Abstract
Familial adenomatous polyposis is characterized by multiple colorectal adenomas and an increased incidence of colorectal carcinomas. Patients also develop various extracolonic tumors, of which, thyroid carcinoma is common in young females. The occurrence of multiple carcinomas in one thyroid is frequently observed, although some carcinomas are solitary. To clarify whether each carcinoma develops independently or metastatically spreads from the first one formed, we analyzed the adenomatous polyposis coli (APC) gene mutation in each carcinoma. We found that each carcinoma had a different somatic mutation of the APC gene. This is molecular confirmation for the multicentric development of thyroid carcinomas in familial adenomatous polyposis through biallelic inactivation of the APC gene.
Familial adenomatous polyposis (FAP) is an autosomal dominant disease characterized by multiple colorectal adenomas and an increased incidence of colorectal carcinomas. It is also accompanied by various benign and malignant extracolonic manifestations, including gastric and duodenal tumors, osteomas, desmoid tumors, retinal pigmentation, and thyroid and adrenocortical tumors. 1-3 We have previously demonstrated that gastric, duodenal, and desmoid tumors, and an adrenocortical carcinoma, in FAP patients develop by inactivation of both alleles of the adenomatous polyposis coli (APC) gene through germline mutation and somatic mutation occurring in the normal allele, 4-6 in the same manner as in colorectal tumors. 7-9 We also demonstrated that thyroid carcinomas from FAP patients had both germline and somatic mutations of the APC gene. 10 Thyroid cancer, being common in young FAP females, develops either as a single carcinoma or as multiple carcinomas in one thyroid. 11-14 However, whether each carcinoma develops independently or metastatically spreads from the first one formed is still unclear. To clarify the mechanism of such multicentric development of thyroid carcinomas, we analyzed somatic mutation of the APC gene in each carcinoma in thyroids from FAP patients.
Materials and Methods
Patients and Samples
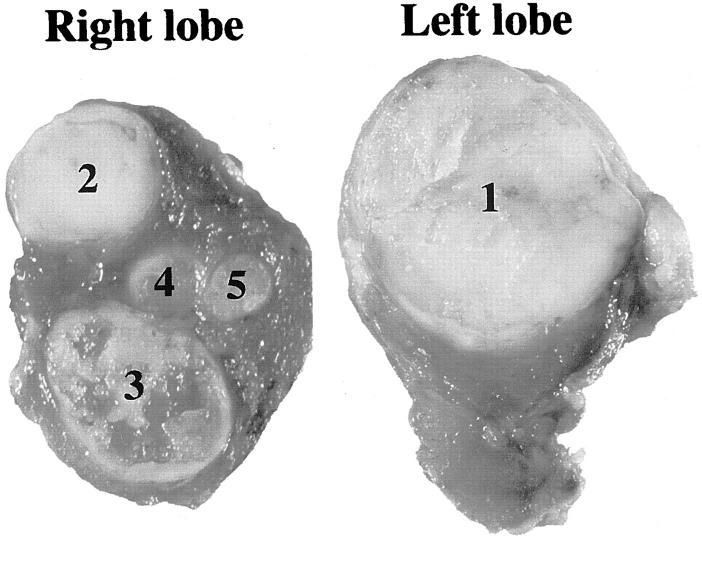
Thyroid carcinomas were obtained from two FAP patients who gave informed consent. Patient PLK29 (a 26-year-old female) had one large carcinoma in the left lobe and multiple carcinomas in the right lobe, as shown in Figure 1 ▶ . Patient PLK294 (a 21-year-old female) had two carcinomas in the left lobe. All carcinomas were histopathologically diagnosed as papillary carcinoma. Genomic DNA was extracted from each carcinoma and corresponding normal tissue, using proteinase K, sodium dodecyl sulfate, and phenol-chloroform.
Figure 1.
Multicentric thyroid carcinomas in FAP patient PLK29. Numbers of carcinomas correspond to those in Table 1 ▶ .
