Abstract
The actin-binding protein ezrin has been associated with motility and invasive behavior of malignant cells. To assess the presence of this protein in human glial cells of the brain and its potential role in benign and malignant glial tumors, we studied ezrin immunoreactivity (IR), proliferation (MIB-1-IR), and apoptosis (terminal dUTP nick-end labeling) in normal human brain tissues from 10 autopsies and tissues from 115 cases of human glial tumors including astro-cytomas, ependymomas, oligodendrogliomas, and glioblastomas. We found weak staining of peripheral processes in normal human brain astrocytes and in World Health Organization grade II benign astrocytomas. Staining was markedly increased in anaplastic astrocytomas (World Health Organization grade III) and clearly strongest in glioblastomas (World Health Organization grade IV). The increase of ezrin-IR correlated significantly with increasing malignancy of astrocytic tumors (P < 0.0001). Statistical analysis revealed a stronger association with increasing malignancy for ezrin-IR than for MIB-1-IR or terminal dUTP nick-end labeling staining. Ezrin-IR was absent in normal oligodendrocytes and in oligodendrogliomas, but pronounced in normal ependymal cells and ependymomas. Ezrin-IR seems to be specific for astrocytes and ependymal glia in the normal brain. Our results indicate that ezrin-IR may provide a useful tool for the distinction of oligodendrogliomas and astrocytomas and for the grading of astrocytic tumors.
The actin-binding proteins ezrin, radixin, and moesin form a subfamily within the band 4.1 superfamily on the basis of sequence homology of the amino-terminal domain with the erythrocyte membrane-cytoskeleton linker protein band 4.1. 1 The ezrin, radixin, and moesin (ERM) proteins, with ezrin as their main representative, accumulate underneath the plasma membrane in cell surface structures such as microvilli, membrane ruffles, and cell-to-cell contact sites. 2-6 In particular, ezrin functions as a linker between the actin cytoskeleton and various membrane-bound molecules. 7,8 Similarly to a related protein, schwannomin (merlin) which is associated with the development of tumors in neurofibromatosis type II, 6,9 ezrin may also be associated with tumorigenesis, and additionally with invasive behavior of tumor cells: Ezrin expression is up-regulated in malignantly transformed fibroblast cultures. 10 With the removal of ezrin expression, invasive behavior of the cells is abolished. 10
Although the presence of ezrin in the brain was established by Western blot, 11 immunocytochemical staining was previously demonstrated only in human ependymal cells but not in other glial cells. 11 However, recent studies demonstrated ezrin in fine peripheral processes of astrocytes in rat brains. 12 We therefore considered the presence of this protein in human astrocytes and astrocytic tumors. Normal and neoplastic astrocytes were investigated for the expression of ezrin by immunocytochemistry using normal human brain tissues from autopsies and tissues from surgically removed glial tumors. We focused on the differentiation of benign World Health Organization (WHO) grade II tumors from anaplastic WHO grade III tumors, because this distinction is of therapeutical relevancy. 13,14 Ezrin immunoreactivity (IR) proved to be specific for ependymal cells and astroglia. We detected a statistically significant correlation between ezrin-IR and histological dignity of astrocytic tumors, whereas ezrin was absent in oligodendrogliomas. Ezrin-IR correlated more strongly with malignancy than proliferation or apoptosis indices.
Materials and Methods
Cases
We performed immunocytochemistry on specimens of paraformaldehyde-fixed, paraffin-embedded normal human brain tissues from autopsies (n = 10, frontal cortex, basal ganglia) and biopsy specimens from astrocytomas, glioblastomas, oligodendrogliomas, and ependymomas with a total of 115 cases. The cases were randomly selected in retrospective. Cases of mixed tumors such as oligo-astrocytomas, glioblastomas with conspicuous oligodendroglial features, or tumors with uncertain diagnosis were excluded. Diagnoses and grading of the tumors according to the WHO classification 13 were obtained by two observers independently. In this study, the tumors were divided into separate groups containing WHO grade I and II (benign) ependymomas; grade II (benign) astrocytomas or oligodendrogliomas; grade III (anaplastic) astrocytomas, ependymomas, or oligodendrogliomas; and WHO grade IV glioblastomas.
Histology and Immunohistochemistry
Five-μm-thick serial sections from paraffin-embedded tissues were cut and processed for staining with hematoxylin and eosin and for immunocytochemistry, which was performed using the indirect avidin-biotin-peroxidase complex method (ABC; Vector Laboratories, Burlingame, CA). Primary antibodies were applied at a concentration of 5 to 25 μg/ml for 1 to 12 hours. These were monoclonal ezrin-3C12 (Sigma Chemical Co., St. Louis, MO) 11,15 and polyclonal glial fibrillary acidic protein (DAKO, Glostrup, Denmark). The proliferation marker monoclonal MIB-1 (DAKO) required pretreatment by microwave at 600 W for 2 × 5 minutes in 10 mmol/L sodium citrate, pH 6.2. Biotinylated pre-adsorbed secondary antibodies, anti-mouse from sheep (Amersham, Arlington Height, IL) and anti-rabbit from swine (DAKO), were used a concentration of 10 μg/ml. Diaminobenzidine (0.05%; Sigma) in 0.02% hydrogen peroxide/phosphate-buffered saline (PBS) served as chromogen. Counterstaining was performed with hematoxylin (Harris) 7 g/L (Merck, Darmstadt, Germany). For blocking of nonspecific activity, nonfat dry milk (1 to 10%), bovine serum albumin (1 to 10%), and normal goat serum (1 to 25%) in PBS were used. To block the endogenous peroxidase, the sections were immersed for 10 to 20 minutes in PBS containing 1% hydrogen peroxide. Cell death (apoptosis) was established using a modified terminal dUTP nick-end labeling (TUNEL) staining method with an anti-digoxigenin-peroxidase system (Oncor, Boehringer, Mannheim, Mannheim, Germany) 16 on sections of paraffin-embedded tissues.
