Abstract
There is limited evidence that inhibition of the activity of the protease calpain I reduces inflammation. Here we investigate the effects of calpain inhibitor I in animal models of acute and chronic inflammation (carrageenan-induced pleurisy and collagen-induced arthritis). We report here for the first time that calpain inhibitor I (given at 5, 10, or 20 mg/kg i.p. in the pleurisy model or at 5 mg/kg i.p every 48 hours in the arthritis model) exerts potent anti-inflammatory effects (eg, inhibition of pleural exudate formation, mononuclear cell infiltration, delayed the development of the clinical signs and histological injury) in vivo. Furthermore, calpain inhibitor I reduced (1) the staining for nitrotyrosine and poly (ADP-ribose) polymerase (immunohistochemistry) and (2) the expression of inducible nitric oxide synthase and cyclooxygenase-2 in the lungs of carrageenan-treated rats and in joints from collagen-treated rats. Thus, prevention of the activation of calpain I reduces the development of acute and chronic inflammation. Inhibition of calpain I activity may represent a novel therapeutic approach for the therapy of inflammation.
It is now widely accepted that the formation of pro-inflammatory cytokines [eg, tumor ecrosis factor-α (TNFα), interleukin IL)−1, IL-6,or IL-8], the expression on endothelium and neutrophils of adhesion molecules (eg, VCAM-1, ICAM-1), and the overproduction of vasoactive mediators [eg, nitric oxide (NO) by inducible NO synthase (iNOS) or eicosanoids by cyclooxygenase-2 (COX-2)] play important roles in the pathophysiology of inflammation. The expression of inducible genes leading to the formation of these proteins relies on transcription factors, which are controlled by (other) inducible genes and, hence, require de novo protein synthesis or alternatively by so-called primary transcription factors. Among the latter, nuclear factor κB (NF-κB) has received a considerable amount of attention because of its unique mechanism of activation and its active role in cytoplasmic/nuclear signaling, and its rapid response to pathogenic stimulation of NF-κB plays a central role in the regulation of many genes responsible for the generation of mediators or proteins in inflammation. These include the genes for TNFα, IL-1, IL-6, IL-8, VCAM-1, ICAM-1, iNOS, and COX-2, to name but a few. 1,2 The discovery in 1997 that inhibition of the activation of NF-κB may be useful in conditions associated with local or systemic inflammation 3 stimulated the search for agents that prevent the activation of NF-κB.
There is evidence that inhibition of the activation of the neutral protease calpain prevents the activation of NF-κB. Calpain is one of the many intracellular proteins, the activity of which is dependent on intracellular calcium levels. To date, two isoforms of calpain have been identified: calpain I (or μ-calpain) and calpain II (or m-calpain), which require, respectively, low and high micromolar concentrations of calcium for their activation. 3,4 After activation by calcium, calpain cleaves a specific subset of cellular proteins. For instance, activation of calpain I leads to the degradation of IκB (IκBα or IκBβ) in the proteasome and, hence, is an essential step in the translocation of NF-κB from the cytosol into the nucleus. 5,6 This calpain-dependent step of the activation of NF-κB is abolished by calpain inhibitor I. 7-10 Thus, calpain inhibitor I prevents the expression of many NF-κB-dependent genes, including those for iNOS 11-13 and COX-2. 14,15 In addition to proteins regulated by NF-κB, the generation of superoxide anions, hydroxyl radicals, and peroxynitrite also plays a pivotal role in the tissue injury associated with inflammation. 16-20 Hydroxyl radicals can cause DNA damage, 21,22 resulting in the activation of the nuclear enzyme poly(ADP-ribose) synthetase (PARS), depletion of NAD and ATP, and ultimately cell death. 23
This study investigates the effects of calpain inhibitor I in animal models of acute (carrageenan-induced pleurisy) and chronic (collagen-induced arthritis) inflammation in the rat. In particular, we investigate the effects of calpain inhibitor I on the lung injury associated with carrageenan-induced pleurisy and the joint injury associated with collagen-induced arthritis. To gain a better insight into the mechanism(s) of action of calpain inhibitor I, we have also investigated the effects of calpain inhibitor I on the expression of iNOS and COX-2, the nitration of cellular proteins by peroxynitrite, and the activation of the nuclear enzyme PARS.
Materials and Methods
Animals
Male Sprague-Dawley and Lewis rats (160–180 g; Charles River, Milan, Italy) were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulations on the protection of animals used for experimental and other scientific purposes (D.M. 116192) as well as with EEC regulations (O.J. of E.C. L 358/1 12/18/1986).
Experimental Groups (Pleurisy Study)
In the treated group of animals, calpain inhibitor I was given as an intraperitoneal (i.p.) bolus 15 minutes before carrageenan (5–20 mg/kg) (CAR + Calp-I group). In a vehicle-treated group of rats, vehicle (the final concentration of ethanol was 1%) was given instead of calpain inhibitor I (CAR group). In separate groups of rats, surgery was performed that was identical in every aspect to the surgery performed on the CAR group, except that saline was injected instead of carrageenan (sham group; Sham). In an additional group of animals, sham surgery was combined with the administration of calpain inhibitor I (5–20 mg/kg) (Sham + Calp-I).
Carrageenan-Induced Pleurisy
Rats were anesthetized with isoflurane, and a skin incision was made at the level of the left sixth intercostal space. The underlying muscle was dissected, and saline (0.2 ml) or saline containing 1% λ-carrageenan (0.2 ml) was injected into the pleural cavity. The skin incision was closed with a suture, and the animals were allowed to recover. At 4 hours after the injection of carrageenan, the animals were killed by inhalation of CO2. The chest was carefully opened, and the pleural cavity was rinsed with 2 ml of saline solution containing heparin (5 U·ml−1) and indomethacin (10 μg·ml−1). The exudate and washing solution were removed by aspiration, and the total volume was measured. Any exudate that was contaminated with blood was discarded. The amount of exudate was calculated by subtracting the volume injected (2 ml) from the total volume recovered. The leukocytes in the exudate were suspended in phosphate-buffered saline (PBS) and counted with an optical microscope in a Burker’s chamber after vital Trypan Blue staining.
Measurement of Nitrite/Nitrate
Nitrite + nitrate production, an indicator of NO synthesis, was measured in the supernatant samples as previously described. 18 Briefly, the nitrate in the supernatant was first reduced to nitrite by incubation with nitrate reductase (670 mU·ml−1) and NADPH (160 μmol/L) at room temperature for 3 hours. The nitrite concentration in the samples was then measured by the Griess reaction, by adding 100 μl of Griess reagent (0.1% naphthylethylendiamide dihydrochloride in H2O and 1% sulfanilamide in 5% concentrated H3PO4; 1:1, v/v) to 100-μl samples. The optical density at 550 nm (OD550) was measured using an enzyme-linked immunosorbent assay microplate reader (SLT- Labinstruments, Salzburg, Austria). Nitrate concentrations were calculated by comparison with OD550 of standard solutions of Dulbecco’s minimum essential medium.
Leukocyte Count
Blood samples (taken from the femoral vein) for a peripheral cell count were taken 4 hours after the administration of carrageenan. The total number of peripheral leukocytes is expressed as white blood cell count (WBC) per μl (mean ± SEM).
Induction of Collagen-Induced Arthritis
Bovine type II collagen (CII) was dissolved in 0.01 mol/L acetic acid at a concentration of 2 mg/ml by stirring overnight at 4°C. Dissolved CII was frozen at −70°C until use. Complete Freund’s adjuvant (CFA) was prepared by the addition of Mycobacterium tuberculosis H37Ra at a concentration of 2 mg/ml. Before injection, CII was emulsified with an equal volume of CFA. Collagen-induced arthritis was induced as previously described. 24 On day 1, Lewis rats were injected intradermally at the base of the tail with 100 μl of the emulsion (containing 100 μg of CII). On day 21, a second injection of CII in CFA was administered. In a separate set of experiments, animals were treated with calpain inhibitor I (n = 10) (5 or 2.5 mg/kg, i.p.) every 48 hours, starting from day 24.
Clinical Assessment of CIA
Rats were evaluated daily for arthritis by using a macroscopic scoring system: 0 = no signs of arthritis, 1 = swelling and/or redness of the paw or one digit, 2 = two joints involved, 3 = more than two joints involved, and 4 = severe arthritis of the entire paw and digits. 24 The arthritic index for each rat was calculated by adding the four scores of individual paws. Clinical severity was also determined by quantitating the change in the paw volume, using plethysmometry (model 7140; Ugo Basile).
