afferent lymphatics predisposes the tonsils to direct interactions with environmental antigens. 2 In this regard, lymphoid cells in the crypt regions of tonsils are closely associated with the surface epithelium. 3-6 The crypts are enriched in microfold or M cells that can transport antigens from the lumen to the underlying subepithelium. 7,8 Tonsils also contain macrophages, human leukocyte antigen (HLA)-DR-positive endothelial cells, and epithelial cells that can potentially process and present antigens to extrafollicular T lymphocytes. 9 Immunoglobulin (Ig)-producing B cells occur in the germinal centers of the lymphoid follicle, the mantle zone, the extrafollicular area, and the reticular sites of the crypt tonsillar epithelium. 10 However, others have reported that antibody-forming cells are confined mainly to the extrafollicular areas. 11 Furthermore, no study to date has directly compared Ig subclass production in adenoid tissues and tonsils.
The question of whether removal of tonsils may compromise protection of the upper respiratory tract and result in humoral immunodeficiency has been the subject of debate. 12-15 Combined adenoidectomy and tonsillectomy were reported to reduce IgA titers in nasopharyngeal secretions to poliovirus and to delay or abrogate the mucosal immune response to subsequent live poliovirus vaccine. 16 This suggested a potential role for these lymphoid tissues in IgA responses and would support the notion that they are NALT. However, adenoids and tonsils were also reported to spontaneously produce lower levels of IgA than IgG. 17 This is in marked contrast to the Ab isotypes associated with mucosal surfaces. 18 Thus, although the tonsils contain the complete set of cellular components necessary for primary 19 and secondary immune responses, 20 the precise contribution of these lymphoid structures to induction and regulation of mucosal and systemic immune responses to inhaled or ingested antigens is still unclear. It is still open to debate whether adenoids and tonsils are sites for induction of immune responses or whether these tissues behave as effector sites for immune responses initiated in systemic or other mucosal compartments. In support of an induction site, tonsillar B cells were reported to proliferate and differentiate into antibody-forming cells after in vitro exposure to respiratory pathogens. 21-24 Furthermore, tonsils were inductive sites for B cell responses after direct antigen stimulation. 25 However, the initiation of immune responses, the dissemination of lymphocytes primed in the human NALT, and the nature of cytokine “help” provided by resident tonsillar T lymphocytes for B cell isotype differentiation all remain poorly defined. It is also unclear whether the epithelium of NALT represents an important site for cell-mediated immunity and cytotoxic T lymphocyte activity, as does its intestinal counterpart. 18
In this study, we analyzed and compared functional characteristics of B and T cells in adenoids and tonsils to determine whether lymphoid cells in these organs display features associated with the systemic or mucosal compartments. For this purpose, B and T cell frequencies and the patterns of Ig isotypes and subclasses of Ig-producing cells were discerned in situ and in freshly dispersed or cultured mononuclear cells isolated from both adenoids and tonsils. The potential contribution of tonsillar T helper cell-derived cytokines to support immune responses in the respiratory tract was also addressed by the analysis of mitogen- and antigen-induced cytokine responses.
Materials and Methods
Nasopharyngeal and Palatine Tonsils
Twenty-four nasopharyngeal (adenoids) and 38 palatine tonsils (tonsils) were obtained from children or adolescents suffering from adenoid hypertrophy or recurrent tonsillitis who underwent adenoidectomy and/or tonsillectomy at the Vanderbilt University Children’s Hospital, Nashville, Tennessee. Fifteen tonsils were also obtained through the Tissue Procurement Core Facility at the University of Alabama at Birmingham (UAB). The study was carried out with full approval of the Human Use Committees at Vanderbilt University and UAB.
Mononuclear Cell Isolation
Adenoids and tonsils from Vanderbilt University were shipped overnight at 4°C in minimum essential medium (Gibco, BRL, Life Technologies, Grand Island, NY) supplemented with 200 U/ml penicillin, 200 μg/ml streptomycin, 1 μg/ml amphotericin B, 50 μg/ml gentamicin, and 290 μg/ml glutamine to the UAB Immunobiology Vaccine Center, where they were processed immediately. Tonsils obtained from the UAB Tissue Procurement Core Facility were processed within 3 hours after surgery. The organs were washed extensively in RPMI 1640 (Cellgro, Mediatech, Washington, DC) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 μg/ml amphotericin B to prevent bacterial and fungal contamination. To isolate tonsillar intraepithelial lymphocytes (IELs), the specimens were incubated overnight on ice in 50-ml centrifuge tubes containing 40 ml of RPMI 1640 and 1 mg/ml of protease (Sigma-Aldrich Chemicals, St. Louis, MO). The tissues were then subjected to gentle agitation for 1 minute and supernatants were collected and stored at 4°C. Fresh medium was added to the tubes and the procedure was repeated 3 times. Hematoxylin and eosin staining of histological sections showed that this procedure removed the epithelial layer without affecting the crypts and the structure of the organs (data not shown). IELs were further purified in discontinuous Percoll gradients where they were collected at the 40 to 55% and 55 to 75% interfaces, as previously described. 26 The subepithelial and germinal center mononuclear cells were obtained by mechanical dissociation of the remaining tonsillar tissues. The residual tissue was minced, teased, and passed through sterile wire mesh. The cell suspensions were washed twice before separation on Ficoll-Histopaque-1077 gradient (Sigma; 400 × g, 30 minutes at 20°C) to obtain mononuclear cells. The mononuclear cells were washed three times with RPMI 1640 medium supplemented with 10 mmol/L HEPES and 10% fetal calf serum (Atlanta Biologicals, Norcross, GA; complete medium) and their viability was assessed by trypan blue dye exclusion. The IEL preparations were 95 to 97% viable, whereas isolated tissue lymphoid cells were >99% viable.
Fluorescence-Activated Cell Sorter (FACS) Analysis
Mononuclear cells were stained with the following monoclonal antibodies (mAbs): anti-CD3 (clone SK7), anti-CD4 (clone SK3), anti-CD8 (clone SK1), anti-T cell receptor (TCR) αβ (clone WT31), anti-TCR γδ (clone 11F2), anti-CD19 (clone 4G7), anti-HLA-DR (clone L243), anti-CD14 (clone M[sws1]P9), anti-CD25 (clone 2A3), from Becton Dickinson (San Jose, CA), and anti-CD28 (clone IOT28) from AMAC, Inc. (Westbrook, ME). Staining of surface Ig was performed with biotinylated anti-μ, -γ, or -α antibodies obtained from Biosource International (Camarillo, CA). Monoclonal antibodies (mAbs) to IgA1 (clone NI 512) and to IgA2 (clone NI 69–11) subclasses were generously provided by Dr. Jiri Mestecky at UAB and those for IgG subclasses (clone HP 6012, HP 6014, HP 6047, and HP 6022 for IgG1, IgG2, IgG3, and IgG4, respectively) were a gift of the Center for Disease Control (Atlanta, GA). For flow cytometry analysis, cell suspensions were incubated for 30 minutes on ice with the appropriate fluorescent labeled mAbs or with biotinylated mAbs, followed by staining with fluorescein isothiocyanate (FITC)-conjugated streptavidin (PharMingen, San Diego, CA). Cells were then washed extensively and analyzed by flow cytometry using a FACScan (Becton Dickinson).