Staining Evaluation
Evaluation of immunostaining was performed by one observer without knowledge of the diagnosis on 5-μm-thick sections, at a magnification of ×20 in the light microscope equipped with a graded ocular lens (10 × 10 squares, corresponding to 500 × 500 μm at ×20 magnification; C. Zeiss, Oberkochen, Germany). For the detection of apoptosis and proliferation, areas with maximal staining were counted and percentages were calculated on the basis of IR-positive and IR-negative cells counted per 100 squares. Positive controls with a known quantity of apoptotic cells were used for each staining series. Samples of positively stained sections were validated by chromatin appearance in semi-thin sections. 16 For the evaluation of ezrin staining, a semiquantitative numeric scoring system based on a combination of quantitative and qualitative criteria was developed. Possible variations in staining intensity between different batches of staining (up to 20 slides each) were evaluated in relation to a positive standard control added to each batch of immunocytochemically labeled slides. To ascertain staining consistency, samples of distinct areas of a given tumor were included in one staining batch, or serial sections of the same area were included in different staining batches. Staining intensity was estimated at ×20 light microscopic magnification as described above. When no staining was visible, the score of 0 was accorded. The score of 0.5 was accorded, when single weakly positive cells were seen between large numbers of entirely negative cells (<5% of the cells). The score of 1 was used for the delicate staining of astrocytic processes in normal tissues or for weak but consistent staining in at least 50% of tumor cells with distribution similar to the normal in individual tumor cells. The scoring values were chosen to yield an average score value of 1 for ezrin-IR in normal tissues. The score of 2 denoted increased staining with partially diffuse cytoplasmatic IR in ∼50 to 70% of the tumor cells and 3 was used for heaviest staining contained in almost continuous areas of primarily cytoplasmic staining in >80% of the tumor cells. Intermediate levels were scored 1.5 and 2.5, respectively.
Statistical Evaluation
For statistical analysis, the univariate chi-square test, the corrected Pearson’s contingency coefficient 17,18 for nonparametric correlation, and the Bayes’ formula for predictive values were applied. For the calculation of predictive values, prevalence data on the occurrence of astrocytomas and oligodendrogliomas were compiled from excerpts of the Central Brain Tumor Registry of the United States data of 1997. 19
Results
Methods
Immunohistochemistry with the anti-ezrin antibody 3C12 was successfully performed on paraffin-embedded, formalin-fixed sections of normal human brain tissues from autopsies and on paraffin-embedded formalin-fixed tissues from surgically removed glial tumors. The intensity of staining on sections of paraffin-embedded tissues proved moderately sensitive to fixation artifacts. Especially underfixed tissues or autopsy tissues which had been immersed in formalin later than 48 hours after the death of the patients yielded no staining at all, overfixation (longer than 48 hours in formalin) or incomplete dehydration had only minor influence on anti-ezrin immunostaining, by slightly decreasing the staining intensity. Suitable concentrations of the primary antibody ranged from 5 to 50 μg/ml without significant difference. Microwave treatment or treatment with protease K at 0.2 mg/ml to 2 mg/ml did not consistently improve staining results. However, for the primary antibody long incubations for at least 12 hours at 4°C were essential. Incubation periods of 1 to 2 hours with otherwise similar methods did not yield any staining in astrocytes. The primary antibody 3C12 tended to lose activity after 2 weeks when stored at more than 0°C. Omission of primary antibody resulted in the absence of labeling in all cases. In some cases, diffuse weak staining of vascular walls or mesenchymal cells was observed, which could be avoided by more extensive blocking of the endogenous peroxidase with 1% hydrogen peroxide for 20 minutes. Differences of immunostaining between different staining series were normalized by positive controls added to each series. Re-evaluation of ezrin-IR revealed an intra-observer variation of 0.5 score points in four cases of IR-scores below 1.5. Higher scores were distributed identically with the exception of one case distributed 2.0/2.5 points, respectively. In these cases, the slides were compared with all other slides of the scores in question and then finally distributed to one score group.
Ezrin-IR in Normal Human Brain Tissues
Strong immunostaining was present in the apical part of ependymal cells (Figures 1A and 2A) ▶ ▶ . We also found delicate staining mostly on peripheral processes of normal human brain astrocytes (mean staining intensity score = 1.0) and in some cases weak staining within the soma and the nuclear membrane of some astrocytes (Figures 1A and 2A) ▶ ▶ . Staining was often very weak and barely visible at low magnification. Positive staining results were seen in a substantial number of cortical and white matter astrocytes, but not all of the studied astrocytes exhibited immunostaining for ezrin. Immunostaining was preferentially located in the molecular layer of the cortex, in deeper cortical areas adjacent to the white matter and in periventricular white matter areas. Ezrin-IR was entirely absent in microglia and in oligodendrocytes (not shown). When, for comparison, reactive astrocytes in a brain of a patient with human immunodeficiency virus-associated encephalitis were stained for ezrin, staining was stronger than in normal astrocytes and detectable in a larger number of astrocytes. The IR distribution was similar to normal astrocytes (Figure 2B) ▶ .
Figure 1.
