Abstract
Uveal melanoma is the most common primary eye cancer, yet its molecular pathogenesis is poorly understood. In this study, we investigated the immunohistochemical expression of proteins in the Rb and p53 tumor suppressor pathways in 33 uveal melanomas from enucleated eyes. Strong nuclear staining for Rb was present in most tumors. However, a few cases displayed weak nuclear staining and strong cytoplasmic staining (possibly indicating Rb mutation), and this aberrant staining correlated strongly with failed radiotherapy or thermotherapy before enucleation. Staining for cyclin D1 was positive in most tumors and was associated with advanced age and larger tumor size, which are both poor prognostic factors. Generally, immunostaining for p53 was weak (suggesting a lack of p53 mutations), although p53 positivity correlated strongly with staining for phosphorylated Rb, supporting the notion that inappropriate phosphorylation of Rb can induce p53. Strong immunostaining for MDM2, which can functionally block p53 activity, was observed in most tumors and correlated significantly with female sex. Strong cytoplasmic staining was observed for Bcl2, which can inhibit both p53-dependent and -independent apoptosis. We conclude that Rb and p53 are mutated infrequently in uveal melanoma, but their respective pathways may be functionally inactivated.
Uveal melanoma is the most common primary malignancy of the eye, yet little is known about its molecular pathogenesis. In contrast to cutaneous melanoma in which significant advances have been made in understanding the molecular etiology, 1 no genes or tumor suppressor pathways have been convincingly linked to uveal melanoma. In addition, most evidence suggests that uveal melanoma differs etiologically from its cutaneous counterpart. Cytogenetic changes commonly found in cutaneous melanoma include loss of 1p, 6q, and 10q, and gain of chromosome 7, 1 whereas the most common changes in uveal melanoma are loss of 3p and 6q, and gain of 6p and 8q. 2-4 In addition, the p16/INK4a tumor suppressor locus on chromosome 9p21 is frequently deleted in cutaneous melanoma, 1 but it is rarely altered in uveal melanoma. 5-8 This void in our understanding of uveal melanoma is complicated by the fact that this tumor is rarely familial and is not amenable to genetic linkage analysis. Therefore, one approach to examining the molecular pathogenesis of uveal melanoma is to study the Rb and p53 tumor suppressor pathways, both of which are commonly disrupted in cancer. 9,10
Mutational deregulation of the cell cycle is a hallmark of cancer. 11 The protein product of the retinoblastoma gene, Rb, is the prototype tumor suppressor gene by virtue of its central role in regulating the cell cycle. 12 The Rb gene is frequently mutated in certain cancers such as retinoblastoma, osteosarcoma, and small-cell lung cancer. 13-16 Further, in most other malignancies Rb is functionally inactivated by inappropriate phosphorylation resulting from deregulation of upstream effectors in the Rb pathway (eg, p16 inactivation or cyclin D overexpression). 9 Recently, we showed that Rb may be functionally inactivated in uveal melanoma as a result of cyclin D-dependent phosphorylation that blocks its tumor suppressor activity. 17 However, it is still unclear whether phosphorylation of Rb is associated with any clinicopathological features of uveal melanoma or whether it correlates with abnormalities in other cancer genes such as p53.
Apoptosis is an important mechanism for maintaining cellular homeostasis, preventing the accumulation of deleterious mutations, and averting malignant transformation. p53 is a key apoptotic regulator that is mutated in more than half of human cancers. 18 It can induce cell-cycle arrest or apoptosis in response to inappropriate cellular proliferation, DNA damage, or a number of other cellular insults. 18 For example, loss of Rb can trigger p53 to induce apoptosis as a means of eliminating cells that have lost proliferative control. 19 Because disruption of the p53 pathway can allow mutations to accumulate and to promote malignant transformation, there is a strong selective pressure in tumors to inactivate p53. These mutations may directly disrupt the p53 gene, or they may functionally inactivate p53 by perturbing upstream or downstream apoptotic regulators. 10 Although p53 mutations have been reported in uveal melanoma, 20 most studies have suggested that p53 mutations are rare in this cancer. 21,22 Other proteins in the p53 pathway, such as MDM2, have not been studied adequately in uveal melanoma.
Because the Rb and p53 pathways form an interconnected tumor suppressor network that is frequently mutated in cancer, our laboratory has been systematically investigating these pathways in uveal melanoma. In the present study, we analyzed the immunohistochemical expression patterns of key proteins in the Rb and p53 pathways in uveal melanoma. Rb and p53 were rarely mutated, but both seemed to be functionally inactivated by deregulation of other proteins in their respective pathways.