Immunohistochemistry
The phenotypes of lymphoid cells in the intraepithelial and subepithelial compartments of adenoids and tonsils, as well as the patterns of surface Ig isotypes, were also analyzed on tissue sections. For this purpose, 4-μm paraffin sections of adenoids and tonsils were deparaffinized, rehydrated, and stained with the following biotinylated mAbs to determine the tissue distribution of T cells (anti-CD3; clone SK7), B cells (anti-CD20; clone L26; Becton Dickinson), and monocytes/macrophages (anti-CD66, clone KP1; Dako, Carpinteria, CA). Furthermore, anti-μ, -γ, and -α Abs (Biosource International) and the anti-IgA subclass α1 (clone NI 512) and α2 (clone NI 69–11) mAbs described above were used to determine the relative numbers of Ig isotype and IgA subclass positive cells in situ. When sections were stained for IgA and one IgA subclass, the two images were merged by use of Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
Detection of Ig-Secreting Cells by Enzyme-Linked Immunospot Assay (ELISPOT)
Frequencies of B cells secreting IgM, IgG, and IgA as well as the subclasses IgG1, IgG2, IgG3, IgG4, IgA1, and IgA2 in adenoids and tonsils were evaluated by a modified solid phase ELISPOT on microtiter plates with nitrocellulose filters. 27 Briefly, the nitrocellulose-based wells (Millipore Corp., Bedford, MA) were coated with the F(ab′)2 fragment of goat anti-human antibodies to μ, γ, or α chains (Jackson ImmunoResearch Inc., West Grove, PA). For IgA subclasses, the α1 mAb (clone NI 512) and α2 mAb (clone NI 69–11) were used as described above. The mAbs were diluted in phosphate-buffered saline (PBS) and incubated overnight at 4°C. After blocking and washing, serial dilutions of cells were distributed to duplicate wells and incubated for 4 hours at 37°C in a humid atmosphere of 5% CO2. The anti-μ, -δ, and -α Abs for detecting IgM, IgG, and IgA, as well as the mAbs specific for IgG- and IgA-subclasses described above, were used in this assay. After addition of appropriate Ab, horseradish peroxidase (HRP)-conjugated avidin (Gibco), was added, followed by HRP-conjugated goat anti-mouse IgG1 Ab (Southern Biotechnology Associates, Birmingham, AL). Finally, the peroxidase substrate 3-amino-9-ethylcarbazole (AEC) (Moss Inc., Pasadena, MD) was added to plates and Ig-secreting cells (IgSCs) were counted with the aid of a dissecting microscope (SZH Zoom Stereo Microscope System, Olympus, Lake Success, NY). To confirm that we were evaluating active Ig secretion and not cytophilic Ig binding to cells with subsequent passive release, an aliquot of each cell preparation was treated with 2 mmol/L cycloheximide 2 hours before and during the incubation period. This treatment inhibited by >90% the numbers of IgSCs.
Lymphoproliferative Responses
Mononuclear cells (2 × 10 5 to 4 × 105/well) were cultured in complete medium in 96-well flat bottom plates (Costar Corp., Cambridge, MA). Mitogen stimulation of T cells was performed with 5 μg/ml of phytohaemagglutinin (PHA; Murex Diagnostics Limited, Dartford, UK). For antigen-specific proliferative assays, cells were cultured with soluble Candida albicans antigen (20 μg/ml; Greer Laboratories, Lenoir, NC), UV-inactivated influenza virus (Wt A/Beijing, H3N2; 1.02 × 10 4 HAU/ml), tetanus toxoid (TT; kindly provided by Dr. Masahiko Mutai, Biken Research Foundation, Osaka University), or diphtheria toxoid (DT; kindly provided by Dr. Freda Pietrobon, Connaught Laboratories, Swiftwater, PA) coated to beads 28,29 at a 10:1 bead:cell ratio. Cells were cultured for 72 hours with mitogen or for 5 days with antigen, and the proliferative responses were determined by the levels of tritiated [3H]thymidine (0.5 μCi/well; Amersham Corp., Arlington Heights, IL) incorporated by cells during the last 18 hours of cell culture.
Cytokine Assessment
Cytokine levels in culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA) using the following mAbs for coating: mouse anti-human interleukin (IL)-4 (8D4–8), rat anti-human IL-5 (TRFK5), rat anti-human IL-10 (JES3–9D7), mouse anti-human IL-12 (C8.3) and mouse anti-human tumor necrosis factor (TNF)-α mAb (PharMingen); mouse anti-human interferon (IFN)-γ (B-B1; Biosource International) and chicken anti-human transforming growth factor (TGF)-β (AB-101-NA; R&D Systems, Minneapolis, MN). Nunc-Immuno plates with a MaxiSorp surface (NalgeNunc, Nasperville, IL) were coated with 100 μl of the appropriate dilution of anti-cytokine antibody in PBS and incubated overnight at 4°C. The wells were blocked with PBS containing either 1% bovine serum albumin or 1% dry milk (for IFN-γ) at 25°C for 1 to 2 hours. Serial twofold dilutions of supernatants were added into duplicate wells and incubated overnight at 4°C. For the analysis of TGF-β, samples were incubated with 1 N HCl for 1 hour at 37°C to activate the TGF-β and the pH neutralized before addition of samples to plates. The plates were next washed and incubated with the following mAbs: rat anti-human IL-4 (MP4–25D2), rat anti-human IL-5 (JES1–5A10), rat anti-human IL-10 (JES3–12G8), mouse anti-human TNF-α (MAβ11), mouse anti-human IL-12 (C8.6) (PharMingen); rabbit anti-human IFN-γ (P-700; Endogen, Woburn, MA); anti-human TGF-β (80-1835-03; Genzyme Corp., Cambridge, MA) diluted in PBS-T containing 1% bovine serum albumin for 1 to 2 hours at 25°C. Finally, HRP-conjugated avidin (Gibco) was added for IL-4, IL-5, IL-10, IL-12, TGF-β, and TNF-α, and goat anti-rabbit IgG HRP conjugate (Southern Biotechnology Associates) was used for assessment of IFN-γ. Standard curves were generated for each cytokine assay with recombinant human cytokines (PharMingen). The ELISA assays were capable of detecting 25 pg/ml of IFN-γ, TNF-α, IL-4, IL-5, IL-10, and IL-12, and 400 pg/ml of TGF-β.
Statistics
The results are expressed as the mean ± SD. Statistical significance (P < 0.05) was analyzed by the Student’s t-test and by analysis of variance followed by the Fisher least significant difference test. The results were analyzed using the Statview II statistical program (Abacus Concepts, Berkeley, CA) adapted for MacIntosh computers.
Results
The Subepithelial and Intraepithelial Regions of Human Adenoids and Tonsils Are Enriched in B Cells
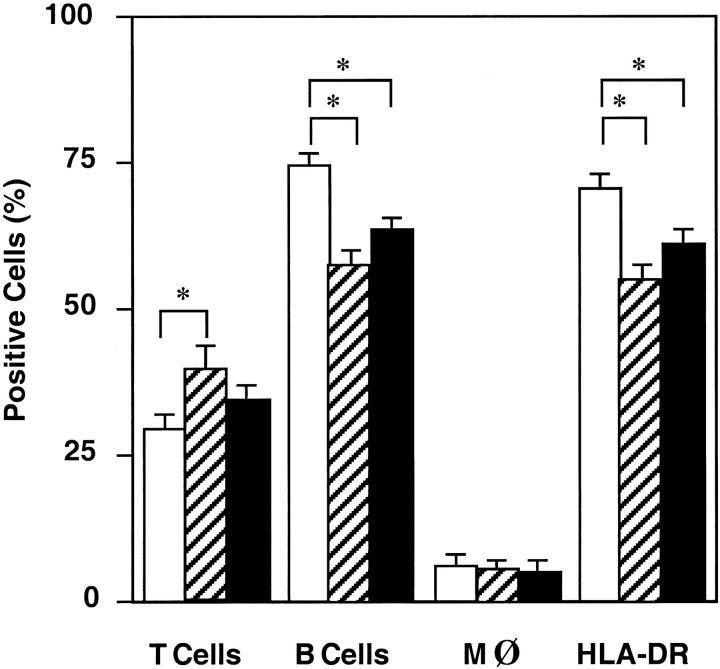
Flow cytometry analysis of mononuclear cells present in the subepithelial compartment of adenoids and tonsils revealed that 25 to 35% were CD3+ T cells, 60 to 75% were CD19+ B cells, and 5 to 8% were M13+ macrophages (Figure 1) ▶ . Comparison of cells from adenoids and tonsils showed higher percentages of B cells (P < 0.05) in tonsils (76%) than in adenoids (64%; Figure 1 ▶ ). When cells isolated from the intraepithelial compartment of tonsils were analyzed, we noted a similar distribution pattern of B cells, T cells, and macrophages as tonsillar subepithelial mononuclear cells (Figure 1) ▶ . However, the relative numbers of T cells were somewhat higher (P < 0.05) in the epithelium. We also confirmed this pattern of cell subsets in fixed sections of adenoid tissue, where B cells were again predominant (Figure 2) ▶ . As shown in the inset of Figure 2, B ▶ cells were also the major lymphocyte population in the epithelium of adenoids.