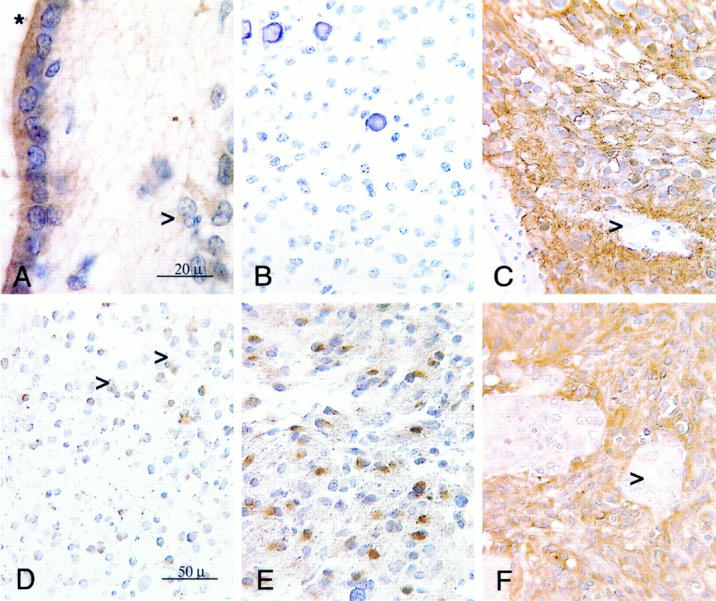
Ezrin-IR in normal brain and various types of gliomas. Immunocytochemistry using the indirect immunoperoxidase method on 5-μm-thick sections of paraffin-embedded normal human brain tissues and tissues from human brain tumors. Diaminobenzidine was used as a chromogen (brown), counterstaining with hematoxylin. A: Ezrin-IR in normal human brain tissue from the periventricular area adjacent to the basal ganglia. Note the strong IR of the ependymal cells, especially their apical region (asterisk) and the much weaker, delicate staining of subependymal astrocytes (arrow). Original magnification, ×100; oil immersion. B: Negative ezrin-IR in a benign oligodendroglioma WHO grade II with entirely absent immunostaining and typical morphology including round monomorphous cells with some calcifications. Original magnification, ×40. C: Strong ezrin-IR in a benign ependymoma WHO grade II with almost regular staining of all tumor cells. Vascular endothelium is excluded (arrow). Original magnification, ×40. D: Weak, almost normal ezrin-IR in a benign astrocytoma WHO grade II with delicate staining of peripheral processes (arrows). Original magnification, ×40. E: Markedly increased ezrin-IR in an anaplastic, malignant astrocytoma WHO grade III with positive staining of cytoplasm and processes. Original magnification, ×40. F: Strong ezrin-IR in a glioblastoma WHO grade IV with even more pronounced staining in comparison with the IR seen in E. All cells, with the exception of proliferated vascular endothelium (arrow), stain positive for ezrin. Finer processes cannot be distinguished from the rather plump cell bodies. Original magnification, ×40.
Figure 2.
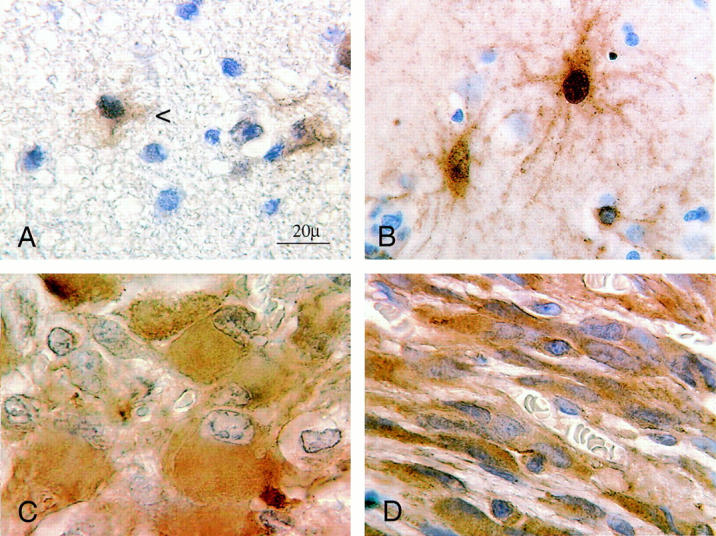
Ezrin-IR in activated astrocytes and in astrocytic tumors. Details of ezrin-IR at high magnification. Immunocytochemistry using the indirect immunoperoxidase method on 5-μm-thick sections of paraffin-embedded tissues with diaminobenzidine as a chromogen (brown), counterstaining with hematoxylin. Original magnification, ×100; oil immersion. A: Detail of ezrin-IR in a benign WHO grade II astrocytoma with delicate sheet-like ezrin-positive processes of neoplastic astrocytes (arrow) which, similarly to normal tissues, are often barely visible at low magnification. Note the faint diaminobenzidine staining which was scored 1.0, may be hard to discern from the paraffin structure. B: Ezrin-IR in reactive astrocytes of a patient with human immunodeficiency virus-encephalopathy in a section of the basal ganglia region. In comparison with A the IR is considerably stronger but shows a similar distribution in long delicate processes and sometimes the nuclear membrane. C: Strong positive ezrin-IR in gemistocytic tumor cells of a glioblastoma WHO grade IV with mostly diffuse cytoplasmatic staining. D: Ezrin-IR in a different glioblastoma containing a fibrillary type of tumor cells with few plump processes which show a strong IR for ezrin which is also diffusely distributed within the cytoplasm.
