Abstract
Skin cells containing excessive ultraviolet (UV) radiation-induced DNA damage are eliminated by apoptosis that involves the p53 pathway and Fas/Fas-Ligand (Fas-L) interactions. To determine whether dysregulation of apoptosis plays a role in skin cancer development through disruption of Fas/Fas-L interactions, hairless SKH-hr1 mice were exposed to chronic UV irradiation from Kodacel-filtered FS40 lamps for 30 weeks. Their skin was analyzed for the presence of sunburn cells (apoptotic keratinocytes) and for Fas and Fas-L expression at various time points. A dramatic decrease in the numbers of morphologically identified sunburn cells and TUNEL-positive cells was detected as early as 1 week after chronic UV exposure began. After 4 weeks of chronic UV exposure, these cells were barely detectable. This defect in apoptosis was paralleled by an initial decrease in Fas-L expression during the first week of chronic UV irradiation and a complete loss of expression after 4 weeks. Fas expression, however, increased during the course of chronic UV exposure. p53 mutations were detected in the UV-irradiated epidermis as early as 1 week after irradiation began and continued to accumulate with further UV exposure. Mice exposed to chronic UV began to develop skin tumors after approximately 8 weeks, and all mice had multiple skin tumors by 24 weeks. Most of the tumors expressed Fas but not Fas-L. We conclude that chronic UV exposure may induce a loss of Fas-L expression and a gain in p53 mutations, leading to dysregulation of apoptosis, expansion of mutated keratinocytes, and initiation of skin cancer.
The incidence of skin cancer far exceeds the incidences of all other human cancers combined. 1,2 Epidemiological, clinical, and biological studies indicate that solar ultraviolet (UV) radiation is the major etiological agent in skin cancer development. 1-4 Wavelengths in the UVB range (280–320 nm) of the solar spectrum are absorbed by the skin, producing erythema, burns, immunosuppression, mutations, and nonmelanoma skin cancers (NMSC). 4-11
Several studies have shown that the p53 tumor suppressor gene is a target for UV-induced mutations and plays a critical role in the induction of NMSC. 4,9-13 In response to DNA damage, the p53 protein transactivates downstream genes such as p21Waf-1/Cip1 that induces cell-cycle arrest at the G1-S phase to allow DNA repair. 14,15 If the damage is not repaired, p53-dependent apoptosis or “cellular proofreading” 16 is triggered to eliminate severely damaged cells. 13,17,18 On repeated exposure to UV radiation, unrepaired DNA damage is transformed into mutations, especially C→T or CC→TT transitions in the p53 gene, thereby initiating the molecular process of skin carcinogenesis. 19
In addition to mutations in p53, defects in Fas/Fas-ligand (Fas-L) interactions may play an important role in the development of UV-induced skin cancer. Fas (APO-1/CD95) and its cognate ligand, play critical roles in the elimination of sunburn cells 20 and self-reactive lymphocytes, 21 the maintenance of immunological privilege, 22,23 the killing of cytotoxic effector cells, 24 and UV-induced immunosuppression. 25 In addition, loss of Fas expression has been shown to correlate with disease progression in colon cancers and melanoma. 26,27 Studies by Owen-Schaub et al 28 have demonstrated that the wild-type p53 protein can up-regulate Fas expression.
We recently found that Fas/Fas-L interactions are essential for the induction of sunburn (apoptosis) in cells from UV-irradiated mouse skin. 20 Although exposure of wild-type mice to a single dose of UV radiation quickly induces the formation of sunburn cells, Fas-L-deficient mice demonstrate very few apoptotic cells in their UV-irradiated skin. In addition, chronic UV irradiation for 1 to 2 weeks results in induction and accumulation of more p53 mutations in Fas-L-deficient mice than in wild-type mice, 20 suggesting that loss of Fas/Fas-L interactions may result in dysregulation of apoptosis and lead to the development of skin cancer. In this study, we tested the hypothesis that loss of Fas/Fas-L interactions (in Fas/Fas-L-proficient mice) is an early event in UV-induced skin carcinogenesis. We believe that the loss results in the inhibition of apoptosis and the stimulation of cell proliferation, ultimately leading to the development of skin cancer.
Materials and Methods
Mice and UV Irradiation
Female, 8-week-old SKH-hr1 mice were obtained from Charles River Laboratories (Wilmington, MA) and housed in cages in a room with controlled temperature and humidity and an alternating 12-hour light and dark cycle. The animals were maintained in facilities approved by the Association for the Assessment and Accreditation of Laboratory Animal Care International and in accordance with current United States Department of Agriculture, Department of Health and Human Services, and National Institutes of Health regulations and standards. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee. The mice were fed ad libitum with a commercial diet and water.