Figure 1.
Lymphoid cell composition of human tonsils and adenoids as determined by FACS analysis. Tonsillar (□) and adenoid (▪) mononuclear cells or tonsillar IELs (▨) were incubated for 30 minutes with FITC-labeled mAbs anti-CD3, anti-CD19, anti-CD14, or anti-HLA-DR for the detection of T cells, B cells, macrophages, and MHC class II molecules. Cells were then washed, fixed with 1% paraformaldehyde, and stored at 4°C until analyzed by flow cytometry. The results are expressed as the mean percentage ± SD of 5 to 12 separate experiments. *P < 0.05.
Figure 2.
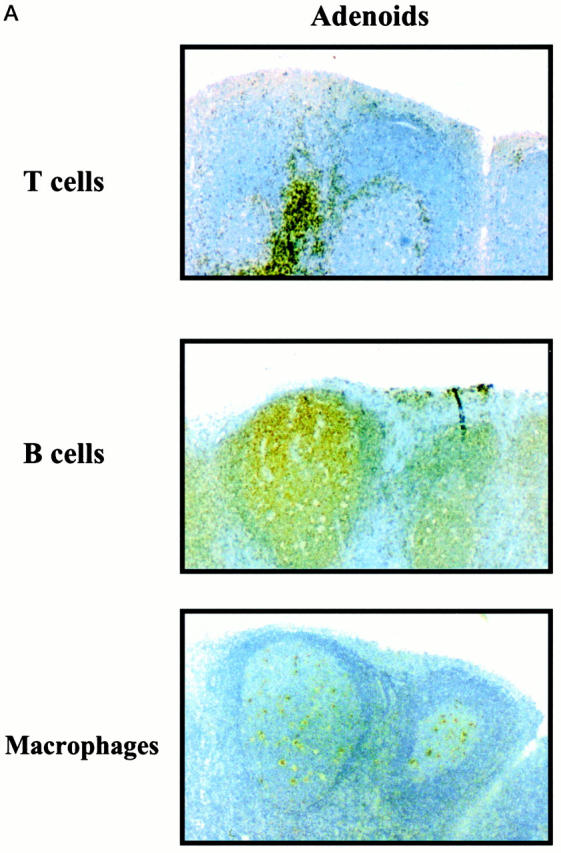
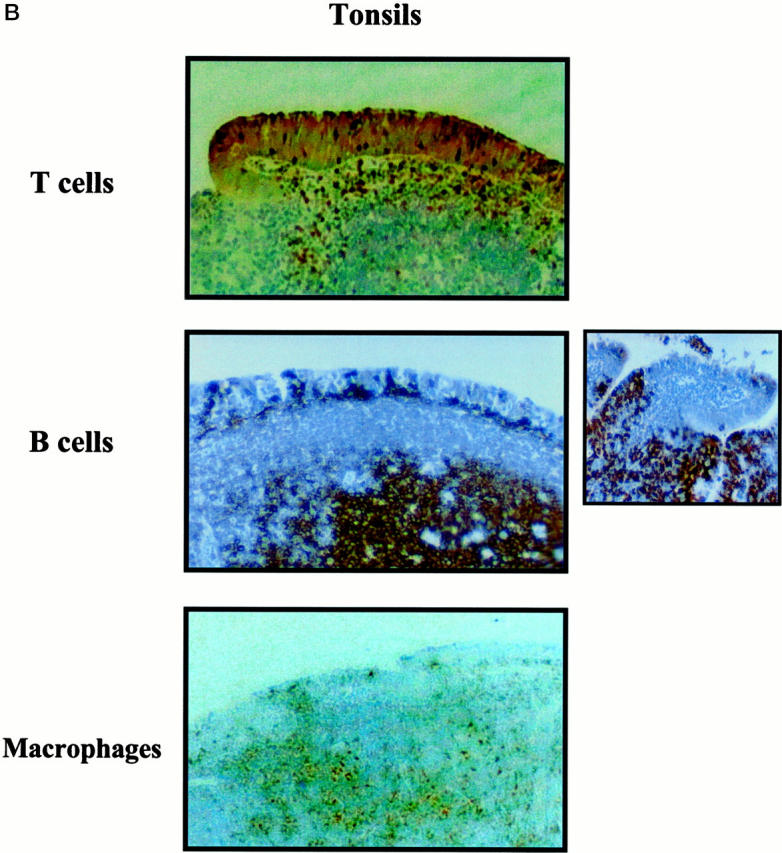
Epithelial and subepithelial distribution of B cells, T cells, and macrophages in sections of human adenoid (A) and tonsils (B). Paraffin sections were incubated with HRP- or biotin-conjugated anti-CD3, anti-CD20, or anti-M13 mAbs followed by streptavidin-HRP. Positive cells appear dark brown or dark red. Original magnification, ×40. Inset: B cells in the tonsil epithelium. Original magnification, ×100.
Distribution of Ig Isotypes in Human Adenoids and Tonsils
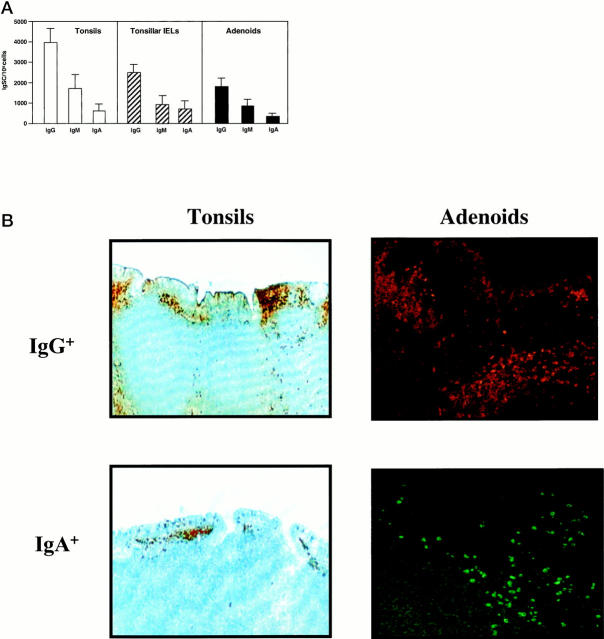
Analysis of surface Ig-positive (sIg+) B cells revealed comparable numbers of both adenoids and tonsils where sIgM+ was the predominant B cell type, followed by sIgG+ and sIgA+ B cells (Table 1) ▶ . IgA subclass analysis showed that >90% of sIgA+ B cells in both tissues were IgA1+ (Table 1) ▶ . We also investigated whether this pattern of sIg expression reflected functional B cell responses by analyzing the frequencies of B cells spontaneously secreting Ig (Ig-secreting cells; IgSCs). Despite individual variations in the total numbers of IgSCs, the Ig isotype- and subclass-specific ELISPOT assays showed a similar distribution of IgG > IgM > IgA IgSCs among different subjects and among autologous adenoid and tonsil mononuclear cell or IEL isolates (Figure 3A) ▶ . Indeed, the IgSCs of IgG isotype accounted for the majority of the IgSCs (approximately 65%) in both adenoids and tonsils, followed by IgM (25–30%) and IgA (7–10%). Interestingly, this same distribution of IgSCs was observed in mononuclear cells isolated from the intraepithelial compartment of tonsils (Figure 3A) ▶ . One may argue that the enzymatic method used in cell isolation could affect the frequency of IgSCs detected. To confirm that this was not the case, we next assessed IgG- and IgA-positive cells in paraffin sections of adenoids and tonsils. As expected, higher numbers of IgG+ than IgA+ B cells were observed in paraffin sections (Figure 3B) ▶ .
