Abstract
Human intestinal lamina propria mesenchymal cells show high surface expression of the α4β1 integrin. Ligation of α4β1 on mesenchymal cell lines with an activating monoclonal anti-α4 antibody or vascular cell adhesion molecule-immunoglobulin (VCAM-IgG) leads to the appearance of activated forms of gelatinase A in culture supernatants, and the de novo expression of activated membrane type-1-matrix metalloproteinase (MT1-MMP). In functional assays, signaling through α4β1 results in an increased capacity of mesenchymal cells to migrate through an artificial extracellular matrix, an effect inhibitable by excess tissue inhibitor of metalloproteinase-2. In organ cultures of human intestine, VCAM-IgG also up-regulates MT1-MMP, and in mucosal ulcers of inflammatory bowel disease patients, MT1-MMP transcripts are abundant, coincident with expression of VCAM-1 on cells at the ulcer margin. Collectively these results suggest that α4β1-induced up-regulation of MT1-MMP may be a crucial factor in the migration of mesenchymal cells into ulcer beds during restitution of diseased gut mucosa.
The α4β1 integrin (very late antigen-VLA-4) is expressed on immune and nonimmune cells throughout the body. On T cells, ligation of α4β1 with the extracellular matrix protein fibronectin or binding to its cell surface ligand, vascular cell adhesion molecule-1 (VCAM-1) on endothelium and macrophages provides a co-stimulatory signal. 1-3 The counter-receptor, VCAM-1, is a member of the immunoglobulin gene superfamily. 4 It is expressed on endothelial cells stimulated by inflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α. 5 It is also present on dendritic cells of the tonsil, spleen, and peripheral lymph nodes, 2,6 on bone marrow stromal cells, and on cytokine-treated neural cells and synoviocytes. 7-10 α4β1 integrin not only serves as a physical link between the extracellular matrix and the cell but via pp125FAK tyrosine phosphorylation after ligand binding can signal changes in the extracellular environment, thereby eliciting changes in cell function. 11,12
Matrix metalloproteinases (MMPs) are a group of Ca2+-dependent, Zn2+-containing enzymes produced by various cell types including mesenchymal cells, T cells, monocytes, macrophages, and neutrophils and are capable of degrading all components of the extracellular matrix. 13-16 Excess MMP activity causes tissue injury in various conditions such as rheumatoid arthritis, osteoarthritis, periodontal disease, tumor progression, bone resorption, and so forth. 17-19 The extracellular activity of MMPs is tightly regulated by tissue inhibitor of metalloproteinase (TIMP).
In inflammatory bowel disease (IBD), MMPs such as stromelysin-1 are produced in excess by mesenchymal cells activated by TNF-α or IL-1β. There is good evidence in model systems and in patients that high expression of stromelysin-1 is important in mucosal degradation and ulcer formation. 20-24 Gelatinase A is produced constitutively by mesenchymal cells and is only marginally up-regulated by pro-inflammatory cytokines. 22 However, its role in gut mucosal inflammation has never been as distinctive as that of stromelysin-1 both ex vivo and in vivo. Whereas the addition of activated stromelysin-1 to explants of human fetal tissue leads to mucosal loss within 24 hours, addition of equivalent amounts of activated gelatinase A has no effect on mucosal structure. 22 Instead, gelatinase A is believed to be more important in the immediate pericellular space and is crucial for tumor cell invasion and metastasis by degrading extracellular matrix components such as type IV collagen, expressed on the basement membrane in the gut. It has also been reported that T cells secrete gelatinase A and that the induction of this MMP in T cells on adhesion to endothelial cells is VCAM-1-dependent. 25
We were interested in determining the regulation of mesenchymal cell MMP production by pathways other than pro-inflammatory cytokines. In this regard, the α4β1 integrin is of interest because it is expressed in lamina propria mesenchymal cells, but not muscularis mucosa cells. 26
Here we show that in contrast to TNF-α or IL-1β, the ligation of α4β1 on mesenchymal cells selectively up-regulates MT1-MMP, increases activation of gelatinase A, and stimulates the cells to migrate through an artificial extracellular matrix. Migration is inhibited by TIMP-2. MT1-MMP mRNA was also detected in an ex vivo intestinal organ culture model after α4β1 ligation. Furthermore, high expression of MT1-MMP and stromelysin-1 mRNA were seen at ulcer edges in IBD along with VCAM-1-positive cells. These data support the notion that signaling through α4β1 integrin on intestinal mesenchymal cells may be important in promoting the migration of mesenchymal cells through granulation tissue during mucosal healing.
Materials and Methods
Monoclonal Antibody and Fusion Protein
α4β1 on mesenchymal cells was ligated using a murine monoclonal IgG1 anti-human VLA-4. 27 A recombinant human VCAM-IgG fusion protein was also used 28 in which the first two Ig domains were linked to human IgG1. As controls for these two reagents, either mouse IgG or human IgG was added at an equivalent concentration. A mutant VCAM-1 fusion protein, VCAM-Ig D40 produced by site-directed mutagenesis of the amino acid residues on the loop between β strands C and D, which does not bind α4β1 was used as a negative control. 29
Isolation, Characterization, and Stimulation of Mucosal Mesenchymal Cells
Human fetal mesenchymal cell lines were isolated and characterized as described previously. 22 Only cells that grew to passage 4 and beyond were used. Each batch of cells was characterized before use. Mesenchymal cells (1 × 105) were seeded into 6-well plates and maintained in minimal essential medium plus 10% fetal calf serum overnight. The cell layer was washed twice with ice-cooled phosphate-buffered saline and stimulated with anti-VLA4 (1 to 10 μg/ml), VCAM-IgG (1 to 10 μg/ml), mouse IgG (10 μg/ml; Sigma, Poole, UK), human IgG (10 μg/ml), IL-1β (1 ng/ml; R & D Systems Europe Ltd., Abingdon, UK), or TNF-α (1 ng/ml; R & D Systems) in serum-free medium for 48 hours. Culture supernatants were removed and spun at 1,200 × g for 10 minutes to remove cell debris before analysis of MMP production.