Ezrin-IR in Human Glial Tumors
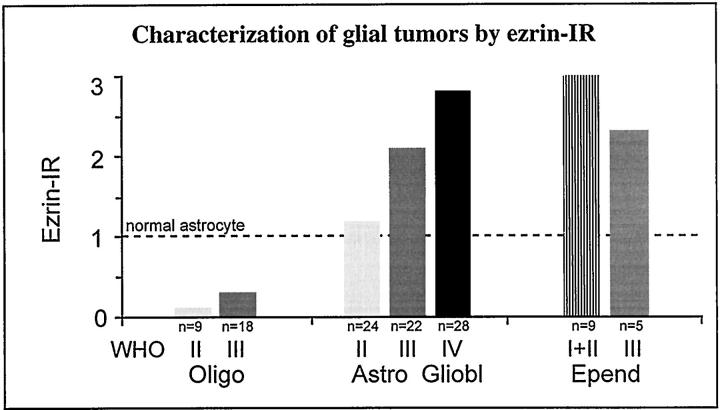
In astrocytomas, varying degrees of staining could be observed (Figure 1, D–F ▶ ; Figures 2 and 3 ▶ ▶ ; and Tables 1 and 2 ▶ ▶ ). WHO grade II benign astrocytomas showed a weak, almost normal staining pattern of cellular processes (mean staining intensity = 1.20) (Figure 1D ▶ , Figure 2A ▶ , and Figure 3 ▶ ; and Tables 1 and 2 ▶ ▶ ). Ezrin-IR may be very faint in normal astrocytes and grade II astrocytomas. This observation was hard to document photographically. At ×100 magnification, the coarse irregular background caused by paraffin-embedding rendered the visible IR rather hazy (Figure 2A) ▶ . Labeling was clearly increased in grade III anaplastic astrocytomas exhibiting a larger proportion of cytoplasmic staining (mean staining intensity = 2.3) (Figure 1E ▶ , and Tables 1 and 2 ▶ ▶ ). WHO grade IV glioblastomas demonstrated a conspicuously more intense labeling than grade III anaplastic astrocytomas (mean staining intensity = 2.8) (Figure 1F ▶ ; Figure 2, C and D ▶ ; and Tables 1 and 2 ▶ ▶ ), with pronounced staining of the cytoplasm and the remaining few plump processes. Gemistocytic astrocytes showed a relatively strong diffusely cytoplasmatic staining (Figure 2C) ▶ , which however, was more pronounced in grade III and grade IV tumors. In relation to the faint staining of normal astrocytes (mean staining intensity = 1.0; Figure 1A ▶ ), both normal oligodendrocytes and WHO grade II oligodendrogliomas showed no immunoreactivity (Figure 1B ▶ ; Tables 1 and 2 ▶ ▶ ). Only few cells of astrocytic shape, which also reacted strongly with glial fibrillary acidic protein (not shown) demonstrated a slight immunoreactivity to ezrin within oligodendrogliomas. Although these cells were included in the evaluation of the tumors, oligodendrogliomas still showed significantly lower staining values (mean staining intensity = 0.1) (Figure 1B ▶ , Table 1 ▶ ) compared with astrocytomas or normal astrocytes. In malignant grade III oligodendrogliomas there were insignificantly more positively staining astrocytes (mean staining intensity = 0.3) (Figure 2) ▶ . Benign WHO grade I and II ependymomas showed strong staining of the whole cytoplasm (mean staining intensity = 3.0) (Figure 1C) ▶ , which was more irregular and slightly weaker in malignant grade III tumors compared with grade II tumors (Figure 3 ▶ , and Tables 1 and 2 ▶ ▶ ). There was a statistically significant correlation between ezrin immunoreactivity and malignancy of astroglial tumors (chi-square, P < 0.0001, corresponding to a likelihood of error <0.01%) (Table 2) ▶ . The strength of this association with malignancy is reflected by a contingency coefficient of 86% when the three grading steps of astrocytic tumors were included (Table 2) ▶ . In addition, the corresponding correlations with only two subsequent grades of astrocytomas were also highly significant and similarly strong (P < 0.0001) (Table 2) ▶ . Ezrin immunoreactivity was also capable of differentiating between oligodendrogliomas (WHO grades II and III) and astrocytomas (WHO grades II and III) with a contingency coefficient of 96% for the association of ezrin immunoreactivity and tumor type (Table 2D) ▶ . Applying Bayes’ formula, high predictive values for astrocytomas and oligodendrogliomas were calculated from excerpts of Central Brain Tumor Registry of the United States data, 19 with a predictive value of 0.93 for astrocytomas and 0.85 for oligodendrogliomas.
Figure 3.
Ezrin-IR in glial tumors. Immunostaining was performed using the indirect immunoperoxidase method with diaminobenzidine as the chromogen and hematoxylin for counterstaining. Staining intensity was evaluated at 20 × light-microscopic magnification. The scoring scale was defined as follows: 0, no staining; 0.5, single weakly positive cells between large numbers of entirely negative cells (<5% of the tumor cells); 1, delicate staining of astrocytic processes in normal tissues or for weak but staining in at least 50% of tumor cells with distribution similar to the normal in individual tumor cells; 2, increased staining of 50 to 70% of the tumor cells; 3, strongly increased staining of at least 80% of the tumor cells. Intermediate levels were scored 1.5 and 2.5, respectively. The scoring values were chosen to yield an average score value of 1 in normal tissues.
Table 1.