Table 1.
Immunoglobulin Isotypes and Subclasses Expressed by Adenoid and Tonsillar B Cells
| Surface Ig-positive (sIg) isotypes | Positive cells (percent) | ||
|---|---|---|---|
| Tonsils | Tonsillar IELs | Adenoids | |
| sIgM+ | 52.5 ± 4.9 (7) | 54.5 ± 3.2 (4) | 55.5 ± 2.3 (4) |
| sIgG+ | 21.2 ± 6.4 (7) | 22.4 ± 2.2 (4) | 21.0 ± 1.9 (4) |
| sIgA+ | 7.8 ± 3.3 (7) | 8.5 ± 1.5 (4) | 7.2 ± 4.2 (4) |
| sIgA1+ | 9.3 ± 4.3 (5) | 8.8 ± 1.9 (3) | 8.2 ± 5.3 (4) |
| sIgA2+ | 0.7 ± 0.3 (5) | 5.0 ± 0.5 (5) | 0.5 ± 3.2 (4) |
| sIgA+/sIgA1+ | 7.3 ± 2.3 (5) | 6.9 ± 2.1 (3) | 7.6 ± 3.2 (4) |
| sIgA+/sIgA2+ | 0.3 ± 0.1 (5) | 0.2 ± 0.2 (5) | 0.3 ± 0.2 (4) |
The frequency and pattern of surface Ig+B cells in adenoids and tonsils were determined by incubating isolated mononuclear cells from these tissues with antibodies to Ig isotypes and subclasses as described in Materials and Methods. The percentage of positive cells was determined by flow cytometry. The results are expressed as the mean percentage ± one SD. The number of samples is indicated in parentheses.
Figure 3.
A: Distribution of Ig-secreting cells (IgSC) in freshly isolated human tonsil (□) or adenoid (▪) mononuclear cells or tonsillar IELs (▨) was determined by isotype-specific ELISPOT assays. The results are expressed as the mean ± 1 SD (n = 10). B: In situ distribution of IgG- and IgA-positive cells. Paraffin sections of tonsils were stained with HRP- or biotin-conjugated anti-IgG or anti-IgA Abs followed by streptavidin-HRP. Positive cells appear dark brown or dark red. Original magnification, ×40. Sections of adenoids were stained with PE-conjugated anti-IgG or FITC-conjugated anti-IgA Abs. Original magnification, ×100.
IgG- and IgA-Subclass Abs Produced by Human Adenoid and Tonsil B Cells
We further assessed B cell responses in these tissues by analyzing IgG- and IgA-subclass-specific IgSCs in subepithelial mononuclear cells and tonsillar IELs by ELISPOT assay. These experiments revealed that IgG1 was the predominant IgG subclass in both adenoids and tonsils followed by IgG3, IgG2, and IgG4 antibody- forming cells (Figure 4A) ▶ . The IgA subclass-specific ELISPOT revealed that both adenoid and tonsillar mononuclear cells secreted almost exclusively IgA1 Abs (Figure 4B) ▶ . The latter observation was consistent with the prevalence of IgA1+ B cells in adenoids and tonsils in situ (Figure 4C) ▶ .
Figure 4.
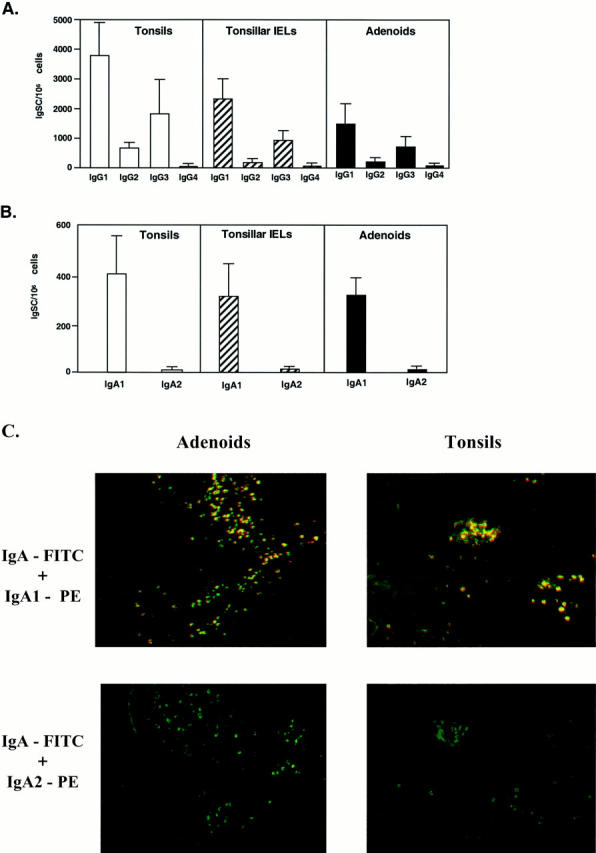
The pattern of IgG (A) or IgA (B) subclass responses in adenoids and tonsils. The distribution of IgSC in freshly isolated human tonsil (□) or adenoid (▪) mononuclear cells or tonsillar IELs (▨) was determined by isotype-specific ELISPOT assays. The distribution of IgG subclass IgSCs (A) and of IgA1 and IgA2 subclass IgSCs (B) are expressed as the mean ± 1 SD (n = 13). In C, the immunofluorescent staining of IgA subclasses in tissue sections of adenoids and tonsils is presented. Paraffin sections were stained with FITC-labeled anti-IgA and PE-labeled anti-IgA1 or anti-IgA2. Original magnification, ×100.
T Cell Subsets in IELs and Subepithelial Mononuclear Cells
The T cell subsets present in adenoids and tonsils were determined by FACS analysis before functional studies. The majority of T cells (80–90%) in these tissues were CD4+ (Table 2) ▶ . In general, adenoid mononuclear cells contained higher numbers of CD4+ T cells than their tonsillar counterpart. No differences were observed in the frequency of CD8+ T cells between the two tissues. Most of the T cells possessed the αβ TCR and only 2 to 3% of the mononuclear cells expressed the γδ TCR (Table 2) ▶ . The CD25 molecule, which characterizes activated cells, was also expressed by cells from both adenoids and tonsils with higher levels (P < 0.05) in adenoids when compared with tonsils (Table 2) ▶ . The CD28 molecule was expressed by virtually all tonsillar and adenoid CD3+ T cells (Table 2) ▶ . Tonsillar IELs contained more T cells than subepithelial mononuclear cells isolated from the same tissue. In fact, tonsillar IELs resembled adenoid mononuclear cells based on the pattern of T cell subsets and activation markers expressed.
Table 2.