Five mice were placed into standard cage. Each cage was separated into five individual compartments with Plexiglas dividers and placed on a shelf 20 cm below the light source. The mice were exposed three times per week (Monday, Wednesday, and Friday) to 2.5 kJ/m 2 UV radiation from a bank of six Kodacel-filtered (290–400 nm) FS40 lamps (Westinghouse Electric Corp., Bloomfield, NJ) for 30 weeks, as described previously. 28 The Kodacel filter (127-μm-thick TA422 cellulose triacetate film; Eastman Kodak, Rochester, NY) removed all UV wavelengths below 290 nm. Because this filter undergoes rapid solarization on exposure to UV radiation, as evidenced by a decrease in the amount of energy that passes through the filter, the film was aged for 1 hour before use, and the spectral output and the intensity of the transmitted light were monitored. Due to solarization with increasing filter use, the amount of energy that passed through the filter decreased over time; however, the spectral distribution of the transmitted wavebands remained the same (data not shown). Measurements of transmitted UVB radiation were made once per week. When the UVB irradiance decreased by 20%, the exposure time was increased in an effort to maintain a relatively constant incident dose of UVB; the Kodacel filter was replaced when the irradiance decreased by more than 50% from the initial reading. The irradiance of the lamps was 5.2 W/m 2 when measured with an IL-700 radiometer with a SEE 240 UVB detector equipped with an A127 quartz diffuser (International Light, Newburyport, MA).
Isolation of Skin Samples
Groups of three mice were sacrificed 6, 12, 24, 48, and 72 hours after the last UV exposure at the end of each week for the first 4 weeks and then at 4-week intervals until tumor development. (The total number of UV-irradiated mice was 105.) These mice were UV-irradiated for a period of 16 weeks for p53 mutation study. A group of 20 UV-irradiated mice was irradiated and monitored for tumor development study for a period of 30 weeks. Fifteen mice were used as controls. The total number of mice used in this study was 140.
Dorsal skin (approximately 2 × 4 cm) was excised from each mouse after sacrifice and cut into two pieces. One piece was immediately fixed in 4% buffered formaldehyde for paraffin-embedded sectioning. The other piece was floated dermis-side down in buffered 0.5 mol/L EDTA solution, pH 7.4, for 1 hour at 37°C to separate the epidermis from the dermis. DNA was extracted from the epidermis by the phenol-chloroform method.
Sunburn Cell Assay
Paraffin-embedded skin was cut into 5-μm sections, deparaffinized, hydrated, dehydrated, and stained with hematoxylin and eosin. The slides were analyzed under a light microscope for the presence of sunburn cells. Typically, sunburn cells exhibit a characteristic morphology of pyknotic nuclei and intensely eosin-stained cytoplasm detectable by light microscopy in hematoxylin-and-eosin-stained epidermal sections. The number of sunburn cells per 100 cells in a field were counted under the microscope. Four fields per mouse were assessed for three mice used at each time point. The mean values and the standard deviations were calculated using StatView 4.0 (Abacus Concepts, Inc., Berkeley, CA).
Apoptosis Assay
Terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay was performed using a commercial kit (Promega Corp., Madison, WI) as described previously. 28 The slides were examined with an Olympus Inverted System Microscope IX70 (Melville, NY), and photos were taken with a Nikon 35-mm camera. The number of TUNEL-positive cells per 100 cells in a field were counted under the microscope. Four fields per mouse were examined for three mice at each time point. The mean values and standard deviations were calculated using StatView 4.0.
Immunohistochemistry
Five-micron sections of paraffin-embedded tissue were analyzed for expression of Fas and Fas-L by immunohistochemistry as described previously. 28 Rabbit polyclonal anti-mouse Fas antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-mouse Fas-L antibody (Santa Cruz Biotechnology) were used as primary antibodies. The sections were incubated with secondary donkey anti-rabbit immunoglobulin horseradish peroxidase (Amersham Life Science, Arlington Heights, IL) for 1 hour at room temperature. The intensity of Fas-L immunostaining (optical density) was measured using Optima version 6.2. Statistical analysis was performed using Statview 4.0.