Contingency Tables: Tumor Type versus Different Stainings (Ezrin-IR, MIB-IR, TUNEL)
| Tumor type | WHO grade | Staining | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ezrin-IR (score values) | ||||||||||||||||||
| 0 | 0.5 | 1 | 1,5 | 2 | 2,5 | 3 | Mean | n | ||||||||||
| Oligodendroglioma | II | 8 | 1 | 0.1 | 9 | |||||||||||||
| III | 11 | 4 | 3 | 0.3 | 18 | |||||||||||||
| Astrocytoma | II | 2 | 13 | 5 | 4 | 1.2 | 24 | |||||||||||
| III | 3 | 14 | 1 | 4 | 2.1 | 22 | ||||||||||||
| Glioblastoma | IV | 5 | 23 | 2.8 | 28 | |||||||||||||
| Ependymoma | I+ II | 9 | 3.0 | 9 | ||||||||||||||
| III | 1 | 1 | 3 | 2.3 | 5 | |||||||||||||
| 21 | 5 | 20 | 6 | 26 | 1 | 36 | 1.7 | 115 | ||||||||||
| MIB (%) | ||||||||||||||||||
| 0–10 | 10–20 | 20–30 | 30–40 | 40–50 | >50 | n.d. | Mean | n | ||||||||||
| Oligodendroglioma | II | 6 | 1 | 2 | 3.5 | 9 | ||||||||||||
| III | 17 | 1 | 17.0 | 18 | ||||||||||||||
| Astrocytoma | II | 22 | 2 | 22.0 | 24 | |||||||||||||
| III | 14 | 2 | 3 | 3 | 6.3 | 22 | ||||||||||||
| Glioblastoma | IV | 2 | 10 | 4 | 5 | 3 | 3 | 1 | 4.5 | 28 | ||||||||
| Ependymoma | I+ II | 8 | 1 | 8.0 | 9 | |||||||||||||
| III | 3 | 1 | 1 | 1.7 | 5 | |||||||||||||
| 72 | 14 | 7 | 5 | 3 | 4 | 10 | 9.0 | 115 | ||||||||||
| TUNEL (%) | ||||||||||||||||||
| 0–5 | 5–10 | 10–15 | 15–20 | 20–25 | >25 | n.d. | Mean | n | ||||||||||
| Oligodendroglioma | II | 9 | 9 | 9 | ||||||||||||||
| III | 16 | 1 | 1 | 8.5 | 18 | |||||||||||||
| Astrocytoma | II | 20 | 4 | 20 | 24 | |||||||||||||
| III | 21 | 1 | 21 | 22 | ||||||||||||||
| Glioblastoma | IV | 16 | 4 | 4 | 3 | 1 | 5.6 | 28 | ||||||||||
| Ependymoma | I+ II | 8 | 1 | 8 | 9 | |||||||||||||
| III | 5 | 5 | 5 | |||||||||||||||
| 95 | 5 | 4 | 3 | 1 | 7 | 11.0 | 115 | |||||||||||
For statistical analysis, parametric (MIB, TUNEL) and nonparametric values (ezrin-IR) were distributed to groups as follows: for ezrin-IR, a semiquantitative scoring system, containing values from 0 to 3.0 with increments of 0.5 (i.e., seven classes) was used. See Materials and Methods section and Figure 3 ▶ for detailed description of the scoring system. The parametric percent values for MIB-1 IR were grouped in six classes (10% each) and those for TUNEL staining in five classes (5% each). For calculation of contingency coefficients, neighboring group cells were fused stepwise if expectancy values were <1. n.d., not documented.
Table 2.
Comparison of Ezrin-IR, Proliferation (MIB-1-IR), and Apoptosis (TUNEL)
| Correlation | P (chi-square) | Contingency coeff. |
|---|---|---|
| A: In astrocytomas (WHO II, III) and glioblastoma (WHO IV) | ||
| With malignancy | ||
| Ezrin-IR* | <0.0001 | 86% |
| MIB-1-IR (%) | <0.0001 | 80% |
| TUNEL (%) | <0.001 | 65% |
| With ezrin-IR | ||
| MIB-1-IR (%) | <0.05 | 49% |
| TUNEL (%) | <0.01 | 45% |
| B: In astrocytomas (WHO II, III) | ||
| With malignancy | ||
| Ezrin-IR | <0.0001 | 82% |
| MIB-1-IR (%) | <0.05 | 53% |
| TUNEL (%) | n.s.c. | |
| With ezrin-IR | ||
| MIB-1-IR (%) | n.s.c. | |
| TUNEL (%) | n.s.c. | |
| C: In astrocytomas (WHO III) and glioblastoma (WHO IV) | ||
| With malignancy | ||
| Ezrin-IR | <0.0001 | 76% |
| MIB-1-IR (%) | <0.001 | 84% |
| TUNEL (%) | <0.01 | 64% |
| With ezrin-IR | ||
| MIB-1-IR (%) | n.s.c. | |
| TUNEL (%) | n.s.c. | |
| D: Differentiation between astrocytomas and oligodendrogliomas | ||
| Astro II versus oligo II | <0.0001 | 92% |
| Astro III versus oligo III | <0.0001 | 96% |
| Astro (II+ III) versus oligo (II+ III) | <0.0001 | 92% |
| Predictive value for astro (II+ III) | 0.93% | |
| Predictive value for oligo (II+ III) | 0.85% |
A–C comparison of ezrin-IR*, proliferation (MIB-1 IR) and apoptosis (TUNEL):
A, in astrocytomoas WHO grades II, III, and IV; B, in astrocytomas WHO grades II and III; C, in astrocytomas WHO grades III and IV; D, Correlation between ezrin-IR* and tumor type.
* Ezrin-IR: semiquantitative scoring system, detailed description in Materials and Methods section Figure 3 ▶ . Parametric values for MIB and TUNEL were grouped as described in Table 1 ▶ . As a measure of association, Pearson’s contingency coefficient was calculated. 18. In order to compare the contingency values of different tables, the contingency coefficients were expressed as percentage of the respective maximal contingency coefficient. For the calculation of predictive values, prevalence data on the occurrence of astrocytomas and oligodendrogliomas were compiled from CBTRUS data. 19.