Immunoglobulin Isotypes and Subclasses Expressed by Adenoid and Tonsillar B Cells
| Positive cells (percent) | |||
|---|---|---|---|
| T cell subsets | Tonsils | Tonsillar IELs | Adenoids |
| CD3+ | 29.2 ± 3.5 (12)* | 38.4 ± 4.0 (4) | 34.4 ± 2.5 (6) |
| CD4+ | 20.8 ± 2.5 (9)* | 31.2 ± 1.6 (4) | 28.2 ± 1.1 (4) |
| CD8+ | 5.2 ± 1.4 (9) | 7.5 ± 2.0 (4) | 6.3 ± 0.7 (4) |
| CD3+/ CD4+ | 20.2 ± 1.2 (5) | 27.3 ± 1.2 (3) | 24.3 ± 2.6 (3) |
| CD3+/ CD8+ | 3.9 ± 1.2 (5) | 4.7 ± 2.0 (3) | 6.2 ± 0.7 (3) |
| TCR αβ+ | 25.0 ± 3.3 (9)* | 30.1 ± 1.5 (4) | 35.0 ± 1.5 (4) |
| TCR + | 2.2 ± 1.6 (9) | 2.7 ± 1.8 (4) | 1.8 ± 1.1 (4) |
| CD25+ | 3.3 ± 2.2 (9)* | 5.1 ± 1.7 (4) | 7.8 ± 1.6 (4) |
| CD28+ | 23.5 ± 1.6 (9)* | 38.3 ± 2.5 (4) | 36.1 ± 2.6 (4) |
The T cells subsets in adenoids and tonsils were determined by incubating cell suspensions with the appropriate FITC-labeled and/or PE-labeled mAbs as described in Materials and Methods. After washing and fixation with 1% paraformaldehyde, the cells were analyzed by flow cytometry. The results are expressed as the mean percentage ± one SD. The number of samples is indicated in parentheses. *P < 0.05 when compared with adenoids.
Antigen-Induced Proliferative Responses and Cytokine Synthesis by Adenoid and Tonsillar T Lymphocytes
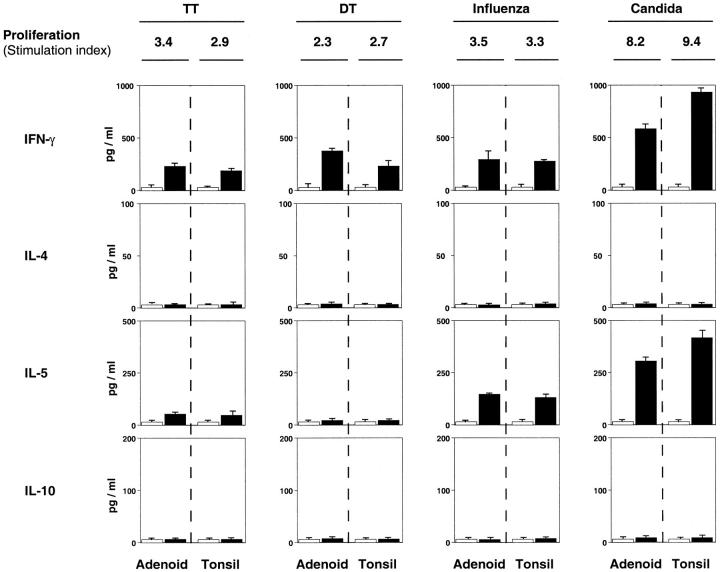
We next measured proliferative responses and cytokine synthesis in response to diphtheria toxoid (DT), tetanus toxoid (TT), influenza virus, or C. albicans anti- gens. We assumed that the subjects had either been vaccinated with these antigens or were exposed to them through infection. Only 8 of 24 adenoids and 10 of 53 tonsils examined responded to DT, TT, or influenza virus antigens. In contrast, responses to C. albicans were observed in 85 to 90% of all samples tested. Furthermore, adenoid and tonsil T cells generally exhibited low responses to DT, TT, or influenza antigens (stimulation index (SI) = 2.7–3.6), whereas C. albicans stimulation consistently resulted in higher proliferative responses (SI = 5.7–9.1). No significant difference was noted between proliferative responses of paired adenoids and tonsils (Figure 5) ▶ or when tonsillar subepithelial mononuclear cells were compared with tonsillar IELs (data not shown).
Figure 5.
Antigen-specific T cell proliferative responses and T helper cell cytokine secretion from paired adenoids and tonsils. Proliferative responses of tonsillar and adenoid mononuclear cells to diphtheria toxoid (DT), tetanus toxoid (TT), influenza virus, or C. albicans antigens were determined by 3H-thymidine uptake of cultured cells. Proliferative responses are expressed as the mean of the stimulation index (SI) and cytokine levels as the mean of duplicate ELISA assays and are representative of paired specimens which, in addition to C. albicans, responded to at least one antigen. The same pair of adenoids and tonsils was used to illustrate responses to influenza and C. albicans.
When Th1- and Th2-type cytokines secreted by paired adenoids or tonsil specimens were analyzed following antigen stimulation, all antigens tested induced the Th1 cytokine IFN-δ (Figure 5) ▶ . In two cases, IFN-γ secretion was detected in the absence of significant 3[H]-thymidine uptake. In contrast, no IL-4 was detected in culture supernatants of adenoid or tonsil mononuclear cells after antigen stimulation (Figure 5) ▶ . However, IL-5 was actively secreted by C. albicans-stimulated cultures and, to a lesser extent, by specimens responding to influenza virus (Figure 5) ▶ . Again, no significant difference was observed between adenoids and tonsils for production of IFN-γ or IL-5. Furthermore, IFN-γ secretion was occasionally enhanced in culture supernatants in the absence of detectable proliferative responses.
Mitogen-Induced Cytokine Responses by Adenoid and Tonsillar Lymphocytes
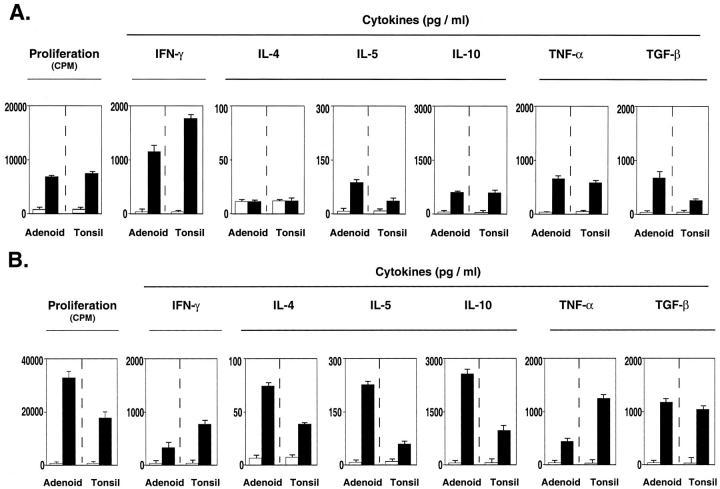
Because IFN-γ and IL-5 were the only cytokines detected in culture supernatants of antigen-stimulated adenoid and tonsillar mononuclear cells, it was important to determine whether these cells possessed the potential to secrete additional Th1- and Th2-type cytokines. We next assessed cytokine profiles after stimulation with PHA. Significant individual variation was observed when paired adenoid and tonsillar cells were stimulated for 72 hours with PHA and stimulation indices (cpm PHA/cpm unstimulated; SI) of responding samples ranged from 10 to 64.
When Th1- and Th2-type cytokines were assessed, Th1- (ie, IFN-γ) and Th2- (IL-4, -5, and -10) type cytokines were induced in cell cultures of both low and high responder populations after mitogenic stimulation with PHA. Interestingly, culture supernatants from low responder cultures contained higher levels of IFN-γ (Th1-type) when compared with Th2-type cytokines (Figure 6A) ▶ , whereas the opposite was noted in high responder cell cultures (Figure 6B) ▶ . Both low and high responder adenoid and tonsillar mononuclear cells actively secreted TNF-α and TGF-β (Figure 6) ▶ , but not IL-12 (data not shown).