Human Fetal Gut Explant Culture
Second trimester human fetal small intestine was obtained within 2 hours of surgical termination from the Medical Research Council Tissue Bank (London, UK). This study received ethical approval from the Hackney and District Health Authority (London, UK). Fetal gut explants were cultured for 2 days in the presence of anti-VLA4 (10 μg/ml) or VCAM-IgG fusion protein (10 μg/ml), mouse IgG or human IgG was used as IgG controls. Culture supernatants and tissue samples were collected and stored at −70°C before analysis.
Flow Cytometry
Mesenchymal cells were released from tissue culture flasks by trypsin-ethylenediaminetetraacetic acid treatment, washed three times, counted, and aliquoted at 5 × 10 5 cells per tube. Cells were then stained with anti-VLA4 (10 μg/ml) and a secondary rabbit anti-mouse antibody conjugated to fluorescein isothiocyanate (Sigma). Mean fluorescence intensity in arbitrary units was recorded on a log scale for each sample.
Western Blotting
Western blotting was performed according to the methods and the reagents described previously 30 except for the monoclonal TIMP-2 antibody (used at 5 μg/ml; Oncogene Research, Nottingham, UK) and polyclonal MT1-MMP antibody (0.125 μg/ml, Chemicon International Inc., Temecula, CA). In all cases, equivalent amounts of protein were loaded onto each lane of the 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were run in reducing conditions. The secondary antibodies, rabbit anti-sheep (1:2,500), goat anti-rabbit (1:3,000), and rabbit anti-mouse (1:1,000) were all conjugated with horse-radish peroxidase (DAKO Ltd., High Wycombe, UK). The bands were visualized by the enhanced chemiluminescence-plus system according to manufacturer’s instructions (Amersham Pharmacia Biotech UK Ltd., Amersham, Buckinghamshire, UK).
MT-MMP Plasmid and Quantification of MT1-MMP Transcripts
To facilitate quantitation of MT1-MMP mRNA by reverse transcriptase-polymerase chain reaction (RT-PCR), we constructed a plasmid that encodes a standard MT1-MMP RNA molecule according to the method of Jung et al. 31 MT1-MMP-specific primer sequences, sense: 5′CGC TAC GCC ATC CAG GGT CTC AAA3′ and antisense: 5′ CGG TCA TCA TCG GGC AGC ACA AAA 3′ 32 were cloned into plasmid pHCQ2, kindly provided by Dr. M. F. Kagnoff (Dept. of Medicine, University of California, San Diego, CA). The sequence of the new construct was confirmed by dideoxy sequencing (Amersham Pharmacia Biotech, Little Chalfont, UK). To generate standard RNA, the plasmid was linearized with HindIII and transcribed in vitro using T7 RNA polymerase under conditions recommended by the supplier (Promega, Southampton, UK). Using the same primer set, RT-PCR of the standard molecule produces a PCR product of 430 bp, whereas the natural target yields a 497-bp fragment. Total RNA was extracted from cells and precipitated according to the method described previously. 22 Total RNA (0.5 μg) was used for first-strand cDNA synthesis together with a serial dilution of synthetic RNA molecules. The standard RNA and test RNA were co-reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (100 U, Life Technologies, Paisley, UK). The thermal cycle was programmed with a hot-start at 94°C for 5 minutes followed by 35 cycles at 94°C for 1.5 minutes, annealing at 58°C for 2 minutes, followed by extension at 72°C for 3 minutes. PCR products were electrophoresed in 0.7% agarose gels containing 0.3 μg/ml ethidium bromide. Bands were visualized and their intensities were quantified by densitometry (Seescan 1D gel analysis package v1.00; Seescan, Cambridge, UK). The ratios of the band intensities of the PCR products from the standard RNA and the target RNA were plotted against the starting amount of standard RNA molecules on a semilogarithmic scale. This lower limit of sensitivity of the assay was set at 1,000 transcripts per microgram of total RNA.
Invasion Chamber Assay
Mesenchymal cells (5 × 104) were seeded in a Biocoat Matrigel invasion chamber (Becton Dickinson, Bedford, MA) with an 8-μm-pore size membrane precoated with Matrigel. Five- percent fetal calf serum was used in the lower chamber as a chemoattractant. Mesenchymal cells were stimulated by adding VCAM-IgG (10 μg/ml) or αVLA-4 (10 μg/ml). Recombinant TIMP-2 (3 μg/ml; Chemicon International Inc., Harrow, UK) was also added to some of the upper chambers. After 48 hours, the upper Matrigel membrane and noninvading cells were removed with cotton wool buds, and the filter was stained with hematoxylin for 10 minutes and mounted on glass slides. The total number of invading cells adherent to the lower side of the membrane was counted by light microscopy.