Materials and Methods
Tissue Samples
Thirty-three enucleated eyes harboring melanomas of the choroid and/or ciliary body were formalin-fixed and paraffin-embedded. Specimens were classified as predominantly spindle, mixed, or epithelioid according to the modified Callendar classification (Morton Smith, MD, University of Wisconsin, Madison, WI). To increase the statistical power of correlation analysis, the specimens were further ranked numerically by cytological severity, as previously described in other pathological tissues. 23 Two independent rankings were highly reproducible, with a correlation coefficient of 0.949. Clinical data (age, sex, eye, largest basal dimension, thickness by ultrasound, location, and previous treatment) were recorded from patient charts (Table 1) ▶ . Survival data were not included because the follow-up interval for most patients was insufficient for analysis.
Table 1.
Clinical, Pathological, and Immunohistochemical Features
| Clinical and pathologic features | Immunohistochemical staining (percent positive tumor cells) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Age | Sex | Eye | LBD (mm) | Location | Thickness (mm) | Pathology | Cytology rank | Prior treatment | Rb | phospho-Rb | p16 | Cyclin D1 | p53 | MDM2 | Bcl-2 |
| 1 | 56 | M | L | 16 | P | 9.2 | Mixed | 24 | None | 58 | 0.4 | 33 | 1 | 0 | 75 | 98 |
| 2 | 79 | F | L | 18 | A | 8.6 | Mixed | 19 | None | 71 | 1.7 | 82 | 58 | 0.2 | 78 | 98 |
| 3 | 28 | M | L | 11 | P | 7.2 | Mixed | 26 | None | 64 | 1.5 | 95 | 1 | 0 | 67 | 99 |
| 4 | 79 | M | L | 24 | P | 10.4 | Mixed | 21 | None | 61 | 0.9 | 97 | 2 | 0.1 | 84 | 99 |
| 5 | 67 | M | R | 16 | P | 2 | Epithelioid | 29 | None | 72 | 1.3 | 82 | 2 | 0 | 75 | 97 |
| 6 | 44 | M | L | 19 | A | 9.6 | Mixed | 16 | None | 74 | 1.1 | 77 | 48 | 0.1 | 65 | 98 |
| 7 | 68 | F | L | 22 | P | 5.5 | Mixed | 7 | None | 66 | ND | 91 | 58 | 0.1 | 68 | 99 |
| 8 | 63 | M | R | 16 | P | 6 | Spindle | 2 | PRT | 19 | 5.8 | 60 | 22 | 0.4 | 74 | 98 |
| 9 | 84 | F | L | 11 | P | 11.1 | Mixed | 6 | None | 71 | 0.9 | 94 | 41 | 0 | 71 | 99 |
| 10 | 66 | M | L | 18 | A | 7.1 | Mixed | 15 | None | 86 | 1.5 | 89 | 23 | 0 | 48 | 98 |
| 11 | 37 | M | R | 15 | A | 8.2 | Spindle | 5 | None | 82 | 0.2 | 70 | 2 | 0 | 65 | 98 |
| 12 | 65 | F | R | 20 | P | 12.6 | Spindle | 1 | None | 63 | 4 | 95 | 20 | 1.1 | 79 | 98 |
| 13 | 73 | M | R | 23 | A | 11.1 | Epithelioid | 32 | None | 59 | 4 | 98 | 3 | 0.1 | 58 | 97 |
| 14 | 70 | M | L | 15 | A | 8.2 | Mixed | 17 | None | 63 | 4.2 | 69 | 19 | 0.1 | 55 | 100 |
| 15 | 77 | M | L | 20 | A | 8 | Epithelioid | 30 | None | 81 | 2.5 | 70 | 64 | 0.1 | 66 | 95 |
| 16 | 79 | F | R | 14 | P | 3.1 | Mixed | 23 | None | 76 | 1.5 | 65 | 1 | 0 | 64 | 98 |
| 17 | 67 | M | L | 12 | P | 3 | Mixed | 9 | PRT | 78 | 7.5 | 95 | 6 | 0 | 76 | 98 |
| 18 | 44 | M | R | 19 | A | 12.2 | Mixed | 18 | None | 53 | 0.4 | 82 | 1 | 0 | 71 | 98 |
| 19 | 82 | M | L | 13 | P | 3.1 | Spindle | 4 | TTT | ND | ND | ND | ND | 0 | ND | 97 |
| 20 | 41 | M | R | 17 | P | 8.9 | Mixed | 20 | None | 24 | 3.6 | 99 | 1 | 0 | 14 | 98 |