Assessment of Arthritis Damage
At day 35, animals were sacrificed while they were under anesthesia, and paws and knees were removed and fixed for histological examination, which was done by an investigator blinded for the treatment regime. The following morphological criteria were considered: score 0, no damage; score 1, edema; score 2, inflammatory cell presence; score 3, bone resorption.
Histological Examination
Lung biopsies were obtained 4 hours after injection of carrageenan, and paws and knees, 35 days after CIA. The biopsies were fixed for 1 week in buffered formaldehyde solution (10% in phosphate-buffered saline) at room temperature, dehydrated by graded ethanol, and embedded in Paraplast (Sherwood Medical, Mahwah, NJ). The paws were trimmed, placed in decalcifying solution for 24 hours, embedded in paraffin, and sectioned at 5 g. Tissue sections were deparaffinized with xylene, stained with trichromic Van Gieson, and studied using light microscopy (Dialux 22 Leitz).
Radiography
The rats were anesthetized with sodium pentobarbital (45 mg/kg, i.p.). Rats were placed on a radiographic box 90 cm from the X-ray source. Radiographic analysis of normal and arthritic rat hind paws was performed by X-ray machine (Philips X12, Germany) with a 40-kW exposition for 0.01 seconds. An investigator blinded for the treatment regime determined the radiograph score. The following radiograph criteria were considered: score 0, no bone damage; score 1, tissue swelling and edema; score 2, joint erosion; score 3, bone erosion and osteophyte formation.
Immunohistochemical Localization of Nitrotyrosine
Tyrosine nitration, an index of the nitrosylation of proteins by peroxynitrite and/or oxygen-derived free radicals, was determined by immunohistochemistry as previously described. 18 At the end of the experiment, the relevant organs were fixed in 10% buffered formaldehyde, and 8-μm sections were prepared from paraffin-embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% H2O2 in 60% methanol for 30 minutes. The sections were permeabilized with 0.1% Triton X-100 in PBS for 20 minutes. Nonspecific adsorption was minimized by incubating the section in 2% normal goat serum in PBS for 20 minutes. Endogenous biotin- or avidin-binding sites were blocked by sequential incubation for 15 minutes with avidin and biotin. The sections were then incubated overnight with a 1:1000 dilution of primary anti-nitrotyrosine antibody or with control solutions. Controls included buffer alone or nonspecific purified rabbit IgG. Specific labeling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex.
Immunohistochemical Localization of PARP
At the specified time after the carrageenan injection, lung tissues were fixed in 10% buffered formalin, and 8-μm sections were prepared from paraffin-embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% H2O2 in 60% methanol for 30 minutes. The sections were permeabilized with 0.1% Triton X-100 in PBS for 20 minutes. Nonspecific adsorption was minimized by incubating the section in 2% normal goat serum in PBS for 20 minutes. Endogenous biotin- or avidin-binding sites were blocked by sequential incubation for 15 minutes with avidin and biotin (DBA, Milan, Italy). The sections were then incubated overnight with a 1:500 dilution of primary anti-poly(ADP-ribose) antibody (DBA) or with control solutions. Controls included buffer alone or nonspecific purified rabbit IgG. Specific labeling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase (DBA).
Myeloperoxidase Activity
Myeloperoxidase (MPO) activity, an indicator of polymorphonuclear leukocyte (PMN) accumulation, was determined as previously described. 25 At the specified time after the intrapleural injection of carrageenan, lung tissues were obtained and weighed. Each piece of tissue was homogenized in a solution containing 0.5% hexadecyltrimethylammonium bromide dissolved in 10 mmol/L potassium phosphate buffer (pH 7) and centrifuged for 30 minutes at 20,000 × g at 4°C. An aliquot of the supernatant was then allowed to react with a solution of tetramethylbenzidine (1.6 mmol/L) and 0.1 mmol/L H2O2. The rate of change in absorbance was measured spectrophotometrically at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide/min at 37°C and was expressed in milliunits per gram weight of wet tissue.
Malondialdehyde Measurement
Malondialdehyde (MDA) levels in the lung tissue were determined as an indicator of lipid peroxidation. 26 Lung tissues, collected at the specified time, were homogenized in 1.15% KCl solution. An aliquot (100 μl) of the homogenate was added to a reaction mixture containing 200 μl of 8.1% sodium dodecyl sulfate, 1500 μl of 20% acetic acid (pH 3.5), 1500 μl of 0.8% thiobarbituric acid, and 700 μl of distilled water. Samples were then boiled for 1 hour at 95°C and centrifuged at 3000 × g for 10 minutes. The absorbance of the supernatant was measured by spectrophotometry at 650 nm.
Determination of Nitric Oxide Synthase Activity
The calcium-independent conversion of l-arginine to l-citrulline in the homogenates of either pleural macrophages or lungs (obtained 4 hours after carrageenan treatment in the presence or the absence of calpain inhibitor I) served as an indicator of iNOS activity. 27 Cells were scraped into a homogenation buffer composed of 50 mmol/L Tris-HCl, 0.1 mmol/L EDTA, and 1 mmol/L phenylmethylsulfonyl fluoride (pH 7.4) and homogenized in the buffer on ice, using a tissue homogenizer. Conversion of [3H]l-arginine to [3H]l-citrulline was measured in the homogenates as described. 28 Briefly, homogenates (30 μl) were incubated in the presence of [3H]l-arginine (10 μmol/L, 5 kBq per tube), NADPH (1 mmol/L), calmodulin (30 nmol/L), tetrahydrobiopterin (5 μmol/L), and EGTA (2 mmol/L) for 20 minutes at 22°C. Reactions were stopped by dilution with 0.5 ml of ice-cold HEPES buffer (pH 5.5) containing EGTA (2 mmol/L) and EDTA (2 mmol/L). Reaction mixtures were applied to Dowex 50W (Na+ form) columns, and the eluted [3H]l-citrulline activity was measured with a Beckman scintillation counter.
Measurement of Prostaglandin E2 in the Pleural Exudate
The amount of prostaglandin E2 (PGE2) present in the pleural fluid was measured by radioimmunoassay without prior extraction or purification. 29
Assessment of COX Activity
Lungs were obtained 4 hours after the induction of pleurisy by carrageenan injection. The material was homogenized at 4°C in a buffer containing protease inhibitors in a ratio of 5:1 (v/w). The protein concentration in the homogenates was measured by the Bradford assay, 30 with bovine serum albumin used as standard. Homogenates were incubated at 37°C for 30 minutes in the presence of excess arachidonic acid (30 μmol/L). The samples were boiled and centrifuged at 10,000 × g for minutes. The concentration of 6-keto-PGF1α present in the supernatant was measured by radioimmunoassay as previously described. 31
Immunohistochemical Localization of COX-1 and COX-2
Lung biopsies were fixed in 10% buffered formalin, and 8-μm sections were prepared from paraffin-embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% H2O2 in 60% methanol for 30 minutes. The sections were permeabilized with 0.1% Triton X-100 in PBS for 20 minutes. Nonspecific binding was minimized by incubating the section in 2% normal goat serum in PBS for 20 minutes. Endogenous biotin- or avidin-binding sites were blocked by sequential incubation for 15 minutes with avidin and biotin (DBA). The sections were then incubated overnight with a 1:500 dilution of the primary anti-COX-1 or anti-COX-2 antibody with (DBA) or with control solutions. Controls included buffer alone or nonspecific, purified rabbit IgG. Specific labeling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase (DBA).
Materials
Unless otherwise stated, all compounds were obtained from Sigma-Aldrich Company (Poole, Dorset, UK). Thiopentone sodium (Intraval Sodium) was obtained from Rhône Mérieux (Harlow, Essex, UK). Biotin blocking kit, biotin-conjugated goat anti-rabbit IgG, primary anti-nitrotyrosine, anti-poly(ADP-ribose) synthetase antibodies, primary anti-iNOS, anti-COX-2, and avidin-biotin peroxidase complex were obtained from DBA. Calpain inhibitor 1 (Cal I-1) was purchased from Calbiochem Novabiochem (Nottingham, UK). Antibodies to IκBα and IκBβ were purchased from Santa Cruz Biotechnology (USA). All other chemicals were of the highest commercial grade available. All stock solutions were prepared in nonpyrogenic saline (0.9% NaCl; Baxter Health Care, Thetford, UK).