In Situ Hybridization
A 1240-bp fragment of MT1-MMP cDNA 33 was subcloned to pGEM vector containing an SP6 RNA polymerase recognition element. When linearized with BgI II, the antisense RNA probe was transcribed in vitro containing 405 bp from 3′UTR of the MT1-MMP cDNA. The specificity of the probe was confirmed by sequencing. As a control for nonspecific hybridization, sections were hybridized with 35S-labeled sense RNA from a bovine tropoelastin cDNA. The validity of this probe as a negative control has been confirmed by Northern 34 and by in situ hybridization assays. 35 The cDNAs were transcribed in vitro using a commercial kit (Promega Corp., Madison, WI) and labeled with 35S-UTP, as previously described. 36
After deparaffinization and rehydration, 5-μm sections were pretreated with 1 mg/ml of proteinase K and washed in 0.1 mol/L triethanolamine containing 0.25% acetic anhydride. Subsequently, sections were hybridized with probes (2.5 to 5 × 10 4 cpm/μl of hybridization buffer) and washed under stringent conditions including treatment with RNase A, as described. 34 Autoradiography was performed for 20 to 45 days. Surgical samples from six ulcerative colitis patients, six Crohn’s patients, three samples from normal colon, three normal ileum, and three normal jejunum were studied. For fetal gut explants, 15 different explants were studied. All samples were processed in at least two experiments and were independently analyzed by two experienced investigators. Samples previously positive for MT1-MMP (breast cancer) were used as positive controls.
Immunohistochemistry
Six-μm sections from Crohn’s or ulcerative colitis resection samples were stained with CD68 (Dako Ltd, Cambridgeshire, UK) anti-VCAM-1 antibody (1:50 dilution; Autoantigen Bioclear UK Ltd., Wiltshire, UK) by the indirect peroxidase method as described previously. 22
Statistical Analysis
Differences between groups were compared using either the Mann Whitney U test, if the data were not normally distributed, or Student’s t-test, if the observations were consistent with a sample from a normally distributed population. Where applicable, results are shown as mean ± 1 SE.
Results
Human Fetal Gut Mesenchymal Cells Are Predominantly VLA-4-Positive
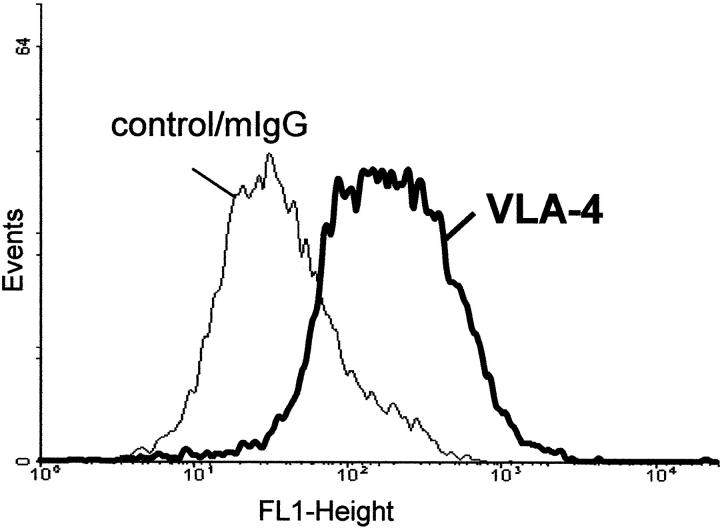
Flow cytometric analysis (Figure 1) ▶ revealed that 80% of gut mesenchymal cells expressed α4β1. Similar findings were made with different mesenchymal cell lines. When mesenchymal cells were plated onto glass coverslips and then stained in situ by immunohistochemistry, >90% of the cells were α4β1-positive (data not shown).
Figure 1.
FAC analysis of α4β1-positive gut mesenchymal cells. Cells were stained with an antibody directed against α4β1 integrin followed by a fluorescein-conjugated secondary antibody. The thin line curve represents the negative mouse IgG control.
The Activated Forms of Gelatinase A Are Up-Regulated after the Ligation of VLA4 on Gut Mesenchymal Cells with Anti-VLA-4 Antibody or VCAM-IgG
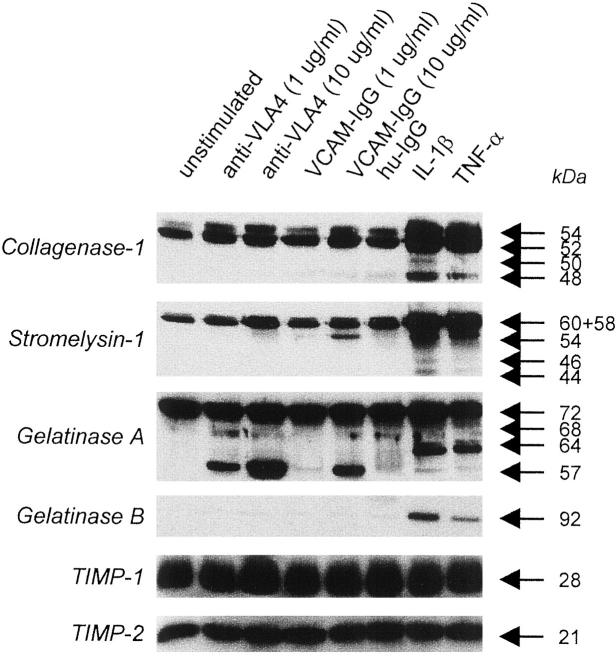
Mesenchymal cells cultured in serum-free media alone produced basal levels of interstitial collagenase, stromelysin-1, gelatinase A, TIMP-1, and TIMP-2 (Figure 2) ▶ . The addition of anti-VLA4 or VCAM-IgG at 1 to 10 μg/ml to mesenchymal cells for 48 hours had little effect on interstitial collagenase, gelatinase B, TIMP-1, and TIMP-2 production and there was a modest up-regulation of stromelysin-1 production, in comparison to TNF-α or IL-1β stimulation. However, anti-VLA4 and VCAM-IgG led to the appearance of two activated forms of gelatinase A (68 kd and 57 kd principally) in culture supernatants in a dose-dependent manner (Figure 2) ▶ .
Figure 2.