| 21 | 51 | F | L | 9 | P | 6.5 | Epithelioid | 31 | None | 64 | 3.1 | 95 | 13 | 0.2 | 75 | 100 |
| 22 | 40 | M | L | 12 | P | 7.7 | Spindle | 3 | None | 68 | 3.8 | 68 | 10 | 0.1 | 64 | 99 |
| 23 | 78 | F | L | 19 | A | 10 | Mixed | 14 | None | 81 | 0.6 | 47 | 60 | 0.1 | 64 | 100 |
| 24 | 52 | F | R | 14 | P | 8.5 | Mixed | ND | None | ND | ND | ND | 1 | 0.1 | 68 | 99 |
| 25 | 76 | F | R | 8 | A | 5 | Mixed | 11 | None | ND | ND | ND | 9 | 0 | 67 | 97 |
| 26 | 75 | M | R | 18 | A | 11 | Mixed | 12 | None | 66 | 3.3 | 80 | 43 | 0 | 64 | 99 |
| 27 | 65 | M | L | 24 | A | 6.2 | Mixed | 13 | None | 73 | 2.3 | 74 | 8 | 0.2 | 63 | 99 |
| 28 | 79 | M | R | 10 | P | 3.5 | Mixed | 25 | TTT | 34 | 1.3 | 31 | 6 | 0.1 | 63 | 99 |
| 29 | 55 | F | L | 20 | A | 10 | Mixed | 27 | None | 92 | 5.6 | 92 | 2 | 0.9 | 78 | 99 |
| 30 | 54 | M | L | 10 | P | 4.8 | Mixed | 22 | None | 67 | 0.6 | 63 | 2 | 0.2 | 66 | 97 |
| 31 | 64 | M | L | 8 | P | 3.1 | Mixed | 28 | None | 73 | 0.2 | 39 | 49 | 0.1 | 66 | 98 |
| 32 | 47 | M | L | 18 | P | 10.8 | Mixed | 10 | PRT | 10 | 0.2 | 67 | 18 | 0.2 | 67 | 99 |
| 33 | 63 | F | R | 9 | P | 5 | Mixed | 8 | None | 76 | 2.9 | 100 | 1 | 0 | 74 | 99 |
M, male; F, female; L, left eye; R, right eye; LBD, largest basal tumor dimension; A, anterior; P, posterior; PRT, plaque radiotherapy (brachytherapy); TTT, transpupillary thermotherapy; ND, not done.
Immunohistochemistry
Method
Immunohistochemistry was performed using the streptavidin-biotin method with the Vector ABC Elite kit (Vector Laboratories, Inc., Burlingame, CA). Nuclear fast red was used for counterstain. Four-micron sections were obtained, deparaffinized, rehydrated with ethanol, and treated with 0.3% hydrogen peroxide and methanol to inhibit endogenous peroxidase activity. Heat-induced antigen retrieval was performed using microwave treatment in citrate buffer (Rb, phospho-Rb, p16, p53, and MDM2) or EDTA (cyclin D1, Bcl2) for 15 minutes. Primary antibodies were applied at 4°C overnight.
Antibodies
Antibodies against Rb (C-15; 1:50 dilution) and p16 (F-12; 1:75 dilution) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The phospho-Rb-serine 807/811 antibody (1:25 dilution; hereafter referred to as “phospho-Rb”) was obtained from New England Biolabs, Inc. (Beverly, MA). Cyclin D1 (NCL-CYCLIN D1-GM; 1:40 dilution) and MDM2 (NCL-MDM2;1:30 dilution) antibodies were obtained from Novocastra Laboratories Ltd. (Newcastle-Upon-Tyne, UK). The p53 antibody (clone 1801; 1:80 dilution) was obtained from Biogenics (Napa, CA). The Bcl2 antibody (1:500) was obtained from DAKO (Glostrup, Denmark). Positive controls included: normal choroidal melanocytes (Rb and p16), a mantle cell lymphoma (cyclin D1), p16-null U20S osteosarcoma cells that hyperphosphorylate Rb (phospho-Rb), and a breast cancer specimen (p53). Negative controls included: Rb-null C33A cervical carcinoma cells (Rb), U2OS cells (p16), and normal choroidal melanocytes in the enucleated eyes (phospho-Rb, cyclin D1, p53, MDM2, and Bcl2). The secondary antibody alone was used as an additional negative control for all antibodies.