Mutation Analysis
DNA samples were amplified using polymerase chain reaction (PCR) and analyzed by the single-strand conformation polymorphism (SSCP) method. Primers for mutation analysis for the APC gene were the same as those previously reported. 15 Conditions for PCR were the same as those previously described. 9 When abnormal bands were detected in the SSCP analysis, single-strand DNA fragments were extracted, amplified by asymmetrical PCR, and then subjected to direct sequencing by dideoxy chain-termination reaction. 9
Loss of Heterozygosity Analysis
Loss of heterozygosity at chromosome 5q near the APC locus was analyzed using D5S346 and (AC)10. Loss of the normal allele was estimated by comparison of intensities between abnormal bands (corresponding to germline mutation) and normal bands in PCR-SSCP analysis.
Results
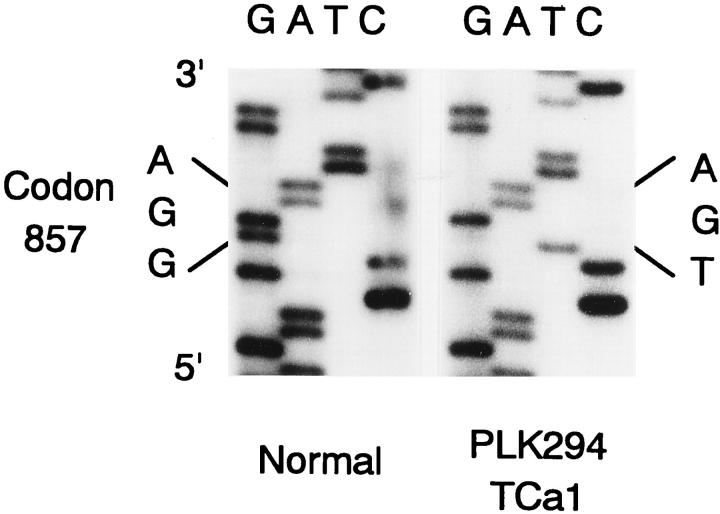
PCR-SSCP analysis and direct sequencing revealed both germline and somatic mutations of the APC gene. Data are shown in Table 1 ▶ and Figure 2 ▶ . Patient PLK29, with a germline mutation of C deletion at codon 175, developed multiple papillary carcinomas in the thyroid (Figure 1) ▶ . One large carcinoma in the left lobe had a somatic APC mutation of CAG to TAG (stop) at codon 886. Two of the multiple carcinomas in the right lobe of the same patient exhibited different somatic mutations of the APC gene, the mutation in one carcinoma (TCa3) being GAA to TAA (stop) at codon 1536, and that in the other (TCa5) being an A insertion at codons 1554 to 1556. Loss of the normal allele was detected in two carcinomas, TCa2 and TCa4. FAP patient PLK294, with a germline mutation of TCA to TGA (stop) at codon 1110, developed two papillary carcinomas in the left lobe. In these carcinomas, different somatic mutations were detected. Mutation in TCa1 was GGA to TGA (stop) at codon 857, and that in TCa2 was AAAAC deletion at codons 1060 to 1063. All of these somatic mutations occurred in exon 15, and formed stop codons resulting in truncated APC protein.
Table 1.
Somatic and Germline Mutations of the APC Gene in Multicentric Thyroid Carcinomas from FAP Patients
| Patient | Thyroid carcinoma | Somatic mutation | Germline mutation |
|---|---|---|---|
| PLK29 | TCa1 | Codon 886 CAG → TAG | Codon 175 C deletion |
| PLK29 | TCa2 | Loss of normal allele | Codon 175 C deletion |
| PLK29 | TCa3 | Codon 1536 GAA → TAA | Codon 175 C deletion |
| PLK29 | TCa4 | Loss of normal allele | Codon 175 C deletion |
| PLK29 | TCa5 | Codon 1554–1556 A insertion | Codon 175 C deletion |
| PLK294 | TCa1 | Codon 857 GGA → TGA | Codon 1110 TCA → TGA |
| PLK294 | TCa2 | Codon 1060–1063 AAAAC deletion | Codon 1110 TCA → TGA |
Figure 2.
Example of sequencing of DNA fragments in SSCP corresponding to somatic mutation of the APC gene in thyroid carcinoma.