Allele-Specific Polymerase Chain Reaction (AS-PCR)
Epidermal DNA was analyzed by AS-PCR for CC → TT mutations at codons 148, 154/155, and 175/176 in exon 5 and for C → T mutations at codons 270 and 275 in exon 8 of the p53 gene, as previously described. 10,20 The mutant-specific forward primers used were: 5′-TTGTGGGTCAGCGCCACTT-3′ for mutations at codon 148, 5′-CCTCCAGCTGGGAGCCGTGCTT-3′ for mutations at codons 154/155, and 5′-TCGTGAGACGCTGCCCCCATT-3′ for mutations at codons 175/176. The reverse primer used for amplification of codons 148, 154/155, and 175/176 was 5′-GCCTGCGTACCTCTCTTTGC-3′. C → T hotspot mutations at codons 270 and 275 were detected using the forward mutant-specific primers 5′-GGACGGGACAGCTTTGAGGTTT-3′ and 5′-GTGTTTGTGCCTGCCT-3′, respectively. The reverse primer used to detect both mutations was 5′-GCCTGCGTACCTCTCTTTGC-3′.
Results
Effects of Chronic UV Exposure on Sunburn Cell Formation
Hairless mouse skin was examined for the presence of sunburn cells at various times after acute and chronic UV irradiation. Photomicrographs of representative unirradiated and UV-irradiated skin sections revealed the presence of numerous (55 ± 2.6 per 100 cells) sunburn cells 24 hours after acute UV irradiation (data not shown). However, the number (6.0 ± 1.6 per 100 cells) of sunburn cells decreased significantly at as little as 1 week of chronic UV exposure (P = 0.0026) and were barely detectable at later time points. The number of detectable sunburn cells did not change between 24 and 72 hours post-chronic UV irradiation (data not shown).
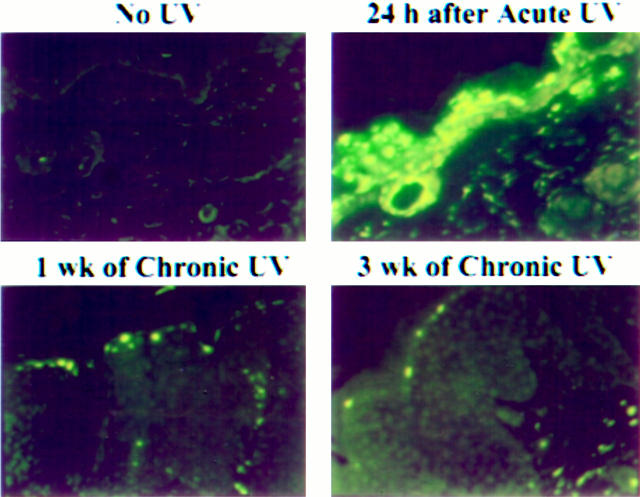
We also examined UV-irradiated mouse skin by TUNEL assay for the presence of apoptotic cells. Immunofluorescence of representative unirradiated skin, acutely irradiated skin, and chronically irradiated skin are shown in Figure 1 ▶ . Although only a few TUNEL-positive cells were observed in unirradiated skin, they were abundant (63 ± 2.0 per 100 cells) in the epidermis 24 hours after a single UV exposure. However, after 1 week of chronic UV exposure, the number of TUNEL-positive cells decreased significantly (P = 0.0017) and after 3 weeks was reduced to basal levels. The number of TUNEL-positive cells remained constant between 24 and 72 hours after chronic UV exposure.
Figure 1.
Induction of TUNEL-positive cells in mouse skin exposed to acute UV and their inhibition by chronic UV irradiation. TUNEL-positive cells were assayed after irradiation, 24 hours after acute exposure, after 1 week of chronic exposure, and after 3 weeks of chronic exposure as described in Materials and Methods. Original magnification, ×200.
Persistence of epidermal hyperplasia and downwardly proliferative projections were also observed in mouse skin chronically UV-irradiated for at least 1 week. Similar changes were observed deep in the dermis where some dilated follicles formed cystic lesions. These changes were accompanied by prominent inflammatory responses involving polymorphonuclear leukocytes and mononuclear cells, by fibrosis, and in some cases by epidermal ulceration.
Effects of Chronic UV Irradiation on Fas and Fas-L Expression
Because elimination of sunburn cells requires Fas/Fas-L interactions, 20 we hypothesized that abrogation of sunburn cell formation and TUNEL-positive cells in chronically UV-irradiated mouse skin might be due to down-regulation or loss of Fas or Fas-L expression. To test this hypothesis, we analyzed unirradiated, acutely irradiated, and chronically irradiated mouse skin for Fas and Fas-L expression at various times post-UV exposure. Immunohistochemistry revealed little or no expression of either Fas or Fas-L in unirradiated skin (Figure 2) ▶ . However, inductions of both Fas and Fas-L were observed as early as 3 hours after acute UV exposure (data not shown) with maximal expression (optical density of 3.23 ± 0.15) 24 hours post-UV irradiation (Figure 2) ▶ .
Figure 2.