Quantitation
The percent positive cells for each antibody was estimated by counting at least 200 cells in at least eight ×40 fields for each specimen. Two independent analyses were performed, and in most cases at least two sections from each tumor were analyzed.
Statistical Analysis
Clinicopathological and immunohistochemical data were analyzed for correlation by Pearson correlation coefficients. Students t-test was used to confirm comparisons of binary variables. Significance was defined as P < 0.05.
Results
Immunohistochemistry of Rb Pathway Proteins
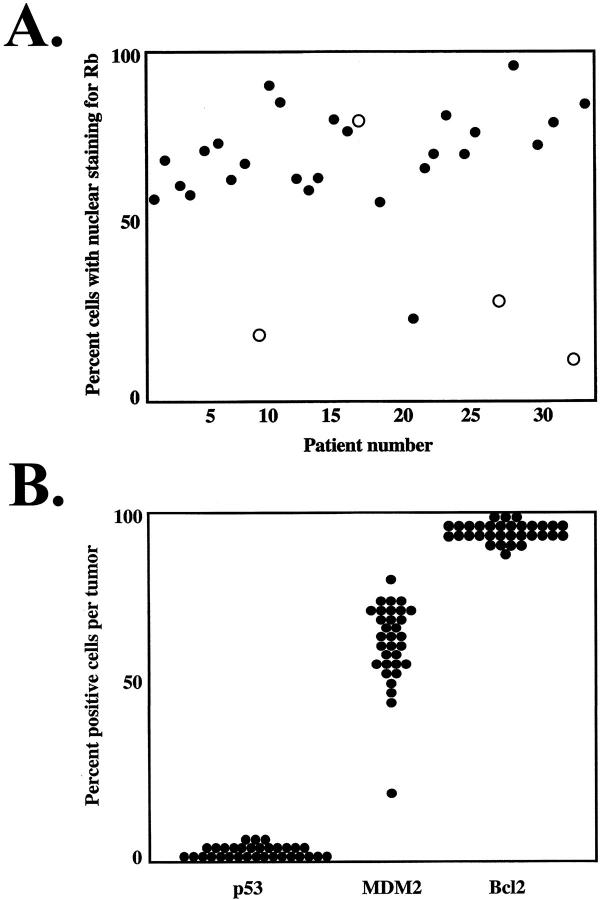
Using an antibody that detects all phosphorylated forms of Rb, most of the tumors had strong nuclear staining for Rb (Table 1 ▶ ; Figure 1A ▶ ). However, four of the tumors had fewer cells (10 to 34%) with nuclear staining and instead had strong cytoplasmic staining for Rb, suggesting that Rb mutations affecting nuclear localization of the protein may have occurred in these tumors (Figure 1B ▶ and Figure 2A ▶ ). Interestingly, three of these four tumors had failed radiotherapy or thermotherapy before enucleation (Table 1) ▶ . Using the phospho-Rb antibody, 0.1 to 1% of tumor cells were positive, whereas all normal choroidal melanocytes were negative (Table 1) ▶ . We previously showed that this phosphorylation of Rb can block its tumor suppressor activity. 17 Strong immunostaining (≥20% positive cells) for p16 was observed in all cases (Table 1) ▶ . Cyclin D1 expression was variable; 41% of tumors were considered strongly positive (≥20% positive cells) (Table 1) ▶ .
Figure 1.