Discussion
The histopathological characteristic of FAP-associated thyroid carcinomas has been reported to be papillary carcinoma with a cribriform pattern and solid areas with a spindle-cell component. 11,13,14 With respect to the pattern of development of these carcinomas, both solitary and multicentric types have been reported. 11 In some cases, more than 10 separate tumors of various sizes have been detected in one thyroid. However, it is difficult to assess, by morphological features, whether these tumors are independent primary tumors or metastatically spread tumors from an originally developed one. We have recently demonstrated that FAP-associated thyroid carcinomas develop by biallelic inactivation of the APC gene through germline and somatic mutations. 10 Accordingly, to clarify the origin of multiple cancer in one thyroid, it is important to examine whether all tumors have the same or different somatic APC mutations. The present study revealed that three of the five carcinomas from a FAP patient with an identified germline mutation had different somatic mutations, and the other two carcinomas exhibited loss of the normal allele of the APC gene. In another patient with a known germline mutation, two carcinomas had different somatic mutations as well. The identification of such different somatic alterations of the APC gene confirms independent development of multicentric thyroid carcinomas in FAP patients. Moreover, all carcinomas were revealed to be formed by biallelic inactivation of the APC gene, because all somatic mutations resulted in truncated APC protein.
The position of somatic mutation within the APC sequence in thyroid carcinoma was not restricted to the region (codons 1281 to 1556) where more than 90% of somatic mutations of gastrointestinal tumors are clustered. 4,7,9 Three of five somatic mutations of thyroid carcinomas in the present study occurred outside of that region (codons 857, 886, and 1061). Somatic mutation of a solitary thyroid carcinoma in an additional patient was at codon 456 (data not shown). Germline mutations in our cases with thyroid carcinomas were at codons 175 and 1110. These patients exhibited sparse-type development of colorectal tumors. Germline mutations in our other cases with thyroid carcinomas were at codons 278, 1061, and 1106 (data not shown), and 848. 16 The position of the germline mutation in our cases is consistent with a recent report that a higher incidence of thyroid cancer has been observed in the patients with germline mutation before codon 1220, 17 different from the position with respect to colorectal tumors. 18,19
Although the range of position of germline and somatic mutations in the APC gene in thyroid carcinoma is somewhat different from that of colorectal tumors, the present results suggest that the phenomenon of multicentricity of thyroid carcinoma formation is analogous to the multiplicity of colorectal tumor formation, because tumors in both cases have different somatic mutations. This molecular evidence for multifocal development of thyroid carcinoma may have value in elucidating the mechanism of thyroid tumorigenesis, and in the diagnosis and treatment of FAP patients. Because FAP-associated thyroid carcinomas occasionally occur before diagnosis of colonic adenomatosis, detection of multiple somatic APC mutations in thyroid carcinomas predicts that the patient is affected by FAP. Moreover, multicentricity of thyroid carcinoma in FAP patients implies the necessity of careful observation after partial thyroidectomy is selected.
Footnotes
Address reprint requests to Michiko Miyaki, Hereditary Tumor Research Project, Tokyo Metropolitan Komagome Hospital, 3-18-22 Honkomagome, Bunkyo-ku Tokyo 113-8677, Japan. E-mail: mmiyaki@opal.famille.ne.jp.
Supported in part by the Project “High-Technology Research Center” from the Ministry of Education, Science, Sport and Culture of Japan.