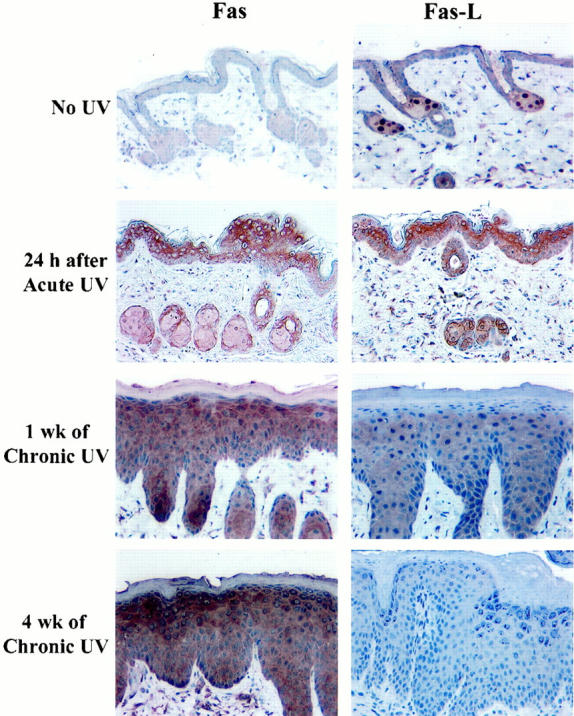
Immunohistochemical analysis for Fas and Fas-L proteins in unirradiated and UV-irradiated hairless mouse skin. Original magnification, ×200.
Expression of Fas continued to increase with chronic UV irradiation (Figure 2) ▶ . However, expression of Fas-L gradually decreased beginning at 1 week of chronic exposure (optical density of 1.2 ± 0.06) and by 4 weeks was reduced to background levels (P = 0.0015) (Figure 2) ▶ . Neither the expression pattern (number of cells and intensity of staining) nor the kinetics of Fas or Fas-L differed substantially between 6 and 72 hours post-chronic UV exposure (data not shown).
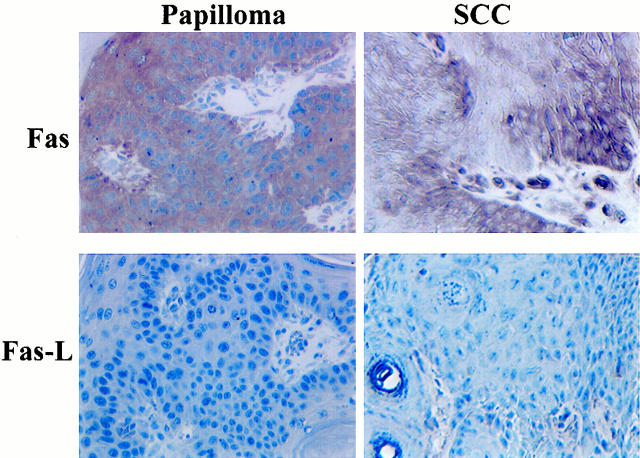
To determine whether Fas/Fas-L interactions were relevant to UV skin tumorigenesis, we analyzed UV-induced skin tumors for Fas and Fas-L expression. Immunohistochemistry revealed that a papilloma that developed after 16 weeks of chronic UV exposure and a squamous cell carcinoma (SCC) that developed after 25 weeks both expressed Fas (Figure 3) ▶ . In contrast, both tumors were negative for Fas-L expression (Figure 3) ▶ . Eleven papillomas and 12 SCCs analyzed for Fas were positive. Seven of 11 papillomas and 10 of 12 SCCs lacked Fas-L expression; the remaining tumors demonstrated low Fas-L expression and the positive cells represented dermal and infiltrating inflammatory cells into the epidermis (data not shown). However, a number of TUNEL-positive cells were detected in both papillomas and SCC (data not shown).
Figure 3.
Immunohistochemical analysis for Fas and Fas-L proteins in UV-induced papilloma and a UV-induced SCC in hairless mice. Original magnification, ×450.