Statistical Evaluation
All values in the figures and text are expressed as mean ± SEM of n observations. For the in vivo studies n represents the number of animals studied. In the experiments involving histology or immunohistochemistry, the figures shown are representative of at least three experiments performed on different experimental days. Data sets were examined by one- or two-way analysis of variance, and individual group means were then compared with Student’s unpaired t-test. For the arthritis studies, a Mann-Whitney U test (two-tailed, independent) was used to compare medians of the arthritic indices. 32 A P value less than 0.05 was considered significant.
Results
Effects of Calpain Inhibitor I in Carrageenan-Induced Pleurisy
All rats that were treated with carrageenan developed an acute pleurisy, characterized by the production of turbid exudate (Table 1) ▶ . When compared with the number of cells collected from the pleural space of sham-operated rats (Table 1) ▶ , injection of carrageenan induced a significant increase in the number of PMNs (Table 1) ▶ . Pretreatment of rats with calpain inhibitor I attenuated the volume of the pleural exudate as well as the number of PMNs within the exudate in a dose-related fashion (Table 1) ▶ . Carrageenan administration also led to a significant increase in the number of circulating leukocytes (WBC count, Table 2 ▶ ). This increase in WBC count caused by administration of carrageenan was not affected by treatment of rats with calpain inhibitor I (Table 2) ▶ .
Table 1.
Effct of Calpain Inhibitor I on Carrageenan-Induced Inflammation, NO Formation, and PG Production in the Pleural Exudate
| Group | Volume exudate (ml) | PMNs infiltration (million cells/rat) | Nitrite/nitrate (nmol/rat) | Lung iNOS activity (fmol/mg/min) | PGE2 (pg/rat) | Lung 6-KETO-PGF1a (pg/mg) |
|---|---|---|---|---|---|---|
| Sham+ vehicle | 0.12 ± 0.05 | 2.5 ± 0.9 | 5.5 ± 1.8 | 4.5 ± 0.9 | N.D. | N.D. |
| Sham+ CALP-I (5 mg/kg) | 0.13 ± 0.06 | 2 ± 0.8 | 4.8 ± 2.1 | 5.8 ± 0.6 | N.D. | N.D. |
| Sham+ CALP-I (10 mg/kg) | 0.1 ± 0.08 | 2.1 ± 0.7 | 5 ± 1 | 4.9 ± 0.9 | N.D. | N.D. |
| Sham+ CALP-I (20 mg/kg) | 0.1 ± 0.04 | 2.2 ± 0.95 | 4.9 ± 1.8 | 5.3 ± 0.85 | N.D. | N.D. |
| CAR+ vehicle | 1.45 ± 0.14* | 95 ± 4.4* | 80 ± 3.3* | 185 ± 4.4* | 200 ± 6.3* | 250 ± 5.4* |
| CAR+ CALP-I (5 mg/kg) | 0.74 ± 0.1† | 65 ± 2.2† | 58 ± 2.2† | 120 ± 2.2† | 110 ± 3.5† | 120 ± 4.2† |
| CAR+ CALP-I (10 mg/kg) | 0.45 ± 0.13† | 43 ± 2.6† | 35 ± 2.4† | 60 ± 5.9† | 75 ± 3.1† | 80 ± 3.9† |
| CAR+ CALP-I (20 mg/kg) | 0.2 ± 0.09† | 20 ± 4.2† | 22 ± 4.1 | 36 ± 4.1† | 48 ± 2.1† | 66 ± 4.1† |
Data are means ± SEM of 10 rats for each group.
*P < 0.01 versus sham.
†P <0.01 versus carrageenan. N.D., not determined.
Table 2.
Peripheral White Blood Cell Count (WBC) of Rats Subjected to Carrageenan-Induced Pleurisy
| Group | WBC (K/μl) | Neutrophils % (range 2–14) | Lymphocytes % (range 55–96) | ||
|---|---|---|---|---|---|
| Sham+ vehicle | 3.6 ± 0.2 | 13 ± 3 | 87 ± 5 | ||
| Sham+ CALP-I | 3.8 ± 0.3 | 14 ± 4 | 88 ± 6 | ||
| CAR+ vehicle | 43.8 ± 6* | 45 ± 9* | 55 ± 4* | ||
| CAR+ CALP-I | 45.2 ± 8* | 42 ± 8.5* | 58 ± 6* |
*P <0.01 versus Sham + vehicle.
The levels of NOx were also significantly (P < 0.01) increased in the exudate obtained from rats challenged with carrageenan (Table 1) ▶ . In the lungs obtained from animals subjected to carrageenan-induced pleurisy, a significant increase in iNOS activity was detected 4 hours after injection of carrageenan (Table 1) ▶ . Pretreatment of rats with calpain inhibitor I significantly reduced (in a dose-dependent fashion) both NOx levels and iNOS activity (Table 1) ▶ . Immunohistochemical analysis of lung sections obtained from carrageenan-treated rats revealed a positive staining for iNOS, which was primarily localized in alveolar macrophages (Figure 1A) ▶ . In contrast, no staining for iNOS was found in the lungs of carrageenan-treated rats that had been pretreated with calpain inhibitor I (20 mg/kg; Figure 1B ▶ ). Staining was absent from control tissue (data not shown). Immunohistochemical analysis of lung sections obtained from rats treated with carrageenan also revealed a positive staining for nitrotyrosine, which was primarily localized in alveolar macrophages and in airway epithelial cells (Figure 2A) ▶ . In contrast, no positive staining for nitrotyrosine was found in the lungs of carrageenan-treated rats that had been pretreated with calpain inhibitor I (20 mg/kg; Figure 2B ▶ ). Immunohistochemical analysis of lung sections obtained from rats treated with carrageenan also revealed a positive staining for PARP (Figure 2C) ▶ . In contrast, no staining for PARP was found in the lungs of carrageenan-treated rats that had been pretreated with calpain inhibitor I (20 mg/kg; Figure 2D ▶ ). Please note that there was no staining for either nitrotyrosine or PARP in lungs obtained from sham-operated rats (data not shown).
Figure 1.

Immunohistochemical localization of iNOS and COX-2 in the lung. A and C: Four hours after carrageenan injection, positive staining for iNOS (A) and COX-2 (C) was localized mainly in macrophages. B and D: There was a marked reduction in the immunostainings in the lungs of carrageenan-treated rats pretreated with calpain inhibitor I (20 mg/kg). Original magnification, ×125. This figure is representative of at least three experiments performed on different experimental days.
Figure 2.
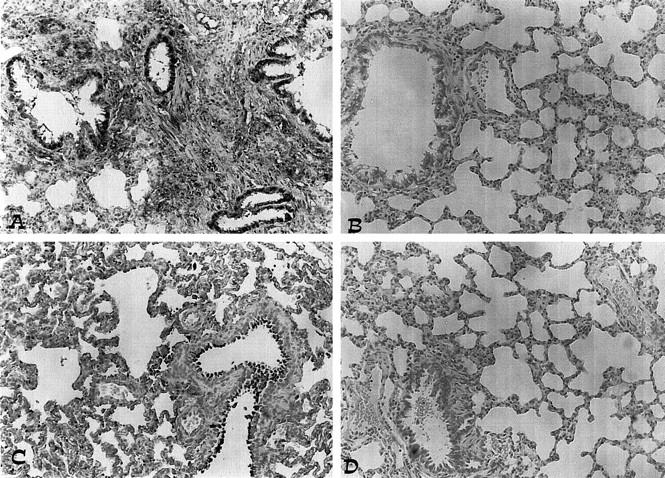
Effect of calpain inhibitor I on nitrotyrosine formation and PARP activation. A and C: Four hours after carrageenan injection, positive staining for nitrotyrosine and for PARP was observed. B and D: There was a marked reduction in the immunostaining in the lungs of carrageenan-treated rats pretreated with calpain inhibitor I (20 mg/kg). Original magnification, ×125. This figure is representative of at least three experiments performed on different experimental days.