References
- 1.Jagelman DG: Extracolonic manifestations of familial polyposis coli. Cancer Genet Cytogenet 1987, 27:319-325 [DOI] [PubMed] [Google Scholar]
- 2.Bülow S: Extracolonic manifestation of familial adenomatous polyposis. Herrera L eds. Familial Adenomatous Polyposis. 1990, :pp 109-114 Alan R. Liss, New York [Google Scholar]
- 3.Iwama T, Mishima Y, Utsunomiya J: The impact of familial adenomatous polyposis on tumorigenesis and mortality at the several organs. Ann Surg 1993, 217:101-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyooka M, Konishi M, Kikuchi-Yanoshita R, Iwama T, Miyaki M: Somatic mutations of the adenomatous polyposis coli gene in gastroduodenal tumors from patients with familial adenomatous polyposis. Cancer Res 1995, 55:3165-3170 [PubMed] [Google Scholar]
- 5.Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Tanaka K, Takahashi H, Muraoka M, Mori T, Konishi F, Iwama T: Coexistence of somatic and germ-line mutations of APC gene in desmoid tumors from patients with familial adenomatous polyposis. Cancer Res 1993, 53:5079-5082 [PubMed] [Google Scholar]
- 6.Seki M, Tanaka K, Kikuchi-Yanoshita R, Konishi M, Fukunari H, Iwama T, Miyaki M: Loss of normal allele of the APC gene in an adrenocortical carcinoma from a patient with familial adenomatous polyposis. Hum Genet 1992, 89:298-300 [DOI] [PubMed] [Google Scholar]
- 7.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y: Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1992, 1:229-233 [DOI] [PubMed] [Google Scholar]
- 8.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thiibodeau SN, Vogelstein B, Kinzler KW: APC mutations occur early during colorectal tumorigenesis. Nature 1992, 359:235-237 [DOI] [PubMed] [Google Scholar]
- 9.Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, Muraoka M, Takahashi H, Amada Y, Fukayama M, Maeda Y, Iwama T, Mishima Y, Mori T, Koike M: Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res 1994, 54:3011-3020 [PubMed] [Google Scholar]
- 10.Iwama T, Konishi M, Iijima T, Yoshinaga K, Tomonaga T, Koike M, Miyaki M: Somatic mutation of the APC gene in thyroid carcinoma associated with familial adenomatous polyposis. Jpn J Cancer Res 1999, 90:372-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harach HR, Williams GT, Williams ED: Familial adenomatous polyposis associated thyroid carcinoma: a distinct type of follicular cell neoplasm. Histopathology 1994, 25:549-561 [DOI] [PubMed] [Google Scholar]
- 12.Giardiello FM, Offerhaus GJA, Lee DH, Krush AJ, Tersmette AC, Booker SV, Kelly NC, Hamilton SR: Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993, 34:1394-1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cetta F, Toti P, Petracci M, Montalto G, Disanto A, Lore F, Fusco A: Thyroid carcinoma associated with familial adenomatous polyposis. Histopathology 1997, 31:231-236 [DOI] [PubMed] [Google Scholar]
- 14.Soravia C, Sugg SL, Berk T, Mitri A, Cheng H, Gallinger S, Cohen Z, Asa SL, Bapat BV: Familial adenomatous polyposis-associated thyroid cancer, a clinical, pathological, and molecular genetics study. Am J Pathol 1999, 154:127-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hunghes JP, Warrington J, McPherson J, Wasmuth J, Le Paslier D, Abderrahim H, Cohen D, Leppert M, White R: Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66:589-600 [DOI] [PubMed] [Google Scholar]
- 16.Kashiwagi H, Konishi F, Kanazawa K, Miyaki M: Sisters with familial adenomatous polyposis affected with thyroid carcinoma, desmoid tumour and duodenal polyposis. Br J Surg 1996, 83:228. [DOI] [PubMed] [Google Scholar]
- 17.Cetta F, Montalto G, Gori M, Curia MC, Cama A, Olschwang S: Germline mutations of the APC gene in patients with familial adenomatous polyposis-associated thyroid carcinoma: results from a European cooperative study. J Clin Endocrinol Metab 2000, 85:286-292 [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y, Mori T, Utsunomiya J, Baba S, Petersen G, Hamilton SR, Kinzler KW, Vogelstein B, Nakamura Y: Germ-line mutations of APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA 1992, 89:4452-4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spirio L, Olschwang S, Groden J, Robertson M, Samowitz W, Joslyn G, Gerbert L, Thliveris A, Carlson M, Otterud B, Lynch H, Watson P, Lynch P, Laurent-Puig P, Burt R, Hughes JP, Thomas G, Leppert M, White R: Alleles of the APC gene: an attenuated form of familial polyposis. Cell 1993, 75:951-957 [DOI] [PubMed] [Google Scholar]