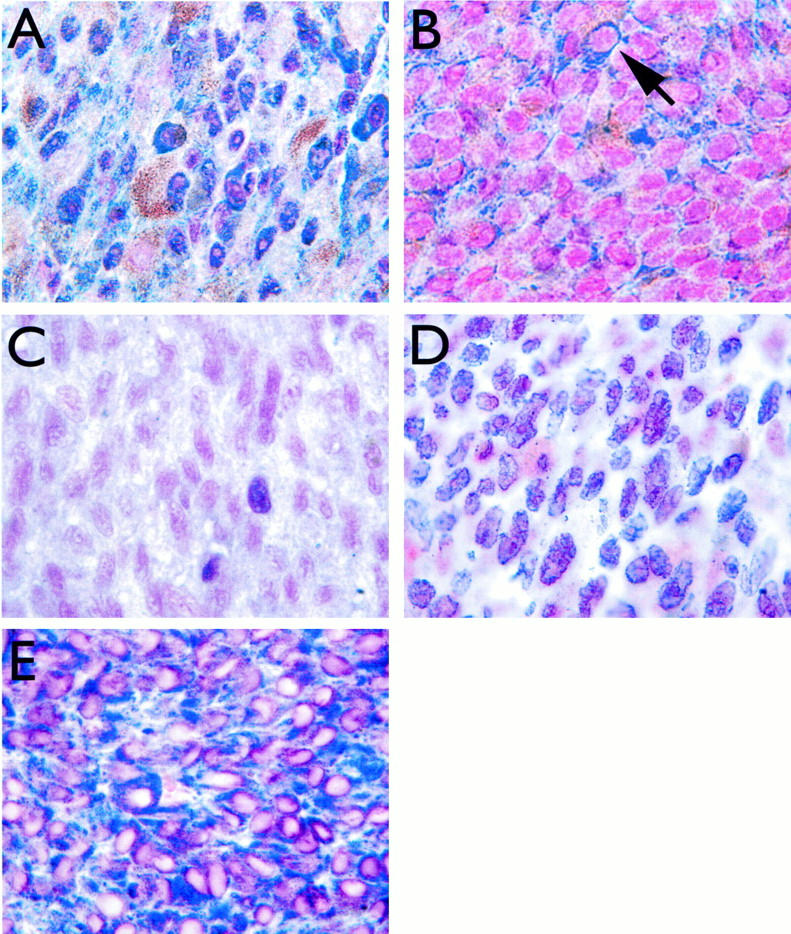
Immunohistochemical staining for Rb, p53, MDM2, and Bcl2. A: Strong nuclear staining for Rb in a uveal melanoma with no local treatment before enucleation. B: Staining for Rb in a uveal melanoma that failed brachytherapy before enucleation (patient no. 32). Note absence of nuclear staining and strong cytoplasmic staining (arrow). C: Weak staining for p53 in a representative uveal melanoma. D: Staining for MDM2 in a representative uveal melanoma, demonstrating strong nuclear expression in most cells. E: Staining for Bcl2 in a representative uveal melanoma, demonstrating strong cytoplasmic expression in most cells. Streptavidin-biotin-peroxidase method, Vector SG peroxidase substrate, and nuclear fast red counterstain (see Materials and Methods). Original magnification, ×100.
Figure 2.
Scatter plots of immunohistochemical staining patterns. A: Nuclear staining for Rb in all tumors examined. Tumors that failed brachytherapy or thermotherapy before enucleation are noted with open circles. B: Immunohistochemical staining patterns for p53, MDM2, and Bcl2.
Immunohistochemistry of p53 Pathway Proteins
Immunostaining for p53 was undetectable in 13 tumors and was weak in the other 19 tumors (overall range, 0 to 1.1% positive cells) (Table 1 ▶ ; Figures 1C and 2B ▶ ▶ ). Most normal choroidal melanocytes had weak or undetectable staining for p53. Strong nuclear staining for MDM2 (≥20% positive cells) was observed in 31 (97%) of 32 specimens (Table 1 ▶ ; Figures 1B and 2B ▶ ▶ ). Most normal choroidal melanocytes had weak or undetectable staining for MDM2. Strong cytoplasmic staining for Bcl2 was found in all tumors, with ≥95% positive cells in each specimen (Table 1 ▶ ; Figures 1C and 2B ▶ ▶ ). Most normal choroidal melanocytes had weak or undetectable staining for Bcl2.
Statistical Analysis
Statistically significant correlations are summarized in Table 2 ▶ . A strong inverse correlation was observed between Rb expression and a history of failed brachytherapy or thermotherapy before enucleation (r = −0.599, P = 0.005) (Figure 2A) ▶ . Significant correlations between proteins included: p53 versus phospho-Rb (r = 0.497, P = 0.006), p53 versus MDM2 (r = 0.393, P = 0.026), and p16 versus phospho-Rb (r = 0.385, P = 0.039). Significant associations between protein immunostaining and clinicopathological features included: MDM2 versus female sex (r = 0.476, P = 0.006), cyclin D1 versus advanced age (r = 0.392, P = 0.026), and p16 versus largest basal tumor dimension (r = 0.382, P = 0.045). In addition, there was a nonsignificant trend for increased p53 expression in thicker tumors (r = 0.312, P = 0.077), and increased cyclin D1 expression among anterior tumors (r = 0.300, P = 0.096).