The COX activity in carrageenan-induced pleural exudate and lung homogenates was assessed by measuring the increase in the formation of PGE2 in the exudate. The amounts of PGE2 found in the pleural exudate of carrageenan-treated rats was increased significantly (n = 6; Table 1 ▶ ). The amounts of PGE2 were significantly lower in the exudate obtained from carrageenan-treated rats that had been pretreated with calpain inhibitor I. In lungs from carrageenan-treated rats, the amount of 6-keto-PGF1α was significantly increased in comparison with sham rats (Table 1) ▶ . The amount of 6-keto-PGF1α was significantly reduced in the lungs from carrageenan-treated rats that had been pretreated with calpain inhibitor I (Table 1) ▶ . Immunohistochemical analysis of lung sections obtained from carrageenan-treated rats revealed a positive staining for COX-2, which was primarily localized in alveolar macrophages (Figure 1C) ▶ . In contrast, no positive COX-2 staining was found in the lungs of from carrageenan-treated rats, which had been pretreated with calpain inhibitor I (Figure 1D) ▶ . Staining was absent in tissue obtained from sham-operated control animals (data not shown).
COX-1 was also detected by immunohistochemical analysis in the lung sections obtained from rats treated with carrageenan, but the degree of staining was similar to that observed in the lungs of sham-operated control animals (data not shown). The degree of staining for COX-1 in lungs of carrageenan-treated rats treated with calpain inhibitor I was similar to that observed in lungs obtained either from carrageenan-treated rats or from sham-operated rats (data not shown).
All rats that were treated with carrageenan exhibited a substantial increase in the activities of MPO and MDA in the lungs (Figure 3, A and B) ▶ . Pretreatment of rats with calpain inhibitor I attenuated the increase in MPO and MDA caused by carrageenan in the lung (Figure 3, A and B) ▶ . In sham-operated rats, calpain inhibitor I had no effect on any of the parameters measured (Figure 3, A and B) ▶ . Histological examination of lung sections of rats treated with carrageenan showed edema, tissue injury, and infiltration of the tissue with PMNs, lymphocytes, and plasma cells (Figure 4A) ▶ . Calpain inhibitor I treatment reduced both lung injury as well as infiltration of the tissue with white blood cells (Figure 4B) ▶ .
Figure 3.

Effect of calpain inhibitor I on myeloperoxidase activity and malondialdehyde levels in the lung. Myeloperoxidase (MPO) activity (A) and malondialdehyde (MDA) levels (B) in the lungs of carrageenan-treated rats killed at 4 hours. MPO activity and MDA levels were significantly increased in the lungs of the carrageenan-treated rats in comparison with sham rats (*P < 0.01). Calpain inhibitor I (5, 10, or 20 mg/kg) reduced the carrageenan-induced increase in MPO activity and MDA levels in a dose-dependent manner. Values are means ± SEM of 10 rats for each group. *P < 0.01 versus sham; °P < 0.01 versus carrageenan.
Figure 4.

Effect of calpain inhibitor I on lung injury. The lung section from a carrageenan-treated rat (A) demonstrates interstitial hemorrhage and polymorphonuclear leukocyte accumulation. The lung section from a carrageenan-treated rat that had received calpain inhibitor I (20 mg/kg) (B) demonstrates reduced interstitial hemorrhage and a lesser cellular infiltration. Original magnification, ×62.5. This figure is representative of at least three experiments performed on different experimental days.
Effects of Calpain Inhibitor I in Collagen-Induced Arthritis
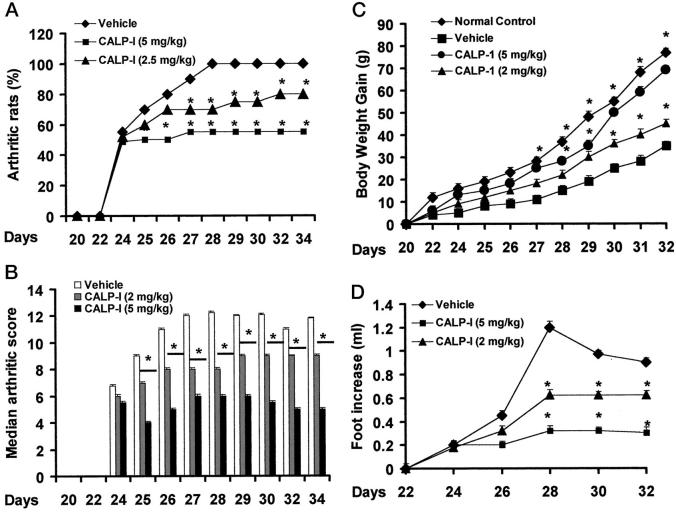
CIA developed rapidly in rats immunized with CII, and clinical signs (periarticular erythema and edema) of the disease (Figure 5A) ▶ first appeared in the hind paws between 24 and 26 days postchallenge. Furthermore, a 100% incidence of CIA was observed by day 27 in CII-immunized rats. Neither the clinical signs nor the histopathological features of CIA were observed in rat forepaws during the 28-day evaluation period. The maximum incidence of CIA in the calpain inhibitor I-treated rats during the 35-day study period was 55% (Figure 5A) ▶ (P < 0.05).
Figure 5.
Effect of calpain inhibitor I on the onset, the secondary lesion, and body weight gain in collagen-induced arthritis. A: The percentage of arthritic rats (rats showing clinical scores of arthritis >1). B: There was a significant increase in the arthritic score from day 26 (P < 0.01). C: Beginning on day 25, the collagen-challenged rats gained significantly less weight than the normal rats, and this trend continued through day 35. D: The swelling in hindpaws over time (ml) was measured at 2-day intervals. Calpain inhibitor I was able to positively affect in a dose-dependent manner the percentage of arthritic rats, the arthritic score, the weight gain, and the paw edema of CII-immunized rats. Values are means ± SEM of 10 animals for each group. *P < 0.01 versus control. °P < 0.01 versus CIA.
Hindpaw erythema and swelling increased in frequency and severity in a time-dependent mode with maximum arthritis indices of approximately 13 observed between 28 to 25 days postimmunization (Figure 5B) ▶ . Calpain inhibitor I attenuated the arthritis index between days 25 and 35 postCII immunization in a dose-dependent fashion (Figure 5B) ▶ . There was no macroscopic evidence of either hindpaw erythema or edema in the normal control rats (Figure 5B) ▶ .
The data in Figure 5D ▶ demonstrate a time-dependent increase in hindpaw (each value represents the mean values of both hind paws) volume (ml) in rats immunized with CII. Maximum paw volume occurred by day 28 in the CII-immunized rats. Calpain inhibitor I significantly suppressed hindpaw swelling from day 24 to day 35 postimmunization in a dose-dependent fashion (Figure 5D) ▶ . A maximum reduction in response hindpaw swelling of 66% was observed from day 28 to day 35. No increase in hindpaw volume over time was observed in normal rats (Figure 5D) ▶ .
The rate and the absolute gain in body weight were comparable in normal Lewis rats and CII-immunized rats for the first week (Figure 5C) ▶ . Beginning on day 25, the collagen-challenged rats gained significantly less weight than the normal rats, and this trend continued through day 35. Calpain inhibitor I attenuated (in a dose-dependent fashion) the weight loss caused by immunization with CII (when compared with the respective control group).
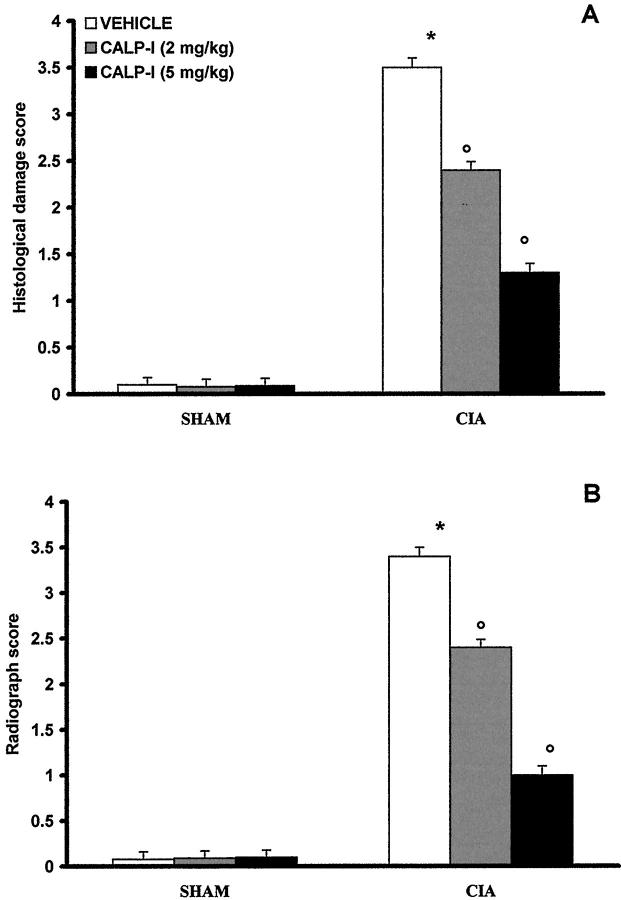
The histological evaluation (at day 35) of the paws in the vehicle-treated arthritic animals revealed signs of severe arthritis, with massive infiltration of the tissue with white blood cells (neutrophils, macrophages, and lymphocytes). In addition, severe or moderate necrosis and sloughing of the synovium were seen, together with the extension of the inflammation into the adjacent musculature, with fibrosis and increased mucous production (Figure 6A ▶ ; see Figure 7A ▶ for the damage score). In the calpain inhibitor I-treated animals, the degree of arthritis was significantly reduced (Figures 6B, 7A) ▶ ▶ .