Ezrin-IR in Comparison with Proliferation and Apoptosis
Apoptosis and proliferation were significantly correlated with malignancy (P = 0.0001 for MIB-1 and P = 0.001 for TUNEL) (Table 2) ▶ when astrocytomas (WHO grade II and III) and glioblastomas (WHO grade IV) were analyzed together. The strength of this association was considerably weaker than in the case of ezrin (Table 2A) ▶ . Correspondingly, when only WHO grade II (benign) and III (anaplastic) astrocytomas were analyzed, both, proliferation and apoptosis were only weakly or not significantly correlated with malignancy (Table 2B) ▶ . To the contrast, the association between ezrin and malignancy was not substantially altered, when only two subsequent astrocytoma grades were analyzed (Table 2 ▶ , B and C). Including all, or only subsequent astrocytoma grades, there was no stringent association between ezrin-IR and MIB-1-IR or TUNEL staining (Table 2 ▶ , A–C).
Discussion
We have demonstrated immunocytochemical staining for the actin-binding protein ezrin in normal human astrocytes where this protein was mostly found in the fine peripheral processes. Ezrin expression was also detected in gliomas of astrocytic but not of oligodendrocytic origin. The intensity of ezrin-staining was strongly and significantly correlated with the grading of these tumors according to the WHO classification. Ezrin-IR permitted a clear distinction between benign grade II astrocytic tumors and anaplastic grade III astrocytomas. Malignant, WHO grade III and IV astrocytic tumors showed an altered distribution of the protein and clearly increased staining intensity compared with benign grade II astrocytomas. Ezrin was highly expressed in ependymomas, but consistently absent in oligodendrogliomas. The association with malignancy of astrocytic tumors was stronger for ezrin-IR than for proliferation or apoptosis.
Methodical Considerations
Ezrin has been detected by Western blot in human brain tissues. 11 The anti-ezrin antibody 3C12 has been extensively characterized 20,21 and has been shown to be monospecific in human brain. 11 Our results demonstrating ezrin staining on astrocytes and ependymal cells of the human brain are in accordance with earlier studies finding ezrin-IR exclusively on ependymal cells 11 and on astrocytes of the rat brain. 12 Also in line with previous data 11 was the failure to detect ezrin on human astrocytes using shorter antibody incubation times. Overnight incubation proved to be essential for the use of the antibody 3C12.
Diagnostic Value of Ezrin Immunoreactivity
The staining for ezrin was specific for the fine processes of normal astrocytes, and the apical surfaces of ependymal cells, because we could not detect this protein in neurons, microglia, or oligodendrocytes. Ezrin-IR varied correspondingly in tumors derived from astrocytes, oligodendroglia, or ependymal cells, denoting an association with the constitutive expression of ezrin in normal cells. Because both, oligodendrogliomas and astrocytomas, express glial fibrillary acidic protein, albeit with some distinctions, 22 their differential diagnosis is conventionally based solely on morphological criteria, which can be problematic in malignant grade III tumors. 13,23 Here, we show that ezrin-IR can distinguish between astrocytomas and oligodendrogliomas with high predictive values. Despite the relatively low case numbers, we regard our findings as valid because of the high significance levels found. The differentiation between astrocytomas and oligodendrogliomas may be of practical use because it may facilitate therapeutical decisions concerning further diagnostic measures and the use of chemotherapy in patients with malignant oligodendrogliomas, which are reported to be more responsive to chemotherapy than astrocytomas. 14,24 Further work will be necessary to establish a potential prognostic role of ezrin-IR.
Our results suggest that ezrin staining may provide a better distinction between grade II benign and grade III anaplastic astrocytomas than apoptosis or proliferation index. 25-29 This study may provide valuable directions, because the differentiation between WHO grade II and grade III marks the difference between benign and anaplastic (malignant) astrocytic tumors, a distinction that is of therapeutical consequence for the patient. 19,23,30 Here we observed a clear-cut distinction between grade II and grade III astrocytomas. However, cases with morphologically ambiguous grading have been excluded from this study, which could have introduced a bias in respect to morphology. The establishment of ezrin immunostaining as a diagnostic tool would require the study of higher numbers of cases and its practicability might be limited by the necessity of overnight staining procedures and the stringent storage requirements for the used antibody.
Ezrin-IR was associated with the development of malignant cellular morphology in astrocytomas. However, the increased ezrin-IR was significantly correlated with proliferation or apoptosis only when grade II, III, and IV astrocytic tumors were analyzed together. The correlation of ezrin with apoptosis or proliferation was weak or not significant when only grade II and III tumors were compared. These findings indicate an indirect relation between ezrin and proliferation or cell death, possibly based on the strong association of each parameter with malignancy. There may be no direct interaction between ezrin and apoptosis or proliferation.