Table 2.
Significant Correlations between Clinicopathological and Immunohistochemical Features
| Correlations | Pearson correlation coefficient, r | P value |
|---|---|---|
| Rb vs. Prior Treatment | −0.599 | 0.005 |
| p53 vs. phospho-Rb | 0.497 | 0.006 |
| MDM2 vs. female sex | 0.476 | 0.006 |
| p53 vs. MDM2 | 0.393 | 0.026 |
| cyclin D1 vs. age | 0.392 | 0.026 |
| phospho-Rb vs. p16 | 0.385 | 0.039 |
| p16 vs. LBD | 0.382 | 0.045 |
LBD, largest basal tumor dimension.
Discussion
In this study, we provide evidence that both the Rb and p53 pathways are disrupted in uveal melanoma. Rb was expressed in all of the tumors, suggesting that Rb mutations are uncommon in this cancer. However, cytoplasmic staining for Rb was observed in conjunction with reduced nuclear expression (suggestive of Rb mutation) in several tumors that had failed previous brachytherapy or thermotherapy. The local failure rate after brachytherapy for uveal melanoma is ∼15%, and resistant tumors are highly metastatic with a poor prognosis. 24 Our finding suggests that mutational inactivation of Rb, although uncommon in primary uveal melanomas, may play a role in the emergence of radioresistance. Further, this finding suggests that mutational inactivation of Rb may provide some additional advantage to the tumor beyond that provided by functional inactivation of Rb as a result of phosphorylation.
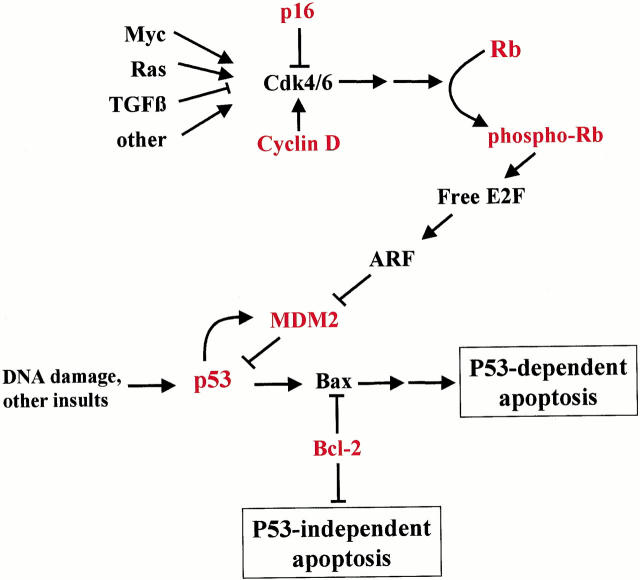
As we previously reported, Rb is often phosphorylated at serine-807 and serine-811 in uveal melanomas, and this phosphorylation can block the repressor function of Rb. 17 Further, we show here that phospho-Rb correlates strongly with increased expression of p53. One explanation for this finding is that phosphorylation of Rb liberates E2Fs, which can then trigger the ARF-MDM2 axis to up-regulate p53 levels (Figure 3) ▶ . 25 The phosphorylation of Rb that we have observed in uveal melanoma may be because of disruption of upstream regulators in the Rb pathway (Figure 3) ▶ . Mutational inactivation of p16/INK4a can allow inappropriate phosphorylation of Rb by allowing unopposed cyclin D-cdk4/6 activity. 26 As we previously reported, 17 there was no evidence for p16/INK4a inactivation in uveal melanoma, although mutation of this gene seems to play an important role in cutaneous melanoma. 1 Consistent with this observation, previous DNA sequence analysis revealed no mutations of the p16/INK4a gene in uveal melanoma. 27 Overexpression of D-type cyclins can also cause inappropriate phosphorylation of Rb by constitutively activating endogenous cdk4 and cdk6. 9 We found increased cyclin D1 immunostaining in many of the tumors as compared to surrounding normal choroidal melanocytes, and this staining was associated with advanced patient age and anterior tumor location, both of which are poor prognostic factors for survival. 28 Similarly, other workers have reported correlations between cyclin D1 expression and epithelioid cell type, anterior tumor location, and increased growth fraction. 29 It will be of interest to determine whether cyclin D1 expression is a significant prognostic factor when longer follow-up is available in this cohort of patients. Cyclin D1 overexpression may be because of gene amplification, chromosomal translocations, or disruption of upstream regulatory pathways (Figure 3) ▶ . 9 For example, c-myc can induce expression of D-type cyclins, 30-32 and this proto-oncogene is commonly expressed in uveal melanomas. 33,34 Further work is needed to determine whether c-myc may be responsible for deregulating cyclin D1 in these tumors.