Figure 6.
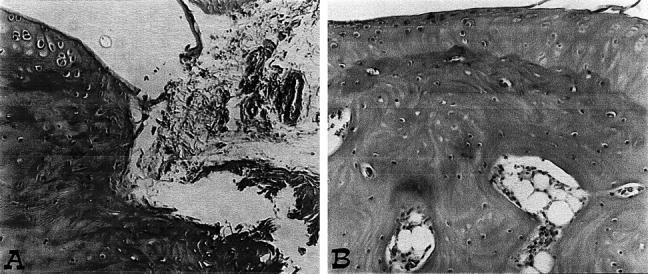
Representative histology of the inflammatory cells infiltration and bone erosion (A) of an arthritic animal. Note the reduction in the degree of inflammatory cells infiltration (B) in the paws of the calpain inhibitor I -treated arthritic animals. Original magnifications, ×100. This figure is representative of at least three experiments performed on different experimental days.
Figure 7.
Effect of calpain inhibitor I treatment on histological damage score (A) and radiograph score (B). Values are means ± SEM of 10 animals for each group. *P < 0.01 versus control. °P < 0.01 versus CIA.
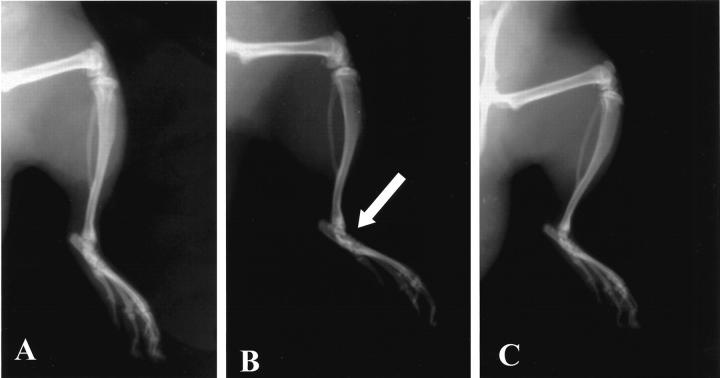
A radiographic examination of hind paws from rats at 35 days after CII immunization revealed bone matrix resorption and osteophyte formation at the joint margin (Figure 8B ▶ ; see Figure 7B ▶ for the radiograph score). There was no evidence of pathology in normal rats (Figures 7B, 8A) ▶ ▶ . Calpain inhibitor I markedly reduced the degree of bone resorption, soft tissue swelling, and osteophyte formation (Figures 7B, 8C) ▶ ▶ .
Figure 8.
Radiographic progression of CIA in the tibiotarsal joint of rats with CIA. A: There is no evidence of pathology in the tibiotarsal joints of normal rats. B: The hindpaws from CII-immunized (35 days) rats demonstrated bone resorption (arrow). C: Calpain inhibitor I suppressed joint pathology (arrow) and soft tissue swelling in the rat hindpaw. This figure is representative of at least three experiments performed on different experimental days.
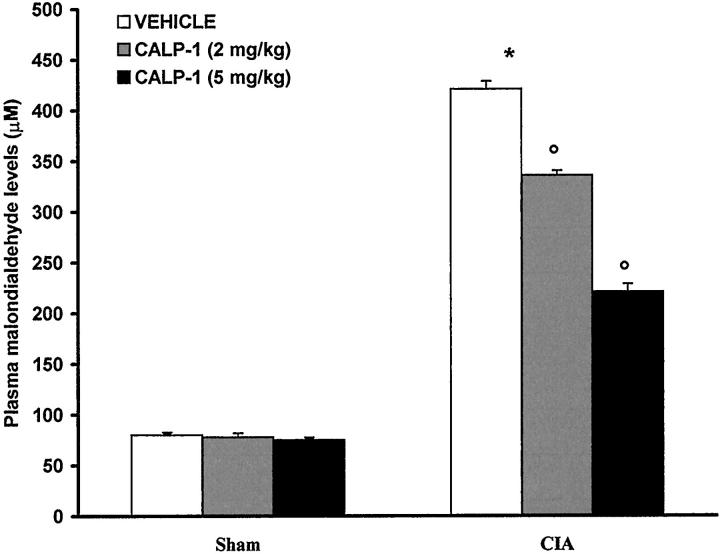
At day 35, all vehicle-treated arthritic animals exhibited a substantial increase in the plasma MDA levels (Figure 9) ▶ . Treatment of rats with calpain inhibitor I significantly attenuated the increase in MDA caused by CIA-induced arthritis (Figure 9) ▶ . No increases in plasma MDA levels were observed with normal rats (Figure 9) ▶ .
Figure 9.
Effect of calpain inhibitor I on malondialdehyde (MDA) levels in plasma: MDA levels in the plasma of CII-immunized rats killed at 35 days. MDA levels were significantly increased in the plasma of CII-immunized rats in comparison to sham rats (*P < 0.01). Calpain inhibitor I reduced the CIA increase in MDA levels. Values are means ± SEM of 10 rats for each group. *P < 0.01 versus control. °P < 0.01 versus CIA.
Immunohistochemical analysis of joint sections obtained from rats treated with collagen type II revealed a positive staining for nitrotyrosine, which was primarily localized in the synovia (Figure 10A) ▶ . In contrast, no positive staining for nitrotyrosine was found in the joints of CIA-treated rats that had been pretreated with calpain inhibitor I (Figure 10B) ▶ . Immunohistochemical analysis of joint sections obtained from rats treated with collagen type II also revealed a positive staining for PARP (Figure 10C) ▶ . In contrast, no specific staining for PARP was found in the joints of CIA-treated rats that had been pretreated with calpain inhibitor I (Figure 10D) ▶ . Please note that there was no staining for either nitrotyrosine or PARP in joints obtained from sham-operated rats (data not shown).
Figure 10.
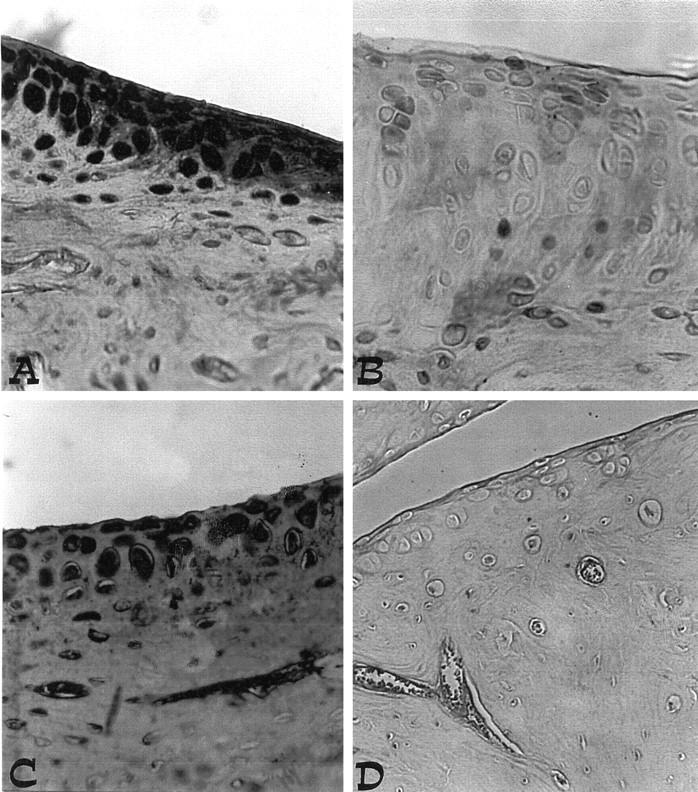
A and C: Nitrotyrosine and PARP immunostaining in the paw of a rat at 35 days of collagen-induced arthritis. A marked increase in nitrotyrosine and PARP stainings is evident in the paws in arthritis. B and D: There was a marked reduction in the immunostaining in the paws of calpain inhibitor I -treated rats. Original magnification, ×125. This figure is representative of at least three experiments performed on different experimental days.