Activated gelatinase A is present in the culture supernatants of gut mesenchymal cells after α4β1 ligation. Mesenchymal cells were stimulated with different concentrations of anti-VLA4 or VCAM-IgG for 2 days. Culture supernatants were collected and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and Western blotting. Human IgG was used as negative control whereas IL-1β and TNF-α were used as positive controls. Arrows pointing to specific bands denote latent and active forms of MMPs. This is a representative figure from five different experiments.
Membrane Type-1 MMP Is Increased after Anti-VLA4 or VCAM-IgG Stimulation of Mesenchymal Cells
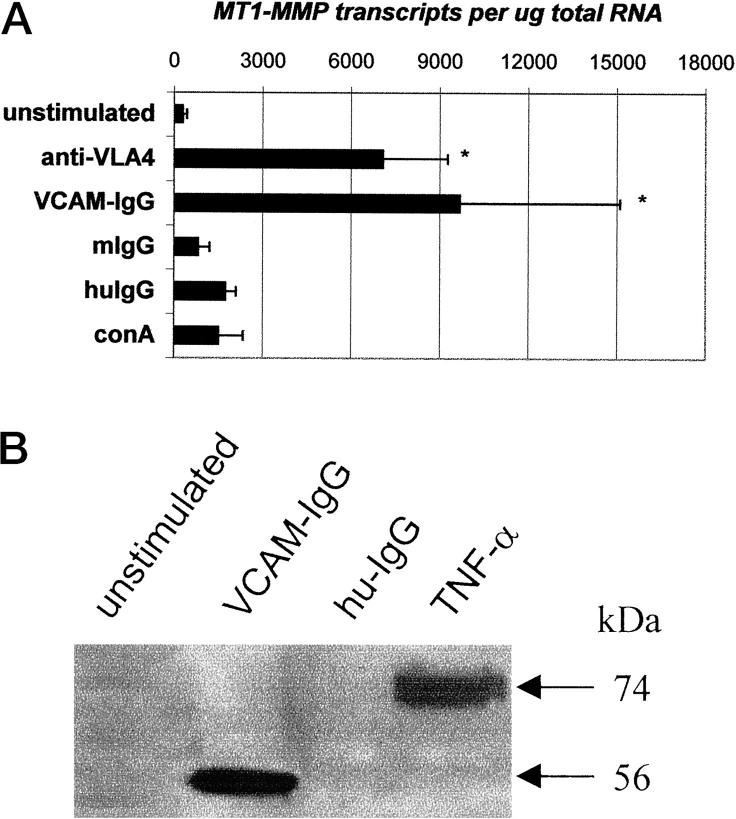
Activation of gelatinase A on the cell membrane is associated with MT1-MMP. Unstimulated mesenchymal cells only expressed low numbers of MT1-MMP transcripts, however, when the cells were stimulated with anti-VLA4 or VCAM-IgG, MT1-MMP transcripts were dramatically increased (Figure 3A) ▶ . ConA and TNF-α also increased the expression of MT1-MMP by mesenchymal cells but only to a small fraction of that seen with VCAM-IgG or anti-α4 antibody. Concomitant with the increased MT1-MMP transcripts, the active form of MT1-MMP protein (56 kd) was also up-regulated after stimulation with VCAM-IgG or recombinant TNF-α as detected by Western blotting in mesenchymal cell lysates (Figure 3B) ▶ .
Figure 3.
A: Induction of MT1-MMP transcripts in gut mesenchymal cells after α4β1 integrin ligation. Gut mesenchymal cells were cultured with anti-VLA4 (10 μg/ml) or VCAM-IgG (10 μg/ml) for 2 days. TNF-α or ConA were used as positive controls. This experiment is one of two separate experiments in which identical results were obtained. B: Induction of MT1-MMP protein by VCAM-IgG or recombinant TNF-α. Mesenchymal cells were stimulated with VCAM-IgG, human-IgG (hu-IgG), or TNF-α for 2 days. Whole cell lysates were used for detecting MT1-MMP by Western blotting. This is a representative of two individual experiments with comparable results.
Anti-VLA4 and VCAM-IgG Induced a Migratory Phenotype
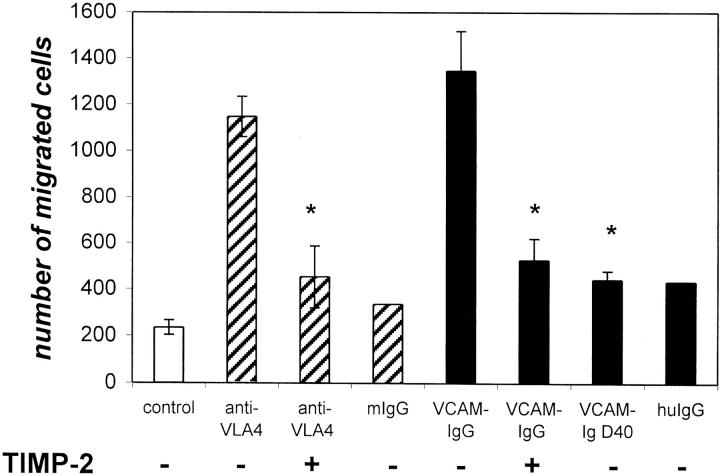
To determine whether activation through α4β1 had functional activity, we plated mesenchymal cells on Matrigel filters and stimulated them with anti-VLA4 or VCAM-IgG. In unstimulated cultures, several hundred cells migrated through the Matrigel and adhered to the basal side of the semipermeable filter. In contrast, when cells were stimulated with either VCAM-IgG or anti-α4β1 antibody, there was a fivefold to sixfold increase in the number of cells adherent to the basal aspect of the filter (Figure 4) ▶ . Anti-α4β1 and VCAM-IgG stimulated migration was significantly inhibited by TIMP-2. Finally, a mutant fusion protein VCAM-Ig D40 that does not bind α4β1 integrins did not induce cell migration.