Figure 3.
Diagram illustrating how the Rb and p53 pathways are linked to form a complex tumor suppressor network. Rb regulates the cell cycle by arresting cells in G1 phase. Rb can be temporarily inactivated to allow cell division by phosphorylation of the protein. Pathological inactivation of the Rb pathway can result from direct mutation of the Rb gene, or from inappropriate phosphorylation of Rb because of disruption of upstream regulators. The ARF-MDM2 axis links the Rb and p53 pathways and can trigger apoptosis as a result of uncontrolled proliferation. Thus, most cancers acquire mutations in both pathways during malignant progression to evade cell cycle control and apoptosis. Proteins in red were analyzed in this study. See text for details.
p53 is the most commonly mutated tumor suppressor in human cancer and is disrupted in >50% of tumors. 35 However, we found no evidence for p53 mutations in uveal melanoma, similar to other studies in both uveal and cutaneous melanoma. 21,22,36 Many p53(+) tumors are functionally p53(−) as a result of mutations in upstream or downstream regulators of the p53 pathway (Figure 3) ▶ . p53 induces expression of MDM2, which in turn interacts with p53 and targets it for degradation, thereby establishing a feedback loop that maintains p53 at low levels under normal conditions. 10,37 Overexpression of MDM2, which has been observed in some cancers, can disrupt this regulatory mechanism and block p53 function under conditions in which the cell should commit to apoptosis. 38 We found strong immunostaining for MDM2 in most of the uveal melanomas, whereas normal choroidal melanocytes had weak or undetectable staining. Consistent with our findings, another group recently demonstrated MDM2 expression in uveal melanoma and found a correlation with poor clinical outcome. 39 MDM2 overexpression may result from amplification, enhanced translation, and other mechanisms, 40 and further work will be needed to determine which of these mechanisms is involved in uveal melanoma.
The Bcl2 family of proteins are important downstream apoptotic regulators, and the interaction of pro- and anti-apoptotic Bcl2 family members can determine the cellular commitment to apoptosis. 41 Bcl2 is anti-apoptotic and can function as a proto-oncogene when inappropriately overexpressed. In contrast, Bax is a pro-apoptotic family member and is a transcriptional target of p53. 42 Deregulation of Bcl2 can promote tumorigenesis by blocking both p53-dependent and -independent apoptosis (Figure 3) ▶ . 43 We found strong immunostaining for Bcl2 in all of the uveal melanomas, similar to findings of other investigators. 21,44 Interestingly, whereas Bcl2 overexpression is the most common molecular abnormality reported to date for uveal melanoma, this alteration seems to be uncommon in cutaneous melanoma. 45
In summary, we have provided evidence for functional abnormalities in both the Rb and p53 pathways in uveal melanoma. These two pathways form an interconnected tumor suppressor network that regulates cellular proliferation (Figure 3) ▶ . A major link between these pathways is the ARF-MDM2 axis. 25 Active Rb is normally bound to E2Fs in a repressor complex. 46 Phosphorylation of Rb disrupts this interaction and can lead to release of free E2Fs, which may then induce ARF, the alternative reading frame of the p16INK4a locus. ARF directly antagonizes MDM2, allowing the accumulation of p53 and induction of growth arrest or apoptosis. Thus, interconnections between the Rb and p53 pathways provide a formidable barrier against tumorigenesis, and indeed many tumors acquire mutations in both pathways during malignant progression. These results provide new insights into the molecular pathogenesis of uveal melanoma and may be useful in the development of novel therapeutic agents.
Acknowledgments
We thank Belinda McMahan in the Immunomorphology Core Laboratory for performing immunohistochemistry; Dr. Mae Gordon in the Biostatistics Core Facility for statistical analysis; and Dr. Morton Smith, University of Wisconsin, for assistance in obtaining tumor specimens.
Footnotes
Address reprint requests to J. William Harbour, MD, Box 8069, 660 S. Euclid Ave., Washington University School of Medicine, St. Louis, MO 63110. E-mail: harbour@vision.wustl.edu.
Supported by National Eye Institute grant K08 EY0038201 (to J. W. H.) and Research to Prevent Blindness, Inc.Career Development Award (to J. W. H.).