Immunohistochemical analysis of joint sections obtained from rats treated with collagen type II revealed a positive staining for iNOS and COX-2 (Figure 11, A and C) ▶ . In contrast, no positive iNOS or COX-2 staining was found in the joints of CIA-treated rats that had been pretreated with calpain inhibitor I (Figure 11, B and D) ▶ .
Figure 11.
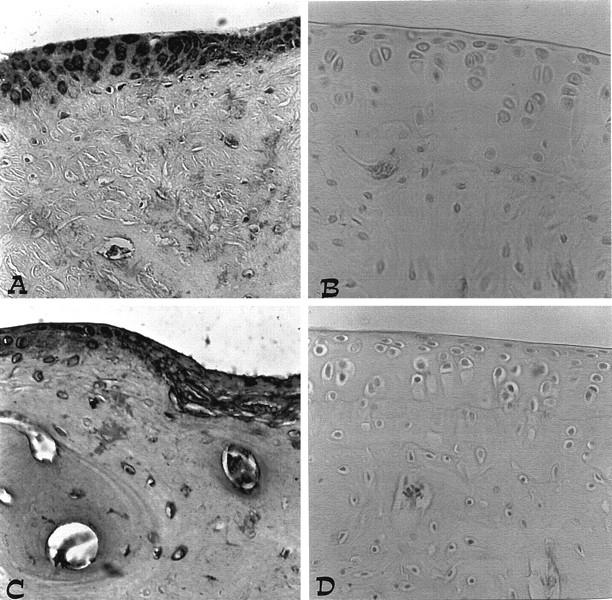
A and C: iNOS and COX-2 immunostaining in the paw of a rat at 35 days of collagen-induced arthritis. A marked increase in iNOS and COX-2 stainings is evident in the paws in arthritis. B and D: There was a marked reduction in the immunostaining in the paws of calpain inhibitor I-treated rats. Original magnification, ×125. This figure is representative of at least three experiments performed on different experimental days.
Discussion
The inflammatory process is invariably characterized by the production of prostaglandins, leukotrienes, histamine, bradykinin, platelet-activating factor (PAF), and IL-1 and by a release of chemicals from tissues and migrating cells. 33,34 Furthermore, there is a large amount of evidence that the production of reactive oxygen species (ROS) such as hydrogen peroxide, superoxide, and hydroxyl radicals at the site of inflammation contributes to tissue damage. 35-39 Inhibitors of NOS activity reduce the development of carrageenan-induced inflammation and support a role for NO in the pathophysiology associated with this model of inflammation. 38-41 In addition to NO, peroxynitrite is also generated in carrageenan-induced inflammation. 38-42 The biological activity and decomposition of peroxynitrite are very much dependent on the cellular or chemical environment (presence of proteins, thiols, glucose, the ratio of NO and superoxide, carbon dioxide levels, and other factors), and these factors influence its toxic potential. 43-45
This study provides the first evidence that pretreatment of rats with calpain inhibitor I attenuates 1) the development of carrageenan-induced pleurisy, 2) the infiltration of the lung with PMNs (histology and MPO activity), 3) the degree of lipid peroxidation in the lung, 4) the degree of lung injury (histology) caused by injection of carrageenan, 5) the development of collagen-induced arthritis, 6) the infiltration of joints with PMNs (histology), 7) the degree of plasma lipid peroxidation, and 8) the degree of joint injury (histology, radiography) in rats treated with type II collagen. All of these findings support the view that calpain inhibitor I attenuates the degree of acute and chronic inflammation in the rat.
What, then, is the mechanism by which calpain inhibitor I protects joints against this inflammatory injury? Activation of the transcription factor NFκB plays an important role in the expression of iNOS. 47-50 An enhanced formation of NO by iNOS may contribute to the inflammatory process. 39-42 This study demonstrates that calpain inhibitor I attenuates the expression of iNOS in the lung from carrageenan-treated rats (Figures 2, 3B) ▶ ▶ and in joints from collagen-treated rats (Figure 11B) ▶ . Thus, the reduction of the expression of iNOS by calpain inhibitor I may contribute to the attenuation by this agent of nitrotyrosine in lungs from carrageenan-treated rats (Figure 4B) ▶ and in joints from collagen-treated rats (Figure 10B) ▶ . Nitrotyrosine formation, along with its detection by immunostaining, was initially proposed as a relatively specific marker for the detection of the endogenous formation “footprint” of peroxynitrite. 51 There is recent evidence, however, that certain other reactions can also induce tyrosine nitration; eg, the reaction of nitrite with hypochlorous acid and the reaction of myeloperoxidase with hydrogen peroxide can lead to the formation of nitrotyrosine. 52 Increased nitrotyrosine staining is considered, therefore, as an indication of “increased nitrosative stress” rather than a specific marker of the generation of peroxynitrite.
ROS and peroxynitrite produce cellular injury and necrosis via several mechanisms, including peroxidation of membrane lipids, protein denaturation, and DNA damage. ROS produce strand breaks in DNA, which trigger energy-consuming DNA repair mechanisms and activate the nuclear enzyme PARP, resulting in the depletion of its substrate NAD in vitro and a reduction in the rate of glycolysis. As NAD functions as a cofactor in glycolysis and the tricarboxylic acid cycle, NAD depletion leads to a rapid fall in intracellular ATP. This process has been termed “the PARP Suicide Hypothesis.” There is recent evidence that the activation of PARP may also play an important role in inflammation. 24,52-55 We demonstrate here that calpain inhibitor I attenuates the increase in PARP activity in lungs from carrageenan-treated rats (Figure 4D) ▶ and in joints from collagen-treated rats (Figure 10D) ▶ .
The promoter region of the murine and human COX-2 genes contains binding sites for NFκB. 56,57 The expression of the COX-2 gene is activated by oxidant stress, 58 and reactive oxygen intermediates cause the activation of NFκB, 59 suggesting that NFκB is one of the transcription factors involved. The increase in prostaglandin formation (COX activity) by murine osteoblasts (cell line MC3T3-E1) involves the activation of NFκB. 60
There is good evidence in this and in other models of inflammation that an enhanced formation of prostanoids after the induction of COX-2 contributes to the pathophysiology of local and chronic inflammation 61,62 and that selective inhibitors of COX-2 exert potent anti-inflammatory effects. 59-61 We demonstrate here that the increase in the levels of PGE2 caused by injection of carrageenan into the pleural cavity is reduced in the exudate of calpain inhibitor I-treated rats. The enhanced formation of PGE2 is secondary to the expression of COX-2 protein, as 1) there was no increase in the expression of COX-1 protein (detected by immunohistochemistry) after carrageenan injection and 2) selective inhibitors of COX-2 activity, including NS-398 (nimesulide) and SC-58125 (Celecoxib), markedly abolished the increase in PGE2 caused by the injection of carrageenan into the pleural space. 64,65 Thus we propose that calpain inhibitor I reduced the expression of COX-2 protein and activity caused by injection of carrageenan into the lung and joints from collagen-treated rats.
Inhibition of calpain I activity reduces the injury associated with ischemia reperfusion of the brain, 65-67 liver, 68 and heart. 69-72 The mechanism by which inhibitors of calpain activity protect tissues/organs against reperfusion injury is not entirely clear. Calpain acts on several substrates causing proteolytic modifications of proteins, which results in changes in their biochemical and morphological parameters, which are highly likely to be implicated in the pathological processes associated with ischemia reperfusion injury. Activation of calpain results in the proteolysis of several cellular proteins, mostly associated with the cellular membrane, including cytoskeletal proteins (eg, spectrin, fodrin, and microtubule-associated proteins), membrane proteins (eg, growth factor receptors, adhesion molecules, and ion transporters), enzymes (kinases, phosphatases and phospholipases), as well as cytokines and transcription factors (reviewed in ref 2 ). Although many of these may be implicated in mechanisms contributing to inflammation, the exact role of calpain activation in inflamed tissues has not been clearly defined. Thus in the present study we demonstrated for the first time that calpain inhibitor I reduced organ injury (lungs and joints) during inflammation.