Effects of Chronic UV Exposure on p53 Mutations
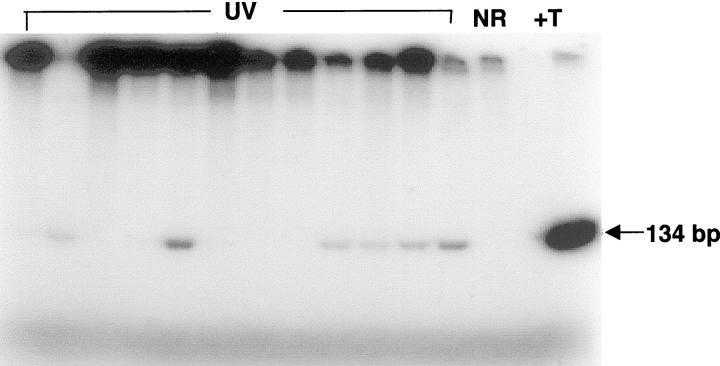
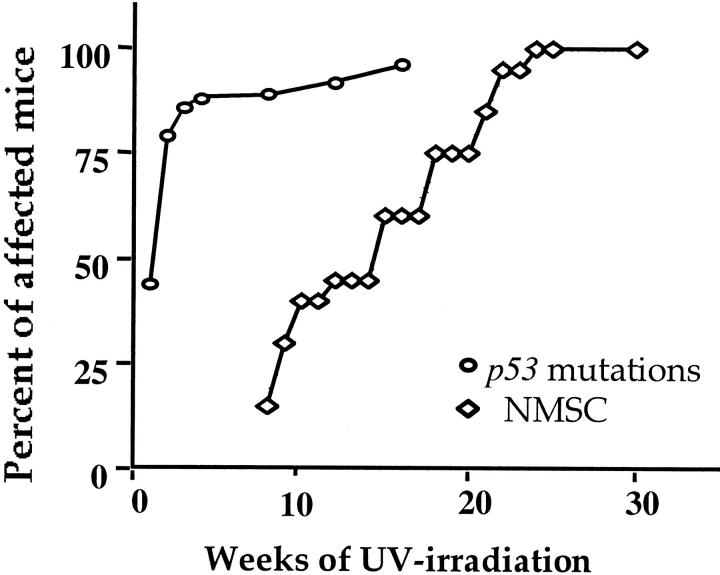
In addition to loss of Fas/Fas-L interactions, loss of p53 function has been shown to inhibit sunburn (apoptotic) cell formation following UV irradiation. 13 We therefore analyzed genomic DNA isolated from the epidermis of chronically UV-irradiated mice for CC → TT hotspot mutations at p53 codons 148, 154/155 and 175/176 and for C → T mutations at codons 270 and 275 by AS-PCR. 10,20 Representative gel electrophoresis data shown in Figure 4 ▶ indicate that the primers specific for mutant p53 sequences at codon 270 amplified genomic DNA from the skin of 6 of 12 mice irradiated with UV for 2 weeks (lanes marked UV). In contrast, the mutant-specific primers did not amplify DNA from unirradiated mouse skin (lane marked NR). As expected, DNA from a UV-induced mouse skin tumor known to contain codon 270 mutation was highly amplified (lane marked +T). Pooled data for all five codons shown in Table 1 ▶ indicate that p53 mutations at one or more of these codons were detected in mouse skin as early as 1 week after the start of chronic UV irradiation. The incidence of mutations increased from 44% after 1 week of irradiation to 80% after 4 weeks of exposure (Table 1 ▶ and Figure 5 ▶ ). Furthermore, incidence exceeded 90% after approximately 8 to 12 weeks (when the mice began to develop skin tumors). In addition, 88% of tumors analyzed had one or more of the p53 mutations normally formed in UV-irradiated skin (data not shown).
Figure 4.
Allele-specific PCR detection of p53 mutations at codon 270 in hairless mouse skin after 2 weeks of chronic UV irradiation. Lanes marked UV represent genomic DNA from UV-irradiated mouse skin. NR, genomic DNA from an unirradiated mouse skin. +T, genomic DNA from a mouse skin tumor containing a high level of p53 mutation at codon 270 (positive control). Arrow represents expected size of PCR products.
Table 1.
p53 Mutations in Hairless Mouse Skin after Chronic UV Exposure
| Weeks of exposure | No. of mice with mutation in a particular codon/no. of mice analyzed | Total mutations (% affected) | ||||
|---|---|---|---|---|---|---|
| 148 CC → TT | 154 /155 CC → TT | 175 /176 CC → TT | 270 C → T | 275 C → T | ||
| 1 | 0 /9 | 4 /9 | 4 /9 | 3 /9 | 0 /9 | 4/9 (44) |
| 2 | 0 /14 | 10 /14 | 9 /14 | 7 /14 | 6 /14 | 11 /14 (79) |
| 3 | 0 /14 | 9 /14 | 10 /14 | 9 /14 | 10 /14 | 12 /14 (86) |
| 4 | 3 /15 | 12 /15 | 5 /15 | 8 /15 | 5 /15 | 12 /15 (80) |
| 8 | 8 /17 | 14 /17 | 13 /17 | 12 /17 | 13 /17 | 15 /17 (88) |
| 12 | 12 /13 | 12 /13 | 12 /13 | 12 /13 | 12 /13 | 12 /13 (92) |
| 16 | 11 /14 | 13 /14 | 13 /14 | 12 /14 | 13 /14 | 13 /14 (93) |
Genomic DNA from UV-irradiated mouse skin was analyzed for CC → TT mutations at codons 148, 154/155, and 175/176, and for C → T mutations at codons 270 and 275 of p53 by allele-specific PCR.