Figure 4.
Gut mesenchymal cells migrate in increased numbers through Matrigel-coated filters after α4β1 ligation. This migration is significantly inhibited by TIMP-2 (3 μg/ml). The mutant fusion protein VCAM-Ig D40 which does not bind α4β1 integrin and does not induce migration. Results shown are the average of two separate experiments in which similar results were obtained with <10% variation between different experiments.
Up-Regulation of MT1-MMP in Intestinal Explants
To determine whether MT1-MMP could be up-regulated by α4β1 ligation of mesenchymal cells in the gut itself, we cultured fetal gut explants with VCAM-IgG. Activated forms of gelatinase A were detected in the culture supernatants of explants stimulated by VCAM-IgG but not in control/unstimulated explants (Figure 5) ▶ . MT1-MMP transcripts were measured by quantitative RT-PCR. Explants which were stimulated with VCAM-IgG for 4 days had ∼17,000 MT1-MMP transcripts per μg total RNA, however, the number of transcripts detected in control explants was <1,000 transcripts per μg total RNA (data not shown). In situ hybridization revealed that MT1-MMP mRNA was expressed on stromal/fibroblast-like cells in the lamina propria of VCAM-IgG-treated explants. These were scattered throughout the lamina propria and are α-smooth muscle actin-positive.
Figure 5.
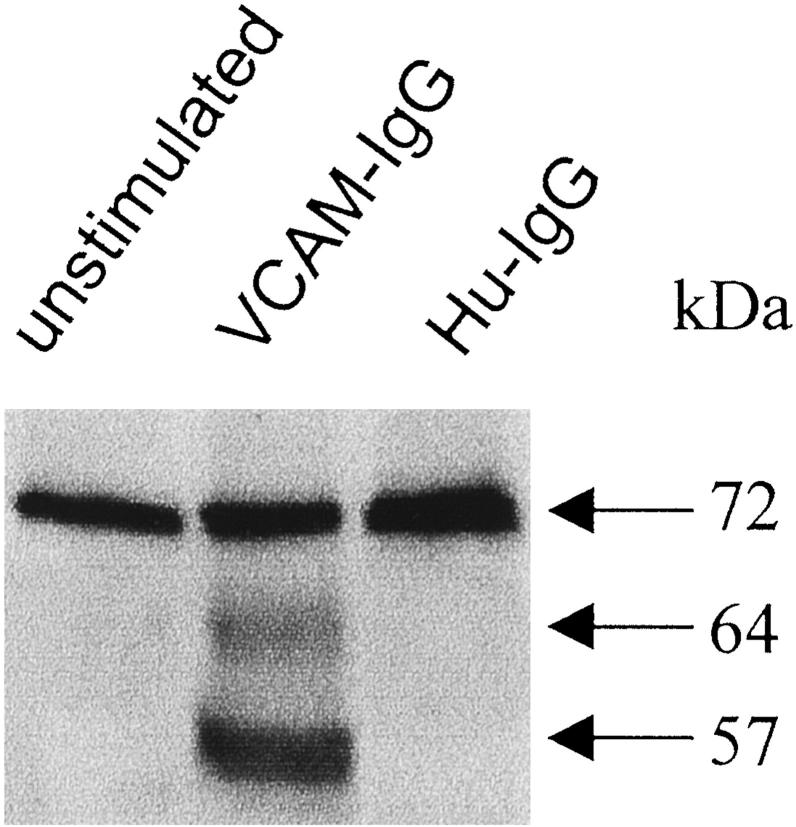
Induction of activated gelatinase A in the supernatants of fetal gut explants after α4β1 ligation. Explants were stimulated with VCAM-IgG or human IgG (Hu-IgG) for 4 days. Culture supernatants were collected for Western blotting analysis. Both 64- and 57-kd active forms of gelatinase A were up-regulated. This is representative of three experiments with similar results.
Localization of MT1-MMP and Stromelysin-1 mRNA Expression and VCAM in Ulcers of Patients with IBD
Weak nonspecific MT1-MMP labeling of the epithelium was seen in normal colon (Figure 6, A and B) ▶ . This probably represents the binding of the probes to mucus. There was no stromelysin-1 signal in control colon as described previously. 21 mRNAs of both MT1-MMP and stromelysin-1 are highly up-regulated in IBD ulcers compared to normal colon. Both MMPs are present at the ulcer edges, with MT1-MMP mRNA-positive cells being spread more deeply in the mucosa (Figure 6, C and D) ▶ . Under high magnification, we found that MT1-MMP mRNA is present in activated fibroblast-like cells (Figure 6E) ▶ . Immunostaining reveals that MT1-MMP expressing cells are neither CD68- (Figure 6, F and G) ▶ nor α-smooth muscle actin-positive (data not shown).
Figure 6.