Figure 6.
Cytokine synthesis and proliferative responses of PHA-stimulated mononuclear cells from paired adenoids and tonsils. Proliferative responses are expressed as the mean cpm ± 1 SD and cytokine levels in culture supernatants as the mean of duplicate ELISA assays. The results are representative of paired adenoids and tonsils exhibiting either low (A) or high (B) proliferative responses after stimulation with PHA for 72 hours.
Discussion
NALT is considered an important component of the common mucosal immune system, which also includes the lymphoid structures of Peyer’s patches and appendix present in the gastrointestinal (GI) tract. 18 However, the contribution of adenoids and tonsils, which represent the human NALT, to systemic and mucosal immune responses remains controversial. 13,15,16,30 In this study, we addressed whether adenoids and tonsils exhibit phenotypic features and patterns of Ig secretion representative of either the systemic or mucosal compartments. We have approached this by assessing IgA and IgG subclasses, the phenotype of isolated cells, and histochemical localization of cells in the intact tonsil and adenoid. These analyses were consistent and showed a remarkable similarity between these two lymphoid tissues. We have also studied the cytokine responses of adenoid and tonsillar mononuclear cells to recall antigens and the subsequent cytokine help provided by T cells for support of immune responses that occur in these two tissues. Indeed, adenoids and tonsils contained a majority of B cells, which, in contrast to B cells in other mucosal compartments, produced more IgG than IgA in both the epithelial and the subepithelial compartments. We also found that both tissues almost exclusively produced IgA1, the IgA subclass prevalent in human mucosal tissues of the upper respiratory and small intestinal tracts. Finally, we showed that adenoid and tonsillar mononuclear cells can develop both Th1 and Th2 cytokine responses to recall antigens to support immune responses that occur in these two tissues.
Different Ig isotypes possess characteristic effector functions and may dominate a particular antigen-specific Ab responses in a defined environment. 31 In this regard, either predominant systemic or mucosal immunity is usually reflected by the ratio of IgG and IgA Abs, because the IgA isotype predominates in mucosal areas, 32,33 whereas IgG provides significant systemic protection. 31 Furthermore, IgA2 Abs increase in the large intestine and genitourinary tracts, whereas IgA1 antibodies are prevalent in other mucosal effector sites. 34-37 In our study, adenoid and tonsillar mononuclear cells did not differ in distribution of T and B cells, supporting previous reports where the two organs were shown to share similar distributions of lymphocyte populations. 4,11,38 We also provide new evidence that, despite a similar pattern of lymphoid cell distribution, the tonsils contain more B cells than the adenoids. Interestingly, in contrast to the mucosal surfaces of the GI tract where T cells usually occur, B cells were the predominant lymphocyte present in both the subepithelial and intraepithelial compartments of tonsils. These observations and the predominance of IgG- versus IgA-secreting cells in both adenoids and tonsils suggest that the MALT is diverse and exhibits different patterns of T cell and B cell Ig isotype responses. In this regard, human adenoids and tonsils contain more IgG secreting cells than does the lamina propria of the GI tract, where the majority of B cells secrete IgA. 27,32 Although the proportion of B and T cells in adenoids and tonsils resemble that of Peyer’s patches in the intestine, B cells in the latter organ primarily secrete IgA, followed by IgM and IgG. 27,32 Thus, when compared to inductive (ie, Peyer’s patches) or effector (ie, lamina propria) sites of the GI tract, adenoids and tonsils should be classified as non-classical mucosal inductive sites. However, the localization of IgA- and large numbers of IgG-secreting cells in the epithelium and subepithelial compartments suggests that the adenoids and tonsils have characteristics of effector sites and that IgG could be a significant component of mucosal immune responses in these tissues. Murine NALT, on the other hand, which is often compared with tonsils and adenoids, has been shown to contain equal numbers of B and T cells, 39,40 whereas Peyer’s patches in the GI tract are enriched in B cells, although significant numbers of T cells are also present. It has also been shown that murine NALT, like Peyer’s patches, contain approximately threefold higher numbers of CD4+ than CD8+ T cells. 18,41 Our studies suggest that adenoids and tonsils resemble murine Peyer’s patches in terms of B and T cell subsets. However, in contrast to murine Peyer’s patches, human adenoids and tonsils are primed for higher IgG than IgA responses. Although NALT, like Peyer’s patches, was shown to produce more IgA than IgG, 42 there are also reports suggesting that NALT produces more IgG than IgA. 40,43 In this study, we have shown that both adenoids and tonsils produced more IgG than IgA, supporting a role for IgG in the protection of mucosal surfaces of the upper respiratory tract.
The patterns of human IgA and IgG subclasses are influenced by the nature of the antigens they encounter. Protein antigens generally stimulate IgG1 and IgG3 subclasses, whereas bacterial polysaccharides and lipopolysaccharides induce IgG2 as the predominant subclass, and parasites or chronic antigenic exposure is often associated with IgG4 antibodies. 31,44 The IgA1 Abs are generally induced by protein antigens, whereas polysaccharides, lipopolysaccharides, and lipoteichoic acid stimulate the IgA2 subclass. 45-47 In our study, both adenoids and tonsils showed a pattern of IgG subclass responses consisting primarily of IgG1 followed by IgG3 Abs. We also found that these tissues contained almost exclusively IgA1 Abs. These observations indicate that both tissues are exposed to the same environment and interact primarily with protein antigens. Further, the predominance of IgA1 subclass-secreting cells in both adenoids and tonsils is consistent with the prevalence of IgA1 Abs in the upper respiratory tract and sets these organs apart from effector sites in the large intestine and genitourinary tracts, which contain higher proportions of IgA2 Abs. 33-37
The ability of adenoid and tonsillar mononuclear cells to respond to antigens is poorly documented and, to our knowledge, no study has directly compared these two tissues. We used four recall antigens to stimulate adenoid and tonsillar cultures assuming that subjects were either systemically vaccinated (eg, TT, DT, and influenza virus) or naturally exposed (eg, C. albicans or influenza virus) to them and thus would possess antigen-specific (memory) lymphocytes. Further, it has been reported that NALT T cells respond to recall antigens for up to 6 months after the initial immunization, 38 thus suggesting that adenoids and tonsils retain memory cells. Both adenoid and tonsillar mononuclear cells proliferated in response to recall antigens from pathogens with tropism for the upper respiratory tract (eg, C. albicans and influenza virus). Interestingly, they also responded to antigens like TT, which are typical of systemic vaccination, suggesting that adenoids and tonsils are also effector sites for at least some immune responses initiated in the systemic compartment. In our study, approximately 80 to 90% of subjects responded to C. albicans, whereas fewer samples responded to DT, TT, or influenza virus. These results are consistent with the limited ability of human adenoid cells to undergo clonal expansion after antigenic stimulation. 24 Indeed, stimulation of adenoid lymphocytes with influenza virus was low in both seronegative and seropositive donors, 24 suggesting that NALT sites are more prone to B than to T cell responses. In this regard, in vitro stimulation of cells from seropositive donors with wild-type influenza virus resulted in secretion of influenza-specific antibodies, whereas low proliferative responses occurred. 24 However, the higher percentage of subjects responding to C. albicans suggests that the potential of adenoid and tonsillar cells to respond to an antigen may depend on the nature of antigen and the extent of previous exposure. Thus, vaccination of patients before they undergo tonsillectomy would allow a precise analysis of vaccine-specific responses of adenoid and tonsillar T cells.