Figure 5.
Time course for induction of p53 mutations and tumor development in UV-irradiated mouse skin. Mouse skin was analyzed for p53 mutations at various time points between 1 and 16 weeks and for 30 weeks for skin tumor development. The incidence of p53 mutations in UV-irradiated mouse skin was obtained from Table 1 ▶ and the number of analyzed mice per each time point varied from 9 to 17. Each point for skin cancer represents data from 20 mice.
Induction of Skin Tumors by Chronic UV Irradiation
The first tumors began to appear after 8 weeks of chronic UV irradiation, and by 25 weeks tumors were present in all UV-irradiated mice (Figure 5) ▶ . Lesions that were approximately 2 mm in diameter had multilayered and hyperplastic epithelia with irregular cells. These lesions were similar to actinic keratoses. After 25 weeks, the mean number of 3- to 5-mm tumors in the irradiated skin was 3.0 ± 1.4 per mouse. The large tumors were diagnosed as well-differentiated SCC.
Discussion
The aim of the present study was to gain insight into the role of Fas/Fas-L interactions in UV-induced carcinogenesis. Our previous findings that UV radiation induces Fas and Fas-L production in mouse skin, that elimination of UV-induced sunburn cells requires Fas/Fas-L interactions, and that UV exposure rapidly results in the accumulation of p53 mutations in the absence of Fas/Fas-L interactions, 20 suggests that loss of these interactions may inhibit UV-induced apoptosis. This could lead to epidermal hyperplasia, expansion of Fas/Fas-L-deficient keratinocytes, and progression to skin cancer. The data presented here support this hypothesis. First, acute UV irradiation induced high levels of Fas and Fas-L expression, which is consistent with our previous observation. 20 However, chronic UV irradiation caused a decrease in Fas-L expression in the epidermis as early as 1 week after UV exposure began. In addition, Fas-L expression continued to decrease further after 2 to 3 weeks of chronic irradiation, and was undetectable after 4 weeks. Second, loss of Fas-L expression was seen in both papillomas and SCCs induced by chronic UV irradiation.
In contrast to loss of Fas-L expression, Fas was expressed at high levels in the epidermis of chronically UV-irradiated skin, papillomas, and SCCs. Because both Fas and Fas-L are required for the elimination of sunburn cells, 20 loss of either one could inhibit apoptosis and lead to the expansion of apoptosis-defective cells that perhaps also contain p53 mutations. Although the resistance of chronically UV-irradiated keratinocytes to cell death is a self-mediated process due to loss of Fas-L expression, these cells could still be sensitive to Fas-L from other sources. In fact, even though the papillomas and carcinomas did not express Fas-L, some of the cells were undergoing apoptosis (data not shown). This may be due to the interaction of Fas (expressed on tumor cells) with its ligand Fas-L (expressed in immune infiltrating cells). Alternatively, apoptosis in papillomas and carcinomas in vivo in chronically irradiated mice may also be mediated by the bax/bcl-2 pathway. Because only a few papillomas progress to carcinomas, it is likely that the rest are eliminated by apoptosis. However, the precise mechanisms involved in the apoptosis of papillomas and carcinoma cells in vivo is unknown.
In addition to loss of Fas-Fas-L interaction, loss of bax/bcl-2 interaction may play a role in dysregulation of apoptosis. Although exposure of hairless mice to a single dose of UV radiation resulted in increased expression of bax and decreased expression of bcl-2, 29 these two proteins were expressed in chronically UV-irradiated mouse skin as well as in papillomas and carcinomas (data not shown). However, no discernable changes in expression of either bax or bcl-2 were noticed. Nonetheless, these data do not exclude the role of bax/bcl-2 pathway in chronic UV-induced apoptosis in hairless mouse skin, but rather favor the importance of Fas/Fas-L interactions in this process.
Analogous to our finding that loss of Fas-L confers resistance to apoptosis in UV-irradiated mouse skin, other studies have shown that Fas/Fas-L interactions play an important role in the apoptosis that helps to maintain homeostasis in several tissues. 30 In addition, mutation or loss of Fas or Fas-L expression can cause hyperplasia in peripheral lymphoid organs and other tissues, thereby accelerating progression of autoimmune diseases and tumorigenesis. 31 These observations support the conclusion that loss of Fas-L expression in mouse keratinocytes after chronic UV exposure results in inhibition of apoptosis.