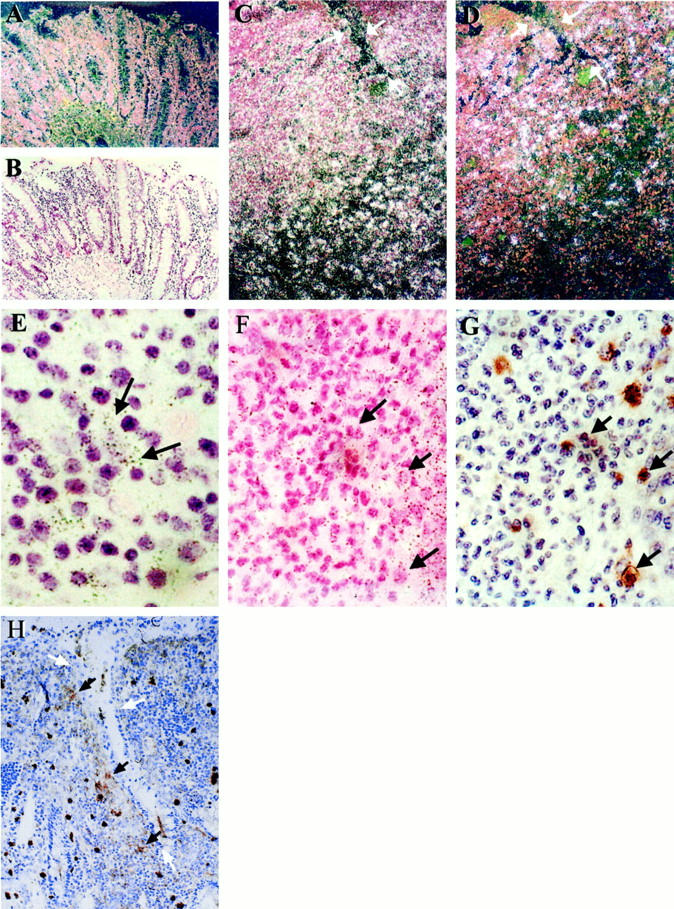
In situ hybridization reveals up-regulation of MT1-MMP mRNA in IBD ulcers. Original magnification, ×100 (unless otherwise stated). A and B: Dark and light field of MT1-MMP mRNA in a normal colon, respectively. C: MT1-MMP mRNA in an UC ulcer. White arrows show the ulcer edge. Note that positive cells are present at the ulcer margin and deep within the mucosa. This is representative of six different UC and six Crohn’s ulcer sections. D: A serial section of C shows MMP-3 mRNA restricted to the ulcer margin. E: MT1-MMP mRNA is expressed by fibroblast-like cells in an ulcer bed. Original magnification, ×400. F: MT1-MMP mRNA in an ulcer bed, arrowheads show MT1-MMP-negative cells. G: A serial section of F stained for CD68, the corresponding arrows, especially the cluster in the middle of the picture, show CD68-positive cells are not co-localized with MT1-MMP expressing cells. H: Immunochemistry shows VCAM-1-positive cells localize in the ulcer edge. White arrows indicate the ulcer edges.
Sections of IBD tissue were also stained with anti-VCAM antibody. There was no staining in control tissue, however, around the ulcer edges, large, strongly positive cells were seen (Figure 6H) ▶ . These cells were probably macrophages.
Discussion
In this study, we have shown that the ligation of α4β1 integrin with an activating antibody or a VCAM-1 fusion protein facilitates mesenchymal cell invasion and migration through extracellular matrix. The migration involves an up-regulation of MT1-MMP and the production of activated gelatinase A. Migration of cells can be inhibited by TIMP-2. Concomitant with these observations, we also showed that MT1-MMP could be up-regulated after VCAM-IgG stimulation in organ cultures of fetal human small intestine. High expression of MT1-MMP and stromelysin-1 mRNA were also detected in activated fibroblast-like cells at the ulcer edge where VCAM-1-positive cells were present. Collectively these results suggest an important role for α4β1 integrin on gut mesenchymal cells, perhaps conferring a migratory phenotype on cells around IBD ulcers.
The important role of myofibroblasts in mucosal inflammation and repair is receiving increasing attention. 37 Our previous results have shown that stromelysin-1 is a potent matrix-degrading enzyme, and it is massively up-regulated during T cell activation in an ex vivo fetal gut explant culture model. 22 Stromelysin-1 is also overexpressed in inflamed mucosa of IBD patients. 21 We initially had hypothesized that signaling through α4β1 would have an effect on a tissue-degrading MMP such as stromelysin-1. However, it was clear from the results (Figure 2) ▶ that the effect on interstitial collagenase, stromelysin-1, gelatinase B, and TIMPs 1 and 2 was minimal and that the most striking feature was the presence of small molecular weight form of gelatinase A.
In this study, we found that activated gelatinase A and MT1-MMP selectively up-regulated after α4β1 ligation led to an increased ability of gut mesenchymal cells to migrate through Matrigel. It has been shown elsewhere that the gelatinase A-related invasiveness of cells is associated with the up-regulation of MT1-MMP and the appearance of the activated form (68 and 64 kd) of gelatinase A. 38 In this study, we not only detected these two active forms but also a smaller active form of gelatinase A (57 kd) in the culture supernatant of anti-VLA4- or VCAM-IgG-stimulated mesenchymal cell cultures. We believe that they were cleavage products generated by the dramatically increased amount of MT1-MMP after stimulation. In addition, we also detected the soluble form of MT1-MMP in culture supernatants of VCAM-IgG-stimulated mesenchymal cells (data not shown). We also found that the migration of gut mesenchymal cells can be inhibited by adding exogenous TIMP-2. This result is consistent with that shown by Sato et al, 38 who also showed that excess TIMP-2 can inhibit the activation of gelatinase A and hence cell migration.
When we ligated α4β1 integrin in whole gut tissue, we also found an activated form of gelatinase A (Figure 5) ▶ in the culture supernatant as well as MT1-MMP mRNA in situ in the VCAM-IgG stimulated fetal gut culture explants. The number of MT1-MMP transcripts was also up-regulated in the culture explants after stimulation. Although it is likely that the MT1-MMP increase was because of an increase in transcripts in resident mesenchymal cells, we cannot exclude the possibility that there may have been a contribution from other cells expressing α4β1in fetal gut, such as T cells or macrophages.