In conclusion, this study demonstrates that the degree of acute and chronic inflammation is significantly attenuated in calpain inhibitor I-treated rats. The mechanisms of the anti-inflammatory effect of calpain inhibitor I are not entirely clear. It appears that calpain inhibitor I reduced the recruitment of neutrophils, the expression of iNOS and COX-2 protein and activity, and ultimately the degree of peroxynitrite formation and tissue injury. This effect of calpain inhibitor I is very likely secondary to the prevention by calpain inhibitor I of endothelial oxidant injury and, hence, to preservation of endothelial barrier function. These results support the view that the overproduction of reactive oxygen or nitrogen free radicals contributes to acute and chronic inflammation. Finally, we propose that calpain inhibitor I may be useful in the therapy of conditions associated with local or systemic inflammation.
Acknowledgments
We thank Fabio Giuffrè and Carmelo La Spada for their excellent technical assistance during this study, Mrs. Caterina Cutrona for secretarial assistance, and Miss Valentina Malvagni for editorial assistance with the manuscript. C. T. is a Senior Fellow of the British Heart Foundation (FS 96/018).
Footnotes
Address reprint requests to Dr. Salvatore Cuzzocrea, Institute of Pharmacology, School of Medicine, University of Messina, Torre Biologica, Policlinico Universitario, via C. Valeria, Gazzi, 98100 Messina, Italy. E-mail: salvator@www.unime.it.
Supported by a grant from the MURST 40%.
References
- 1.Bauerle PA, Henkel T: Function and activation of NF-kB in the immune system. Annu Rev Immunol 1994, 2:141-179 [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Karin M: Nuclear factor k B: a pivotal transcription factor in chronic inflammatory disease. N Engl J Med 1997, 336:1066-1071 [DOI] [PubMed] [Google Scholar]
- 3.Ruetten H, Thiemermann C: Effect of calpain inhibitor I, an inhibitor of the proteolysis of IκB, on the circulatory failure and multiple organ dysfunction caused by endotoxin in the rat. Br J Pharmacol 1997, 121:695-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melloni E, Pontremoli S: The calpains. Trends Neurosci 1989, 12:438-444 [DOI] [PubMed] [Google Scholar]
- 5.Saido TC, Sorimachi H, Suzuki K: Calpain: new perspectives in molecular diversity and physiological-pathophysiological involvement. FASEB J 1994, 8:814-822 [PubMed] [Google Scholar]
- 6.Wang KK, Yuen PW: Calpain inhibition: an overview of its therapeutic potential. Trends Pharmacol Sci 1994, 15:412-419 [DOI] [PubMed] [Google Scholar]
- 7.Baeuerle PA, Baltimore D: Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell 1988, 53:211-217 [DOI] [PubMed] [Google Scholar]
- 8.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U: Mutual regulation of the transcription activator NF-kappa B and its inhibitor, I kappa B. Proc Natl Acad Sci USA 1993, 90:2532-2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henkel T, Machleidt T, Alkalay I, Kroenke M, Benneriah Y, Bauerle PA: Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature 1993, 365:182-185 [DOI] [PubMed] [Google Scholar]
- 10.Siebenlist U, Franzoso G, Brown K: Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol 1994, 10:405-455 [DOI] [PubMed] [Google Scholar]
- 11.Griscavage JM, Wilk S, Ignarro LJ: Inhibitors of proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-kappa B. Proc Natl Acad Sci USA 1996, 93:3308-3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kengatharan M, De Kimpe SJ, Thiemermann C: Analysis of the signal transduction in the induction of nitric oxide synthase by lipoteichoic acid in macrophages. Br J Pharmacol 1996, 117:1163-1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milligan SA, Owens MW, Grisham MB: Inhibition of IkappaB-alpha and Ikappa-B-beta proteolysis by calpain inhibitor I blocks nitric oxide synthase synthesis. Arch Biochem Biophys 1996, 335:388-395 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Arakawa T, Ueda N, Yamamoto S: Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor-alpha-dependent induction of cyclooxygenase-2 in MC3T3–E1 cells. J Biol Chem 1995, 270:31315-31320 [DOI] [PubMed] [Google Scholar]
- 15.Crofford LJ, Tan B, McCarthy CJ, Hla T: Involvement of nuclear factor kappa B in the regulation of cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum 1997, 40:226-236 [DOI] [PubMed] [Google Scholar]
- 16.Moncada S, Palmer RMJ, Higgs EA: Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 1991, 43:109-142 [PubMed] [Google Scholar]
- 17.Nathan C: Nitric oxide as a secretory product of mammalian cells. FASEB J 1996, 6:3051-3064 [PubMed] [Google Scholar]
- 18.Cuzzocrea S, Zingarelli B, Gilard E, Hake P, Salzman AL, Szabó C: Anti-inflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic Biol Med 1998, 24:450-459 [DOI] [PubMed] [Google Scholar]
- 19.Youn YK, Lalonde C, Demling R: Use of antioxidant therapy in shock and trauma. Circ Shock 1991, 35:245-249 [PubMed] [Google Scholar]
- 20.Mccord J: Oxygen-derived free radicals. New Horizons 1993, 1:70-76 [PubMed] [Google Scholar]
- 21.Inoue S, Kawanishi S: Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett 1995, 371:86-88 [DOI] [PubMed] [Google Scholar]
- 22.Salgo MG, Bermudez E, Squadrito G, Pryor W: DNA damage and oxidation of thiols peroxynitrite causes in rat thymocytes. Arch Biochem Biophys 1995, 322:500-505 [DOI] [PubMed] [Google Scholar]
- 23.Zingarelli B, O’Connor M, Wong H, Salzman AL, Szabó C: Peroxynitrite-mediated DNA strand breakage activates poly-ADP ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol 1996, 156:350-358 [PubMed] [Google Scholar]
- 24.Szabó C, Viràg L, Cuzzocrea S, Scott GS, Hake P, O’Connor M, Zingarelli B, Ma Y, Hirsch R, Boiovin GP, Salzman AL, Kun E: Protection against peroxynitrite-induced fibroblast injury and arthritis development by inhibition of poly (ADP-ribose) synthetase. Proc Natl Acad Sci USA 1998, 9:3867-3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullane KM, Kraemer R, Smith B: Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods 1985, 14:157-167 [DOI] [PubMed] [Google Scholar]
- 26.Ohkawa H, Ohishi N, Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979, 95:351-358 [DOI] [PubMed] [Google Scholar]
- 27.Szabó C, Mitchell JA, Thiemermann C, Vane JR: Nitric oxide-mediated hyporeactivity to norepinephrine precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol 1993, 108:786-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heller B, Wang ZQ, Wagner EF, Radons J, Burkle A, Fehsel K, Burkart V, Kolb H: Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem 1995, 270:11176-11180 [DOI] [PubMed] [Google Scholar]
- 29.Sautebin L, Ialenti A, Ianaro A, Di Rosa M: Modulation by nitric oxide of prostaglandin biosynthesis in the rat. Br J Pharmacol 1995, 114:323-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 31.Sautebin L, Ianaro A, Rombolà L, Ialenti, Sala A, Di Rosa M: Cyclooxygenase-2-dependent generation of 8-epiprostaglandin F2 alpha by lipopolysaccharide-activated J774 macrophages. Inflam Res 1999, 48:503-508 [DOI] [PubMed] [Google Scholar]
- 32.Hughes C, Wolos JA, Giannini EH, Hirsh R: Induction of T helper cell hyporesponsiveness in an experimental model of autoimmunity by using nonmitogonic anti-CD3 monoclonal antibody. J Immunol 1994, 153:3319-3325 [PubMed] [Google Scholar]
- 33.Tomlinson A, Appleton I, Moore AL, Gilroy DW, Willis D, Mitchell JA, Willoughby DA: Cyclo-oxygenase and nitric oxide synthase isoforms in rat carrageenan-induced pleurisy. Br J Pharmacol 1994, 113:693-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vane J, Botting R: Inflammation and the mechanism of action of antiinflammatory drugs. FASEB J 1987, 1:89-96 [PubMed] [Google Scholar]
- 35.Ohishi S, Hayashi I, Hayashi M, Yamaki K, Utsunomiya I: Pharmacological demonstration of inflammatory mediators using experimental inflammatory models: rat pleurisy induced by carrageenan and phorbol myristate acetate. Dermatologica 1989, 179(Suppl 1):68-71 [DOI] [PubMed] [Google Scholar]