The mechanism for loss of Fas-L expression in this scenario is presently unknown. However, it is possible that chronic UV irradiation causes malfunctioning of upstream elements that regulate Fas-L expression. For example, mitogen-activated protein kinases 32 and transcription factors such as nuclear factor of activated T lymphocytes (NFAT), 33 nuclear factor (NF)-κB, and activator protein-1 (AP-1) 34 may be inactivated by chronic UV irradiation, leading to a failure of Fas-L up-regulation. In addition, overexpression of Bcl-2 can lead to down-regulation of Fas-L because Bcl-2 can bind and sequester calcineurin, a calcium-dependent phosphatase that dephosphorylates NFAT to prevent its translocation to the nucleus. 35 However, it is also important to note that Fas-L expression can be specifically affected by different mechanisms without any changes in Fas expression. 36 Interestingly, although we have shown that wild-type p53 is a transcriptional activator of Fas, 28 Fas was expressed in chronically UV-irradiated mouse skin and in tumors arising in UV-irradiated mice despite the high incidence of p53 mutations. In this regard, it is important to note that Fas can be up-regulated by additional mechanisms in the absence of wild-type p53. 37
In addition to loss of Fas-L expression, loss of wild-type p53 function can prevent elimination of UV-induced sunburn cells. An important relationship between UV-induced apoptosis and skin carcinogenesis was proposed by Zeigler et al, 13 who showed that UV irradiation of normal mouse skin induced the formation of sunburn cells. However, when p53−/− animals were irradiated, few sunburn cells were observed. This suggests that p53 may act as a guardian of the tissue by inducing apoptosis in UV-damaged keratinocytes, whereas p53 mutations induced by subsequent radiation favor continued cell division and accumulation of additional genetic alterations. The data presented here are consistent with this hypothesis and further suggest that loss of Fas-L expression and loss of wild-type p53 function (due to mutation) can inhibit apoptosis and lead to the expansion of keratinocytes and initiation of skin cancer. It is important to note that the p53 mutations arose very early, well before the development of skin tumors, suggesting that p53 mutations is an early event in photocarcinogenesis, which is in agreement with previous findings in humans 12 and mice. 10,38
In summary, the results of this study indicate that loss of Fas-L expression and gain of p53 mutations occur very early during UV skin carcinogenesis. Because these two events can prevent the elimination of sunburn cells containing DNA damage induced by repeated exposure to UV radiation, the cells undergo expansion, acquire additional genetic alterations, and progress into skin cancers.
Acknowledgments
We thank Stephen Ullrich and Cora Bucana for technical advice.
Footnotes
Address reprint requests to Dr. Honnavara N. Ananthaswamy, Department of Immunology, Box 178, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030. E-mail: hanantha@mail.mdanderson.org.
Supported by National Cancer Institute grants CA 46523 (to H. N. A) and CA 16672 (Institutional Core Grant). A. O. was supported by a McCarthy postdoctoral fellowship.
References
- 1.Miller DL, Weinstock MA: Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol 1994, 30:774-778 [DOI] [PubMed] [Google Scholar]
- 2.Urbach F: Incidence of nonmelanoma skin cancer. Dermatol Clin 1991, 9:751-755 [PubMed] [Google Scholar]
- 3.Urbach F: Ultraviolet radiation and skin cancer of humans. J Photochem Photobiol B 1997, 40:3-7 [DOI] [PubMed] [Google Scholar]
- 4.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J: A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA 1991, 88:10124-10128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilchrest BA: Actinic injury. Annu Rev Med 1990, 41:199-210 [DOI] [PubMed] [Google Scholar]
- 6.Young AR: Cumulative effects of ultraviolet radiation on the skin: cancer and photoaging. Semin Dermatol 1990, 9:25-31 [PubMed] [Google Scholar]
- 7.Kripke M: Immunological effects of ultraviolet radiation. J Dermatol 1991, 18:429-433 [DOI] [PubMed] [Google Scholar]
- 8.de Gruijl FR, Forbes PD: UV-induced skin cancer in a hairless mouse model. Bioessays 1995, 17:651-660 [DOI] [PubMed] [Google Scholar]
- 9.Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ: Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Invest Dermatol Symp Proc 1996, 1:136-142 [PubMed] [Google Scholar]
- 10.Ananthaswamy HN, Loughlin SM, Cox P, Evans RL, Ullrich SE, Kripke ML: Sunlight and skin cancer: inhibition of p53 mutations in UV-irradiated mouse skin by sunscreens. Nat Med 1997, 3:510-514 [DOI] [PubMed] [Google Scholar]
- 11.Kanjilal S, Pierceall WE, Cummings KK, Kripke ML, Ananthaswamy HN: High frequency of p53 mutations in ultraviolet radiation-induced murine skin tumors: Evidence for strand bias and tumor heterogeneity. Cancer Res 1993, 53:2961-2964 [PubMed] [Google Scholar]