The up-regulation of gelatinase A and MT1-MMP is also seen in skin wound healing, where there are many migratory phenotypes of skin fibroblasts; 39 in angiogenesis, where the endothelial cells migrate and the matrix remodels; 40 in airway wall and lumen, where there is infiltration of lymphocytes and eosinophils; 41 and in various neoplastic conditions. 42 Gelatinase A also appears to be localized and activated through an interaction with other integrins such as αvβ3 on the cell surface of both invading tumor cells and angiogenic vessels. 43 Recently gelatinase A and MT1-MMP null mice have been produced. The gelatinase A-deficient mouse shows no impairment of development and reproduction. 44 However, MT1-MMP deficiency causes craniofacial dysmorphism, arthritis, osteopenia, dwarfism, and soft tissue fibrosis to the animals 45 implicating its importance in growth and development.
Following on from the experiments with the fetal gut explants we used in situ hybridization to examine MT1-MMP and stromelysin expression in the intestine of patients with IBD. High levels of stromelysin-1 and MT1-MMP mRNA were localized in the same area near the ulcer edges. When we identified the positive cells at higher magnification, we found that most of the MT1-MMP-positive cells are activated fibroblast-like cells, and they are neither CD68 nor α-smooth muscle actin-positive cells. It was noticeable however that whereas stromelysin-1-positive cells were only around ulcers, MT1-MMP-positive cells extended deep into the tissue. If indeed MT1-MMP expression is related to migratory activity, this would indicate that extensive tissue remodeling is occurring deep below sites of obvious inflammation. We were however only able to visualize VCAM-1-positive cells at ulcer edges and could not see positive cells deeper in the mucosa. We would like to point out that unlike those in IBD ulcer, MT1-MMP mRNA-expressing cells in the fetal gut are α-smooth muscle actin-positive. Fetal gut culture tissue and chronic ulcer tissue are different, it is not surprising that one is smooth muscle actin-positive, the other is not. Further studies are needed to resolve the relative roles of fibronectin, abundant in inflamed gut, and VCAM-1-positive cells in the induction of MT1- MMP in vivo.
One of the most striking aspects of the natural history of IBD and the ulcers associated with the condition, is the ability of the gut to heal itself without medical intervention. There has been a great deal of interest in recent years on the factors which make epithelial cells migrate across diseased tissue 46,47 however a key part of healing must be the ability of mesenchymal cells to migrate through granulation tissue at ulcer edges and heal the ulcer bed before re-epithelialization and restoration of barrier function. Our results are to our knowledge, the first to demonstrate a potential mechanism by which mesenchymal cells could achieve this.
Acknowledgments
The authors thank Dr. J. Lohi, Haartman Institute of University of Helsinki, for the MT1-MMP cDNA. We are grateful to Dr. L. Wong, MRC Tissue Bank (London) for supplying human fetal tissue.
Footnotes
Address reprint requests to Dr. Sylvia L. F. Pender, Ph.D., Centre for Infection, Allergy, Inflammation, and Repair, Mailpoint 813, Level E, South Academic Block, Southampton General Hospital, Tremona Road, Southampton SO16 6YD, UK. E-mail: s.pender@soton.ac.uk.
Supported by Biotechnology and Biological Sciences Research Council (to S. P. and C. M.), European Union Grant ERBFMRXCT9 (to G. M.), Pfizer Ltd. (to D. S.), the Crohn’s in Childhood Research Association UK, (to T. T. M.), the Academy of Finland (to M. S. and U. S.), the Sigrid Juselius Foundation (to M. S. and U. S.), and the Helsinki University Central Hospital Research Foundation (to M. S. and U. S.).
References
- 1.Holzmann B, McIntyre BW, Weissman IL: Identification of a murine Peyer’s patch-specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4 alpha. Cell 1989, 56:37-46 [DOI] [PubMed] [Google Scholar]
- 2.Rice GE, Munro JM, Corless C, Bevilacqua MP: Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol 1991, 138:385-393 [PMC free article] [PubMed] [Google Scholar]
- 3.Sato T, Tachibana K, Nojima Y, D’Avirro N, Morimoto C: Role of the VLA-4 molecule in T cell costimulation. Identification of the tyrosine phosphorylation pattern induced by the ligation of VLA-4. J Immunol 1995, 155:2938-2947 [PubMed] [Google Scholar]
- 4.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R: Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 1989, 59:1203-1211 [DOI] [PubMed] [Google Scholar]
- 5.Thornhill MH, Haskard DO: IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol 1990, 145:865-872 [PubMed] [Google Scholar]
- 6.Freedman AS, Munro JM, Rice GE, Bevilacqua MP, Morimoto C, McIntyre BW, Rhynhart K, Pober JS, Nadler LM: Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science 1990, 249:1030-1033 [DOI] [PubMed] [Google Scholar]
- 7.Miyake K, Medina K, Ishihara K, Kimoto M, Auerbach R, Kincade PW: A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. J Cell Biol 1991, 114:557-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyake K, Weissman IL, Greenberger JS, Kincade PW: Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med 1991, 173:599-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan DH, Nuccie BL, Abboud CN, Winslow JM: Vascular cell adhesion molecule-1 and the integrin VLA-4 mediate adhesion of human B cell precursors to cultured bone marrow adherent cells. J Clin Invest 1991, 88:995-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birdsall HH, Lane C, Ramser MN, Anderson DC: Induction of VCAM-1 and ICAM-1 on human neural cells and mechanisms of mononuclear leukocyte adherence. J Immunol 1992, 148:2717-2723 [PubMed] [Google Scholar]
- 11.Maguire JE, Danahey KM, Burkly LC, van Seventer GA: T cell receptor- and beta 1 integrin-mediated signals synergize to induce tyrosine phosphorylation of focal adhesion kinase (pp125FAK) in human T cells. J Exp Med 1995, 182:2079-2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T: Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 1995, 377:539-544 [DOI] [PubMed] [Google Scholar]
- 13.Woessner JF, Jr: Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991, 5:2145-2154 [PubMed] [Google Scholar]