- 36.Da Motta JI, Cunha FQ, Vargaftig BB, Ferreira SH: Drug modulation of antigen-induced paw oedema in guinea-pigs: effects of lipopolysaccharide, tumour necrosis factor and leucocyte depletion. Br J Pharmacol 1994, 112:111-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson J, Sedgwick AD, Edwards JC, Lees P: A comparative study of the cellular, exudative and histological responses to carrageenan, dextran and zymosan in the mouse. Int J Tissue React 1999, 13:171-185 [PubMed] [Google Scholar]
- 38.Cuzzocrea S, Zingarelli B, Gilard E, Hake P, Salzman AL, Szabó C: Protective effect of melatonin in carrageenan-induced models of local inflammation. J Pineal Res 1997, 23:106-116 [DOI] [PubMed] [Google Scholar]
- 39.Salvemini D, Wang ZQ, Wyatt P, Bourdon DM, Marino MH, Manning PT, Currie MG: Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol 1996, 118:829-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tracey WR, Nakane M, Kuk J, Budzik G, Klinghofer V, Harris R, Carter G: The nitric oxide synthase inhibitor, l-NG-monomethylarginine, reduces carrageenan-induced pleurisy in the rat. J Pharmacol Exp Ther 1995, 273:1295-1299 [PubMed] [Google Scholar]
- 41.Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY: Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 1995, 375:408-411 [DOI] [PubMed] [Google Scholar]
- 42.Cuzzocrea S, Costantino G, Mazzon E, Caputi AP: Beneficial effects of raxofelast (IRFI 016), a new hydrophilic vitamin e-like antioxidant, in carrageenan-induced pleurisy. Br J Pharmacol 1999, 126:407-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pryor W, Squadrito G: The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol 1995, 268:L699-L772 [DOI] [PubMed] [Google Scholar]
- 44.Beckman JS, Beckman TW, Chen J, Marshall PA, Freman BA: Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990, 87:1620-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villa LM, Salas E, Darley-Usmar M, Radomski MW, Moncada S: Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc Natl Acad Sci USA 1994, 91:12383-12387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA: Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 1994, 269:26066-26075 [PubMed] [Google Scholar]
- 47.Xie Q, Kashiwabara Y, Nathan C: Role of transcription factor NF-κB/Rel in induction of nitric oxide. J Biol Chem 1994, 269:4705-4708 [PubMed] [Google Scholar]
- 48.Griscavage JM, Wilk S, Ignarro LJ: Serine and cysteine proteinase inhibitors prevent nitric oxide production by activated macrophages by interfering with transcription of inducible NO synthase gene. Biochem Biophys Res Commun 1995, 215:721-729 [DOI] [PubMed] [Google Scholar]
- 49.Ruetten H, Thiemermann C: Effect of calpain inhibitor I, an inhibitor of the proteolysis of I kappa B, on the circulatory failure and multiple organ dysfunction caused by endotoxin in the rat. Br J Pharmacol 1997, 121:695-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu ZQ, Kunimatsu M, Yang JP, Ozaki Y, Sasaki M, Okamoto T: Proteolytic processing of nuclear factor kappa B by calpain in vitro. FEBS Lett 1996, 385:109-113 [DOI] [PubMed] [Google Scholar]
- 51.Beckman JS: Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol 1996, 9:836-844 [DOI] [PubMed] [Google Scholar]
- 52.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, Van der Vliet A: Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 391:393-397 [DOI] [PubMed] [Google Scholar]
- 53.Cuzzocrea S, Caputi AP, Zingarelli B: Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy. Immunology 1998, 93:96-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuzzocrea S, Zingarelli B, Gilard E, Hake P, Salzman AL, Szabó C: Protective effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthase in carrageenan-induced models of local inflammation. Eur J Pharmacol 1998, 342:67-76 [DOI] [PubMed] [Google Scholar]
- 55.Szabó C, Lim LHK, Cuzzocrea S, Getting SJ, Zingarelli B, Flower RJ, Salzman AL, Perretti M: Inhibition of poly (ADP-ribose) synthetase exerts anti-inflammatory effects and inhibits neutrophil recruitment. J Exp Med 1997, 186:1041-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sirois J, Levy LO, Simmons DL, Richards JS: Characterization and hormonal regulation of the promoter of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Identification of functional and protein-binding regions. J Biol Chem 1993, 268:12199-12206 [PubMed] [Google Scholar]
- 57.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T: Structure of the human cyclooxygenase-2 gene. Biochem J 1994, 302:723-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB: Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α, and lipopolysaccharide. J Clin Invest 1995, 95:1669-1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schreck R, Rieber P, Baeuerle PA: Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 1991, 10:2247-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wadleigh DJ, Herschman HR: Transcriptional regulation of the cyclooxygenase-2 gene by diverse ligands in murine osteoblasts. Biochem Biophys Res Commun 1999, 264:865-870 [DOI] [PubMed] [Google Scholar]
- 61.Salvemini D, Manning P, Zweifel BS, Seibert K, Connor J, Currie MG, Needleman P, Masferrer JL: Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J Clin Invest 1995, 196:301-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sautebin L, Ialenvi A, Ianavo A, Di Rosa M: Relationship between nitric oxide and prostaglandins in carrageenin pleurisy. Biochem Pharmacol 1998, 55:1113-1118 [DOI] [PubMed] [Google Scholar]
- 63.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR: Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA 1993, 24:11693-11697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harada Y, Hatanaka K, Kawamura M, Saito M, Ogino M, Majima M, Ohno T, Yamamoto K, Taketani Y, Yamamoto S, Katori M: Role of prostaglandin H synthase-2 in prostaglandin E2 formation in rat carrageenin-induced pleurisy. Prostaglandin 1996, 51:19-33 [DOI] [PubMed] [Google Scholar]
- 65.Bartus RT, Elliott PJ, Hayward NJ, Dean RL, Harbeson S, Straub JA, Li Z, Powers JC: Calpain as a novel target for treating acute neurodegenerative disorders. Neurol Res 1995, 17:249-258 [DOI] [PubMed] [Google Scholar]
- 66.Wang K, Nath KR, Posner A: An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci USA 1996, 93:6687-6692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markgraf CG, Velayo NL, Johnson MP, McCarthy DR, Medhi S, Koehl JR, Chmielski PA, Linnik MD: Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke 1998, 29:152-158 [DOI] [PubMed] [Google Scholar]
- 68.Kohli V, Madden JF, Bentley RC, Clavien PA: Calpain mediates ischemic injury of the liver through modulation of apoptosis and necrosis. Gastroenterology 1999, 116:168-178 [DOI] [PubMed] [Google Scholar]
- 69.Atsma DE, Bastiaanse EM, Jerzewski A, Van der Valk LJ, Van der Laarse A: Role of calcium-activated neutral protease (calpain) in cell death in cultured neonatal rat cardiomyocytes during metabolic inhibition. Circ Res 1995, 76:1071-1078 [DOI] [PubMed] [Google Scholar]
- 70.Yoshida K, Inui M, Harada K, Saido TC, Sorimachi Y, Ishihara T, Kawashima S, Sobue K: Reperfusion of rat heart after brief ischemia induces proteolysis of calspectin by calpain. Circ Res 1995, 77:603-610 [DOI] [PubMed] [Google Scholar]
- 71.Yoshida K, Yamasaki Y, Kawashima S: Calpain activity alters rat myocardial subfractions after ischemia and reperfusion. Biochim Biophys Acta 1993, 1182:215-220 [DOI] [PubMed] [Google Scholar]
- 72.Iwamoto H, Miura T, Okamura T, Shirakawa K, Iwatate M, Kawamura S, Tatsuno H, Ikeda Y, Matsuzaki M: Calpain inhibitor I reduces infarct size and DNA fragmentation of myocardium in ischemic/reperfused hearts. J Cardiovasc Res 1999, 33:580-586 [DOI] [PubMed] [Google Scholar]