Antigen stimulation of tonsillar and adenoid cells in vitro resulted in enhanced IFN-γ production, whereas secretion of IL-5 was seen in response to C. albicans and less frequently in response to other recall antigens. IFN-γ has been reported to stimulate the growth and differentiation of Ig-secreting tonsillar B cells. 48 On the other hand, IL-5 was shown to augment IgM secretion by human B cells. 49 We also noted that PHA-stimulated tonsillar and adenoid cells secreted additional Th2-type cytokines (ie, IL-4 and -10) that support Ab responses. Furthermore, TNF-α and other inflammatory cytokines have been shown to support the clonal expansion of antigen-activated CD4+ T cells and to provide help for antigen-specific Ab responses. 50 Interestingly, mitogen stimulation of adenoid and tonsillar mononuclear cells resulted in high levels of TNF-α synthesis, suggesting that this molecular pathway may support Ab responses in vivo when NALT sites are exposed to appropriate immunogens. Our results of cytokine responses after antigen stimulation are consistent with previous findings where peripheral blood mononuclear cells from TT-vaccinated 51 and normal donors 52 were shown to secrete IFN-γ following in vitro stimulation with TT or C. albicans, respectively. Thus, similar patterns of antigen-specific cytokine profiles may occur in both human peripheral blood mononuclear cells and NALT (adenoids and tonsils) compartments. Our findings also suggest that cytokine help provided by resident adenoid and tonsillar T lymphocytes for B cell responses is similar to that of murine NALT where a Th0 environment occurs. 41
We have shown that, unlike murine NALT but similar to Peyer’s patches, mononuclear cells from adenoids and tonsils are predominantly B cells. However, in contrast to the small intestine and other mucosal effector sites, these two tissues contain more IgG- than IgA-producing cells. This distribution of B cell subsets may play an important role in the protection of the upper respiratory tract. The almost exclusive expression of IgA1 and the pattern of IgG subclass characterized by IgG1 and IgG3 identify these two tissues as distinct from mucosal immune compartments in the GI and genitourinary tracts and suggest that they respond to predominantly protein antigens in vivo. We have also shown that the human NALT displays a CD4+/CD8+ T cell ratio similar to the murine Peyer’s patches. Further, both NALT and Peyer’s patches provide a Th0 environment for B cell responses. Taken together, our findings show major similarities between adenoids and tonsils and suggest that these strategically placed tissues could play a role as inductive sites for nasal vaccines as well as effector sites for both IgG and IgA antibody responses.
Acknowledgments
We thank Ms. Sheila D. Turner for preparation of this manuscript.
Footnotes
Address reprint requests to Dr. Jerry R. McGhee, Department of Microbiology and the Immunobiology Vaccine Center, The University of Alabama at Birmingham, Bevill Biomedical Research Building, Room 761, 845 19th Street South, Birmingham, AL 35294-2170. E-mail: mcghee@uab.edu.
Supported by National Institute of Allergy and Infectious Diseases Respiratory Pathogens Research Unit contract NO1 AI 65298, and by National Institutes of Health grants AI 43197, AI 18958 DE 12242, and DK 44240.
P. N. B., P. F. W., and M. M. contributed equally to this paper.
Current address for M. M. and S. Di F.: Istituto Superiore di Sanita, Rome, Italy.
Current address for H. F. S.: Center for AIDS Research, Duke University, Durham, NC.
References
- 1.Kuper CF, Koornstra PJ, Hameleers DM, Biewenga J, Spit BJ, Duijvestijn AM, van Breda Vriesman PJ, Sminia T: The role of nasopharyngeal lymphoid tissue. Immunol Today 1992, 13:219-224 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein JM, Corfien J, Brandtzaeg P: Immunobiology of tonsils and adenoids. Ogra PL Mestecky J Lamm ME Strober W McGhee JR Bienenstock J eds. Mucosal Immunology. 1999, :pp 1339-1362 Academic Press, San Diego [Google Scholar]
- 3.Brandtzaeg P: Immune functions and immunopathology of palatine and nasopharyngeal tonsils. Bernstein J Ogra PL eds. Immunology of the Ear. 1987, :pp 63-106 Raven Press, New York [Google Scholar]
- 4.Brandtzaeg P, Surjan L Jr, Berdal P: Immunoglobulin-producing cells in clinically normal, hyperplastic and inflamed human palatine tonsils. Acta Otolaryngol 1979 (suppl.) 360:211–215 [DOI] [PubMed]
- 5.Bernstein JM, Scheeren R, Schoenfeld E, Albini B: The distribution of immunocompetent cells in the compartments of the palatine tonsils in bacterial and viral infections of the upper respiratory tract. Acta Otolaryngol (suppl.) 1988, 454:153-162 [DOI] [PubMed] [Google Scholar]
- 6.Okamoto Y, Brodsky L, Bernstein JM, Ogra PL: Characteristics of in vitro production of mucosal antibody to respiratory syncytial virus in tonsillar tissue lymphocytes. Clin Immunol Immunopathol 1988, 49:299-307 [DOI] [PubMed] [Google Scholar]
- 7.Olah I, Everett NB: Surface epithelium of the rabbit palatine tonsil: scanning and transmission electron microscopic study. J Reticuloendothel Soc 1975, 18:53-62 [PubMed] [Google Scholar]
- 8.Howie AJ: Scanning and transmission electron microscopy on the epithelium of human palatine tonsils. J Pathol 1980, 130:91-98 [DOI] [PubMed] [Google Scholar]
- 9.Richtsmeier WJ, Shikhani AH: The physiology and immunology of the pharyngeal lymphoid tissue. Otolaryngol Clin N Am 1987, 20:219-228 [PubMed] [Google Scholar]
- 10.Brandtzaeg P, Surjan L, Jr, Berdal P: Immunoglobulin system of human tonsils. I. Control subjects of various ages: quantification of Ig-producing cells, tonsillar morphometry and serum Ig concentrations. Clin Exp Immunol 1978, 31:367-381 [PMC free article] [PubMed] [Google Scholar]
- 11.Hoefakker S, van’t Erve EH, Deen C, van den Eertwegh AJ, Boersma WJ, Notten WR, Claassen E: Immunohistochemical detection of co-localizing cytokine and antibody producing cells in the extrafollicular area of human palatine tonsils. Clin Exp Immunol 1993, 93:223-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolen WK, Spofford B, Selner JC: The hidden tonsils of Waldeyer’s ring. Ann Allergy 1990, 65:244-248 [PubMed] [Google Scholar]
- 13.D’Amelio R, Palmisano L, Le Moli S, Seminara A, Aiuti F: Serum and salivary IgA levels in normal subjects: comparison between tonsillectomized and non-tonsillectomized subjects. Int Arch Allergy Appl Immunol 1982, 68:256-259 [DOI] [PubMed] [Google Scholar]
- 14.Donovan R, Soothill JF: Immunological studies in children undergoing tonsillectomy. Clin Exp Immunol 1973, 14:347-357 [PMC free article] [PubMed] [Google Scholar]
- 15.Wingerd J, Sponzilli EE: Concentration of serum protein fractions in white women: effects of age, weight, smoking, tonsillectomy and other factors. Clin Chem 1977, 23:1310-1317 [PubMed] [Google Scholar]
- 16.Ogra PL: Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody response to poliovirus. N Engl J Med 1971, 284:59-64 [DOI] [PubMed] [Google Scholar]
- 17.Harabuchi Y, Hamamoto M, Kodama H, Kataura A: Spontaneous immunoglobulin production by adenoidal and tonsillar lymphocytes in relation to age and otitis media with effusion. Int J Pediatr Otorhinolaryngol 1996, 35:117-125 [DOI] [PubMed] [Google Scholar]