Cell Biological and Functional Considerations
Immunocytochemistry provides rather indirect data concerning the expression of a protein. However, ezrin has been shown to participate in changes of shape and motility of several cell types in cell culture 2,3,5,8,10,31-41 . The increased staining intensity and the altered pattern of staining in malignant astrocytomas and glioblastomas might therefore reflect the altered cellular shape of malignant astrocytes, which have a more plump cell body and less processes compared with normal or reactive astrocytes. 23 The high correlation of ezrin-IR with malignancy may indicate a functional role of this protein in malignant transformation of astrocytes. The increased ezrin-IR in malignant astrocytes may also relate to the altered biological behavior of these cells, namely their increased motility and invasivity. Similar observations have been reported from the investigation of cultivated malignantly transformed fibroblasts where transformation was accompanied by an up-regulation of ezrin expression, 10 and the loss of ezrin protein resulted in a loss of invasive behavior. 10 Ezrin, which is contained in normal astrocytes, is obviously increasingly dysregulated in astrocytomas with the development of malignant morphology. In this context, one might speculate that the loss of cellular processes, accompanying malignant transformation of astrocytes could be related to the altered distribution of ezrin in these cells, but also to the lack of signals limiting the spread of tumor cells. A possible association between ezrin and pathological expression of the epidermal growth factor receptor in malignant gliomas 2,42 is the subject of ongoing research. Although ezrin-IR is strongly correlated with malignancy of astrocytic tumors in this study, up-regulation of ezrin may not be specific to malignancy. Potentially reactive astrocytes of oligodendrogliomas and reactive astrocytes in human immunodeficiency virus-encephalopathy also seem to show increased immunoreactivity. Additionally, ezrin has also been linked to activation of lymphocytes. 43 This does, however, not question the validity of the present findings, in particular because always central tumor areas with typical morphology were chosen for evaluation
Ezrin may have distinct functional properties in different glial cell types, because in ependymomas, staining tends to get weaker with increasing malignancy. Ezrin staining intensity also appears to be more irregular in malignant ependymomas as compared with benign ependymal tumors. It is possible that ezrin-IR may be dependent on its constitutive expression in a given cell type, and it may be down-regulated during dedifferentiation in distinct tumor entities.
We demonstrated immunoreactivity for the actin-binding protein ezrin, an ERM protein, in astrocytes of paraffin-embedded human tissue sections with increased IR in malignant astrocytic tumors but not in tumors of oligodendrocytic origin. Although the measurement of immunostaining represents a semiquantitative method, and the present study includes a relatively low number of cases, immunocytochemical staining may provide a useful diagnostic tool for the grading of astrocytic tumors and for the distinction of astrocytomas and oligodendrogliomas. The expression of ezrin is likely to be linked to malignant transformation of astrocytes. The possible important role of actin-binding proteins in malignancy of astrocytic tumors merits further research.
Acknowledgments
We thank Brigitte Georgiewa, Magdalena Heinz, Barbara Lafferton, and Ursel Rech for excellent technical help.
Footnotes
Address reprint requests to Dr. Kathrin Geiger, Edinger-Institute, JWG-University, Deutschordenstr. 46, D-6528 Frankfurt, Germany. E-mail: k.geiger@em.uni-frankfurt.de.
Supported by the Edinger Foundation, Frankfurt, Germany and the Deutsche Forschungs Gemeinschaft, De 676/2–1.
References
- 1.Gould KL, Bretscher A, Esch FS, Hunter T: cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to the band 4.1. EMBO J 1989, 8:4133-4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bretscher A: Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol 1989, 108:921-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bretscher A, Reczek D, Berryman M: Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci 1997, 110:3011-3018 [DOI] [PubMed] [Google Scholar]
- 4.Tsukita S, Hieda Y, Tsukita S: A new 82 kD-barbed end-capping protein localized in the cell-to-cell adherens junction: purification and characterization. J Cell Biol 1989, 108:2369-2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukita S, Yonemura S, Tsukita S: ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol 1997, 9:70-75 [DOI] [PubMed] [Google Scholar]
- 6.Vaheri A, Carpén O, Heiska L, Helander TS, Jääskeläinen J, Majander-Nordenswan P, Sainio M, Timonen T, Turunen O: The ezrin protein family: membrane-cytoskeleton interactions and disease associations. Curr Cell Biol 1997, 9:659-666 [DOI] [PubMed] [Google Scholar]
- 7.Helander TS, Carpén O, Turunen O, Kovanen PE, Vaheri A, Timonen T: ICAM-2, redistributed by ezrin as a target for killer cells. Nature 1996, 382:265-268 [DOI] [PubMed] [Google Scholar]
- 8.Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S: Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmatic domain of CD 44, CD 43, and ICAM-2. J Cell Biol 1998, 140:885-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sainio M, Jääskeläinen J, Pihlaja H, Carpén O: Mild familial neurofibromatosis 2 associates with expression of merlin with altered COOH-terminus. Neurology 2000, 54:1132-1138 [DOI] [PubMed] [Google Scholar]
- 10.Lamb RF, Ozanne BW, Roy C, McGarry L, Stipp C, Mangeat P, Jay DG: Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Curr Biol 1997, 7:682-688 [DOI] [PubMed] [Google Scholar]
- 11.Böhling T, Turunen O, Jääskeläinen J, Carpén O, Sainio M, Wahlström T, Vaheri A, Haltia M: Ezrin expression in stromal cells of capillary hemangioblastoma. An immunohistochemical survey of brain tumors. Am J Pathol 1996, 148:367-373 [PMC free article] [PubMed] [Google Scholar]