- 12.Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, Leffell DJ, Tarone RE, Brash DE: Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci USA 1996, 93:14025-14029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE: Sunburn and p53 in the onset of skin cancer. Nature 1994, 372:773-776 [DOI] [PubMed] [Google Scholar]
- 14.Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB: Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA 1992, 89:7491-7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Mitchell DL, Ho VC, Reed JC, Tron VA: Decreased DNA repair but normal apoptosis in ultraviolet-irradiated skin of p53-transgenic mice. Am J Pathol 1996, 148:1113-1123 [PMC free article] [PubMed] [Google Scholar]
- 16.Brash DE: Cellular proofreading. Nat Med 1996, 2:525-526 [DOI] [PubMed] [Google Scholar]
- 17.Tron VA, Trotter MJ, Tang L, Krajewska M, Reed JC, Ho VC, Li G: p53-regulated apoptosis is differentiation dependent in ultraviolet B-irradiated mouse keratinocytes. Am J Pathol 1998, 153:579-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith ML, Fornace AJ, Jr: p53-mediated protective responses to UV irradiation. Proc Natl Acad Sci USA 1997, 94:12255-12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leffell DJ, Brash DE: Sunlight and skin cancer. Sci Am 1996, 275:52–53, 56–59 [DOI] [PubMed]
- 20.Hill LL, Ouhtit A, Loughlin SM, Kripke ML, Ananthaswamy HN, Owen-Schaub LB: Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science 1999, 285:898-900 [DOI] [PubMed] [Google Scholar]
- 21.Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC: CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature 1995, 376:181-184 [DOI] [PubMed] [Google Scholar]
- 22.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC: A role for CD95 ligand in preventing graft rejection. Nature 1995, 377:630-632 [DOI] [PubMed] [Google Scholar]
- 23.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA: Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270:1189-1192 [DOI] [PubMed] [Google Scholar]
- 24.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P: Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 1994, 265:528-530 [DOI] [PubMed] [Google Scholar]
- 25.Hill LL, Shreedhar VK, Kripke ML, Owen-Schaub LB: A critical role for Fas ligand in the active suppression of systemic immune responses by ultraviolet radiation. J Exp Med 1999, 189:1285-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F: The Fas counter attack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996, 184:1075-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen-Schaub LB, van Golen KL, Hill LL, Price JE: Fas and Fas ligand interactions suppress melanoma lung metastasis. J Exp Med 1998, 188:1717-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, Roth JA, Deisseroth AB, Zhang WW, Kruzel E: Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 1995, 15:3032-3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouhtit A, Muller HK, Davis WD, Ullrich SE, McConkey D, Ananthaswamy HN: Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol 2000, 156:201-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S: Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet 1995, 11:294-300 [DOI] [PubMed] [Google Scholar]
- 31.Nagata S: Fas-induced apoptosis, and diseases caused by its abnormality. Genes Cells 1996, 1:873-879 [DOI] [PubMed] [Google Scholar]
- 32.Hsu SC, Gavrilin MA, Tsai MH, Han J, Lai MZ: p38 mitogen-activated protein kinase is involved in Fas ligand expression. J Biol Chem 1999, 274:25769-25776 [DOI] [PubMed] [Google Scholar]
- 33.Latinis KM, Norian LA, Eliason SL, Koretzky GA: Two NFAT transcription factor binding sites participate in the regulation of CD95 (Fas) ligand expression in activated human T cells. J Biol Chem 1997, 272:31427-31434 [DOI] [PubMed] [Google Scholar]
- 34.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR: DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell 1998, 1:543-551 [DOI] [PubMed] [Google Scholar]
- 35.Srivastava RK, Sasaki CY, Hardwick JM, Longo DL: Bcl-2-mediated drug resistance: inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J Exp Med 1999, 190:253-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R, Brunner T, Zhang L, Shi Y: Fungal metabolite FR901228 inhibits c-Myc and Fas ligand expression. Oncogene 1998, 17:1503-1508 [DOI] [PubMed] [Google Scholar]
- 37.Chan HB, Bartos DP, Owen-Schaub LB: Activation-dependent transcriptional regulation of the human Fas promoter requires NF-kappaB p50–p65 recruitment. Mol Cell Biol 1999, 19:2098-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg RJW, van Kranen HJ, Rebel HG, de Vries A, van Vloten WA, van Kreijl CF, van der Leun JC, de Gruijl FR: Early p53 alterations in mouse skin carcinogenesis by UVB radiation: immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells. Proc Natl Acad Sci USA 1996, 93:274-278 [DOI] [PMC free article] [PubMed] [Google Scholar]