- 14.Matrisian LM: The matrix-degrading metalloproteinases. [Review] [50 refs]. Bioessays 1992, 14:455-463 [DOI] [PubMed] [Google Scholar]
- 15.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993, 4:197-250 [DOI] [PubMed] [Google Scholar]
- 16.Nagase H, Woessner JFJ: Matrix metalloproteinases. J Biol Chem 1999, 274:21491-21494 [DOI] [PubMed] [Google Scholar]
- 17.Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF, Jr: Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest 1989, 84:678-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meikle MC, Atkinson SJ, Ward RV, Murphy G, Reynolds JJ: Gingival fibroblasts degrade type I collagen films when stimulated with tumor necrosis factor and interleukin 1: evidence that breakdown is mediated by metalloproteinases. J Periodontal Res 1989, 24:207-213 [DOI] [PubMed] [Google Scholar]
- 19.Murphy G, Reynolds JJ: Extracellular matrix degradation. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects. Edited by PM Royce, B Steinmann. New York: Wiley-Liss, Inc., 1993, pp 287–316
- 20.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA: Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. J Clin Pathol 1994, 47:113-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saarialho-Kere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg ML: Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol 1996, 148:519-526 [PMC free article] [PubMed] [Google Scholar]
- 22.Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT: A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol 1997, 158:1582-1590 [PubMed] [Google Scholar]
- 23.Pender SL, Breese EJ, Gunther U, Howie D, Wathen NC, Schuppan D, MacDonald TT: Suppression of T cell-mediated injury in human gut by interleukin-10: role of matrix metalloproteinases. Gastroenterology 1998, 115:573-583 [DOI] [PubMed] [Google Scholar]
- 24.Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U: Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol 1998, 152:1005-1014 [PMC free article] [PubMed] [Google Scholar]
- 25.Romanic AM, Madri JA: The induction of 72-kD gelatinase in T cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol 1994, 125:1165-1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choy MY, Richman PI, Horton MA, MacDonald TT: Expression of the VLA family of integrins in human intestine. J Pathol 1990, 160:35-40 [DOI] [PubMed] [Google Scholar]
- 27.Jackson DY, Quan C, Artis DR, Rawson T, Blackburn B, Struble M, Fitzgerald G, Chan K, Mullin S, Burnier JP, Fairbrother WJ, Clark K, Berisini M, Chui H, Renz M, Jones S, Fong S: Potent alpha 4 beta 1 peptide antagonists as potential anti-inflammatory agents. J Med Chem 1997, 40:3359-3368 [DOI] [PubMed] [Google Scholar]
- 28.Renz ME, Chiu HH, Jones S, Fox J, Kim KJ, Presta LG, Fong S: Structural requirements for adhesion of soluble recombinant murine vascular cell adhesion molecule-1 to alpha 4 beta 1. J Cell Biol 1994, 125:1395-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu HH, Crowe DT, Renz ME, Presta LG, Jones S, Weissman IL, Fong S: Similar but nonidentical amino acid residues on vascular cell adhesion molecule-1 are involved in the interaction with alpha 4 beta 1 and alpha 4 beta 7 under different activity states. J Immunol 1995, 155:5257-5267 [PubMed] [Google Scholar]
- 30.Monteleone G, MacDonald TT, Wathen NC, Pallone F, Pender SL: Enhancing lamina propria Th1 cell responses with interleukin 12 produces severe tissue injury. Gastroenterology 1999, 117:1069-1077 [DOI] [PubMed] [Google Scholar]
- 31.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF: A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 1995, 95:55-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giambernardi TA, Grant GM, Taylor GP, Hay RJ, Maher VM, McCormick JJ, Klebe RJ: Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol 1998, 16:483-496 [DOI] [PubMed] [Google Scholar]
- 33.Lohi J, Lehti K, Westermarck J, Kahari VM, Keski-Oja J: Regulation of membrane-type matrix metalloproteinase-1 expression by growth factors and phorbol 12-myristate 13-acetate. Eur J Biochem 1996, 239:239-247 [DOI] [PubMed] [Google Scholar]
- 34.Prosser IW, Stenmark KR, Suthar M, Crouch EC, Mecham RP, Parks WC: Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol 1989, 135:1073-1088 [PMC free article] [PubMed] [Google Scholar]
- 35.Saarialho-Kere UK, Chang ES, Welgus HG, Parks WC: Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest 1992, 90:1952-1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC: Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest 1993, 92:2858-2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB: Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 1999, 277:C183-C201 [DOI] [PubMed] [Google Scholar]
- 38.Sato H, Takino T, Okada Y, Cao J, Shinagaura A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells [see comments]. Nature 1994, 370:61-65 [DOI] [PubMed] [Google Scholar]
- 39.Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P: Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol 1997, 137:67-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas TL, Davis SJ, Madri JA: Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem 1998, 273:3604-3610 [DOI] [PubMed] [Google Scholar]
- 41.Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K: Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol 1999, 162:4212-4219 [PubMed] [Google Scholar]
- 42.Okada A, Bellocq JP, Rouyer N, Chenard M, Rio M, Chambon P, Basset P: Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci USA 1995, 92:2730-2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetter-Stevenson WG, Quigley JP, Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 1996, 85:683-693 [DOI] [PubMed] [Google Scholar]
- 44.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S: Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 1997, 272:22389-22392 [DOI] [PubMed] [Google Scholar]
- 45.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey G, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H: MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999, 99:81-92 [DOI] [PubMed] [Google Scholar]
- 46.Mahida YR, Galvin AM, Gray T, Makh S, Mcalindon ME, Sewell HF, Podolsky DK: Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol 1997, 109:377-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podolsky DK: Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol 1999, 277:G495-G499 [DOI] [PubMed] [Google Scholar]