- 12.Derouiche A, Tsukita S: ERM-Proteins in the CNS: specializations of astrocyte filopodia and lamellate processes. Eur J Neurosci 1998, 10(European Neuroscience Forum, suppl abstracts):A.145.16
- 13.Kleihues P, Cavenee WK (Eds): World Health Organization classification of tumors. Tumors of the Nervous System. Lyon, IARC-Press, 2000
- 14.Pech IV, Peterson K, Cairncross JG: Chemotherapy for brain tumors. Oncology 1998, 12:537-553 [PubMed] [Google Scholar]
- 15.Yao X, Cheng L, Forte JG: Biochemical characterization of ezrin-actin interaction. J Biol Chem 1996, 271:7224-7229 [DOI] [PubMed] [Google Scholar]
- 16.Geiger KD, Bloom FE, Sarvetnick NE: Methods for detection of apoptosis in the CNS. Neuromethods. Apoptosis Techniques and Protocols, vol 29. Edited by J Poirier. Totowa, NJ, Humana Press, 1997, pp 217–235
- 17.Pawlik K: The maximal contingency coefficient in the case of nonquadratic contingency tables. Metrika 1959, 2:123-126 [Google Scholar]
- 18.Sachs L: Applied Statistics: Augewandfe Statistik: Auwendung von statistischen Methoden [Application of Statistical Methods.] Berlin, Heidelberg, New York, Springer, 1992, pp 476
- 19.Davis FG, Preston-Martin S. Epidemiology. Russel and Rubinstein’s Pathology of Tumors of the Nervous System. Edited by DD Bigner, RE McLendon, JM Bruner. London, Sydney, Auckland, Arnold, 1998, pp 5–46
- 20.Turunen O, Winqvist R, Grzeschik KH, Pakkanen R, Wahlström T, Vaheri A: Cytovillin, a microvillar Mr 75,000 protein: cDNA sequence, prokaryotic expression, and chromosomal localization. J Biol Chem 1989, 264:16727-16732 [PubMed] [Google Scholar]
- 21.Turunen O, Wahlström T, Vaheri A: Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol 1994, 126:1445-1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luider TM, Kros JM, Sillevis-Smith PA, van den Bent MJ, Vecht CJ: Glial fibrillary acidic protein and its fragments discriminate astrocytoma from oligodendroglioma. Electrophoresis 1999, 20:1087-1091 [DOI] [PubMed] [Google Scholar]
- 23.Lantos PL, VandenBerg SR, Kleihues P: Tumors of the nervous system. Greenfield’s Neuropathology. Edited by DI Graham, PL Lantos PL. London, Sydney, Auckland, Arnold, 1997, pp 583–880
- 24.Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB: Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 2000, 18:636-645 [DOI] [PubMed] [Google Scholar]
- 25.Heesters MA, Koudstaal J, Go KG, Molenaar WM: Analysis of proliferation and apoptosis in brain gliomas: prognostic and clinical value. J Neurooncol 1999, 44:255-266 [DOI] [PubMed] [Google Scholar]
- 26.Schiffer D, Cavalla P, Migheli A, Chio A, Giordana MT, Marino S, Attanasio A: Apoptosis and cell proliferation in human neuroepithelial tumors. Neurosci Lett 1995, 195:81-84 [DOI] [PubMed] [Google Scholar]
- 27.Litfsky NS, Mix TC, Baker SP, Recht LD, Smith TW: Ki-67 (clone MIB-1) proliferation index in recurrent glial neoplasms: no prognostic significance. Surg Neurol 1998, 50:579-585 [DOI] [PubMed] [Google Scholar]
- 28.Yew DT, Wang HH, Zheng DR: Apoptosis in astrocytomas with different grades of malignancy. Acta Neurochir 1998, 140:341-347 [DOI] [PubMed] [Google Scholar]
- 29.Korshunov A, Golanov A, Sycheva R, Pronin I: Prognostic value of tumor associated antigen immunoreactivity and apoptosis in cerebral glioblastomas: an analysis of 168 cases. J Clin Pathol 1999, 52:574-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daumas-Duport C: Histological grading of gliomas. Curr Opin Neurol Neurosurg 1992, 5:924-931 [PubMed] [Google Scholar]
- 31.Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M: Ezrin contains cytoskeleton and membrane binding domains accounting for it’s proposed role as a membrane-cytoskeletal linker. J Cell Biol 1993, 120:129-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berryman M, Franck Z, Bretscher A: Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci 1993, 105:1025-1043 [DOI] [PubMed] [Google Scholar]
- 33.Bretscher A: Microfilament structure and function in the cortical cytoskeleton. Ann Rev Cell Biol 1991, 4:337-374 [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Crohn JA, Mandel LJ: Dephosphorylation of ezrin as an early event in renal microvillar breakdown and anoxic injury. Proc Natl Acad Sci USA 1995, 92:7495-7499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR: Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J 1997, 16:35-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goslin K, Birgbauer E, Banker G, Solomon F: The role of cytoskeleton in organizing growth cones: a microfilament-associated groth cone component depends upon microtubules for it’s localization. J Cell Biol 1989, 109:1621-1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jooss KU, Müller R: Deregulation of genes encoding microfilament-associated proteins during Fos-induced morphological transformation. Oncogene 1995, 10:603-608 [PubMed] [Google Scholar]
- 38.Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F: Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett 1999, 147:31-38 [DOI] [PubMed] [Google Scholar]
- 39.Spacek J: Three-dimensional analysis of dendritic spines. III Glial sheats. Anat Embryol 1985, 171:245-252 [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S: Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol 1994, 125:1371-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsukita S, Yonemura S, Tsukita S: ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci 1997, 22:53-58 [DOI] [PubMed] [Google Scholar]
- 42.Jiang WG, Hiscox S: Cytokine regulation of ezrin expression in the human colon cancer cell line HT29. Anticancer Res 1996, 16:861-865 [PubMed] [Google Scholar]
- 43.Serrador JM, Nieto M, Alonso-Lebrero JL, del Pozo MA, Calvo J, Furthmayr H, Schwartz-Albiez R, Lozano F, Gonzalez-Amaro R, Sanchez-Mateos P, Sanchez-Madrid F: CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood 1991, 91:4632-4644 [PubMed] [Google Scholar]