- 18.McGhee JR, Lamm ME, Strober W: Mucosal immune responses: an overview. Ogra PL Mestecky J Lamm ME Strober W McGhee JR Bienenstock J eds. Mucosal Immunology. 1999, :pp 485-506 Academic Press, San Diego [Google Scholar]
- 19.Watanabe T, Yoshizaki K, Yagura T, Yamamura Y: In vitro antibody formation by human tonsil lymphocytes. J Immunol 1974, 113:608-616 [PubMed] [Google Scholar]
- 20.Platts-Mills TA, Ishizaka K: IgA and IgA diphtheria antitoxin responses from human tonsil lymphocytes. J Immunol 1975, 114:1058-1064 [PubMed] [Google Scholar]
- 21.Drucker MM, Agatsuma Y, Drucker I, Neter E, Bernstein J, Ogra PL: Cell-mediated immune response to bacterial products in human tonsils and peripheral blood lymphocytes. Infect Immun 1979, 23:347-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morag A, Morag B, Bernstein JM, Beutner K, Ogra PL: In vitro correlates of cell-mediated immunity in human tonsils after natural or induced rubella virus infection. J Infect Dis 1975, 131:409-416 [DOI] [PubMed] [Google Scholar]
- 23.Rynnel-Dagoo B: Polyclonal activation to immunoglobulin secretion in human adenoid lymphocytes induced by bacteria from nasopharynx in vitro. Clin Exp Immunol 1978, 34:402-410 [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards KM, Snyder PN, Stephens DS, Wright PF: Human adenoid organ culture: a model to study the interaction of influenza A with human nasopharyngeal mucosa. J Infect Dis 1986, 153:41-47 [DOI] [PubMed] [Google Scholar]
- 25.Quiding-Jarbrink M, Granstrom G, Nordstrom I, Holmgren J, Czerkinsky C: Induction of compartmentalized B-cell responses in human tonsils. Infect Immun 1995, 63:853-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawabata S, Boyaka PN, Coste M, Fujihashi K, Yamamoto M, McGhee JR, Kiyono H: Intraepithelial lymphocytes from villus tip and crypt portions of the murine small intestine show distinct characteristics. Gastroenterology 1998, 115:866-873 [DOI] [PubMed] [Google Scholar]
- 27.Fujihashi K, McOhee JR, Lue C, Beagley KW, Taga T, Hirano T, Kishimoto T, Mesteeky J, Kiyono H: Human appendix B cells naturally express receptors for and respond to interleukin 6 with selective IgA1 and IgA2 synthesis. J Clin Invest 1991, 88:248-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu-Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, Burrows PD, Elson CO, Pillai S, McGhee JR: Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med 1993, 178:1309-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee JR: Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol 1995, 155:4621-4629 [PubMed] [Google Scholar]
- 30.Yarchoan R, Barrow LA, Kurman C, Strober W, Nelson DL: Human peripheral blood mononuclear cells produce IgA anti-influenza antibody in a secondary in vitro antibody response. J Immunol 1985, 135:1033-1039 [PubMed] [Google Scholar]
- 31.Jefferis R, Kumararatne DS: Selective IgG subclass deficiency: quantification and clinical relevance. Clin Exp Immunol 1990, 81:357-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mestecky J, McGhee JR: Immunoglobulin A (IgA): Molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol 1987, 40:153-245 [DOI] [PubMed] [Google Scholar]
- 33.Mestecky J, Russell MW: IgA subclasses. Monogr Allergy 1986, 19:277-301 [PubMed] [Google Scholar]
- 34.Mestecky J, Czerkinsky C, Russell MW, Brown TA, Prince SJ, Moldoveanu Z, Jackson S, Michalek SM, McGhee JR: Induction and molecular properties of secretory and serum IgA antibodies specific for environmental antigens. Ann Allergy 1987, 59:54-59 [PubMed] [Google Scholar]
- 35.Crago SS, Kutteh WH, Moro I, Allansmith MR, Radl J, Haaijman JJ, Mestecky J: Distribution of IgA1-, IgA2- and J chain-containing cells in human tissues. J Immunol 1984, 132:16-18 [PubMed] [Google Scholar]
- 36.Kett K, Brandtzaeg P, Radl J, Haaijman JJ: Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol 1986, 136:3631-3635 [PubMed] [Google Scholar]
- 37.Kutteh WH, Hatch KD, Blackwell RE, Mestecky J: Secretory immune system of the female reproductive tract. I. Immunoglobulin and secretory component-containing cells. Obstet Gynecol 1988, 71:56-60 [PubMed] [Google Scholar]
- 38.Nadal D, Soh N, Schlapfer E, Bernstein JM, Ogra PL: Distribution characteristics of immunoglobulin-secreting cells in adenoids: relationship to age and disease. Int J Pediatr Otorhinolaryngol 1992, 24:121-130 [DOI] [PubMed] [Google Scholar]
- 39.Wu H-Y, Russell MW: Nasal lymphoid tissue, intranasal immunization and compartmentalization of the common mucosal immune system. Immunol Res 1997, 16:187-201 [DOI] [PubMed] [Google Scholar]
- 40.Asanuma H, Thompson AH, Iwasaki T, Sato Y, Inaba Y, Aizama C, Kurata T, Tamura S-I: Isolation and characterization of nasal-associated lymphoid tissue. J Immunol Methods 1997, 202:123-131 [DOI] [PubMed] [Google Scholar]
- 41.Hiroi T, Iwatani K, Ijima H, Kodama S, Yanagita M, Kiyono H: Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passages, respectively. Eur J Immunol 1998, 28:3346-3353 [DOI] [PubMed] [Google Scholar]
- 42.Wu H-Y, Nguyen HH, Russell MW: Nasal lymphoid tissue (NALT) as a mucosal inductive site. Scand J Immunol 1997, 46:506-513 [DOI] [PubMed] [Google Scholar]
- 43.Asakura K, Saito H, Hata M, Kataura A: Antigen-specific IgA response of NALT and cervical lymph node cells in antigen-primed rats. Acta Otolaryngol 1998, 118:859-863 [DOI] [PubMed] [Google Scholar]
- 44.Rautonen N, Pelkonen J, Sipinen S, Kayhty H, Maleka O: Isotype concentrations of human antibodies to group A meningococcal polysaccharide. J Immunol 1986, 137:2670-2675 [PubMed] [Google Scholar]
- 45.Russell MW, Lue C, van den Wall Bake AW, Modoveanu Z, Mestecky J: Molecular heterogeneity of human IgA antibodies during an immune response. Clin Exp Immunol 1992, 87:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarkowski A, Lue C, Moldoveanu Z, Kiyono H, McGhee JR, Mestecky J: Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J Immunol 1990, 144:3770-3778 [PubMed] [Google Scholar]
- 47.Moldoveanu Z, Brown TA, Ventura MT, Michalek SM, McGhee JR, Mestecky J: IgA subclass responses to lipopolysaccharide in humans. Adv Exp Med Biol 1987, 216B:1199-1205 [PubMed] [Google Scholar]
- 48.Jelinek DF, Splawski JB, Lipsky PE: The roles of interleukin 2 and interferon-γ in human B cell activation, growth and differentiation. Eur J Immunol 1986, 16:925-932 [DOI] [PubMed] [Google Scholar]
- 49.Bertolini JN, Sanderson CJ, Benson EM: Human interleukin-5 induces staphylococcal A Cowan 1 strain-activated human B cells to secrete IgM. Eur J Immunol 1993, 23:398-402 [DOI] [PubMed] [Google Scholar]
- 50.Pape KA, Khoruts A, Mondino A, Jenkins MK: Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol 1997, 159:591-598 [PubMed] [Google Scholar]
- 51.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ: Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis 1996, 173:269-272 [DOI] [PubMed] [Google Scholar]
- 52.Levitz SM, North EA: Gamma interferon gene expression and release in human lymphocytes directly activated by Cryptococcus neoformans and Candida albicans. Infect Immun 1996, 64:1595-1599 [DOI] [PMC free article] [PubMed] [Google Scholar]