References
- 1.Chin L, Merlino G, DePinho RA: Malignant melanoma: modern black plague and genetic black box. Genes Dev 1998, 12:3467-3481 [DOI] [PubMed] [Google Scholar]
- 2.Prescher G, Bornfeld N, Friedrichs W, Seeber S, Becher R: Cytogenetics of twelve cases of uveal melanoma and patterns of nonrandom anomalies and isochromosome formation. Cancer Genet Cytogenet 1995, 80:40-46 [DOI] [PubMed] [Google Scholar]
- 3.Sisley K, Parsons MA, Garnham J, Potter AM, Curtis D, Rees RC, Rennie IG: Association of specific chromosome alterations with tumour phenotype in posterior uveal melanoma. Br J Cancer 2000, 82:330-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speicher MR, Prescher G, du Manoir S, Jauch A, Horsthemke B, Bornfeld N, Becher R, Cremer T: Chromosomal gains and losses in uveal melanomas detected by comparative genomic hybridization. Cancer Res 1994, 54:3817-3823 [PubMed] [Google Scholar]
- 5.Singh AD, Wang MX, Donoso LA, Shields CL, De Potter P, Shields JA: Genetic aspects of uveal melanoma: a brief review. Semin Oncol 1996, 23:768-772 [PubMed] [Google Scholar]
- 6.Wang X, Egan KM, Gragoudas ES, Kelsey KT: Constitutional alterations in p16 in patients with uveal melanoma. Melanoma Res 1996, 6:405-410 [DOI] [PubMed] [Google Scholar]
- 7.Soufir N, Bressac-de Paillerets B, Desjardins L, Levy C, Bombled J, Gorin I, Schlienger P, Stoppa-Lyonnet D: Individuals with presumably hereditary uveal melanoma do not harbour germline mutations in the coding regions of either the P16INK4A, P14ARF or cdk4 genes. Br J Cancer 2000, 82:818-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohta M, Nagai H, Shimizu M, Rasio D, Berd D, Mastrangelo M, Singh AD, Shields JA, Shields CL, Croce CM, et al: Rarity of somatic and germline mutations of the cyclin-dependent kinase 4 inhibitor gene, CDK4I, in melanoma. Cancer Res 1994, 54:5269-5272 [PubMed] [Google Scholar]
- 9.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 10.Prives C, Hall PA: The p53 pathway. J Pathol 1999, 187:112-126 [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 2000, 100:57-70 [DOI] [PubMed] [Google Scholar]
- 12.Bartek J, Bartkova J, Lukas J: The retinoblastoma protein pathway in cell cycle control and cancer. Exp Cell Res 1997, 237:1-6 [DOI] [PubMed] [Google Scholar]
- 13.Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP: A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 1986, 323:643-646 [DOI] [PubMed] [Google Scholar]
- 14.Fung YK, Murphree AL, T’Ang A, Qian J, Hinrichs SH, Benedict WF: Structural evidence for the authenticity of the human retinoblastoma gene. Science 1987, 236:1657-1661 [DOI] [PubMed] [Google Scholar]
- 15.Lee WH, Bookstein R, Hong F, Young LJ, Shew JY, Lee EY: Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science 1987, 235:1394-1399 [DOI] [PubMed] [Google Scholar]
- 16.Harbour JW, Lai SL, Whang-Peng J, Gazdar AF, Minna JD, Kaye FJ: Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science 1988, 241:353-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brantley MA, Harbour JW: Inactivation of Rb in uveal melanoma by phosphorylation of sites in the C-terminal region. Cancer Res 2000, 60:4320-4323 [PMC free article] [PubMed] [Google Scholar]
- 18.Levine AJ: p53, the cellular gatekeeper for growth and division. Cell 1997, 88:323-331 [DOI] [PubMed] [Google Scholar]
- 19.Symonds H, Krall L, Remington L, Saenz-Robles M, Lowe S, Jacks T, Van Dyke T: p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 1994, 78:703-711 [DOI] [PubMed] [Google Scholar]
- 20.Tobal K, Warren W, Cooper CS, McCartney A, Hungerford J, Lightman S: Increased expression and mutation of p53 in choroidal melanoma. Br J Cancer 1992, 66:900-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chana JS, Wilson GD, Cree IA, Alexander RA, Myatt N, Neale M, Foss AJ, Hungerford JL: c-myc, p53, and Bcl-2 expression and clinical outcome in uveal melanoma. Br J Ophthalmol 1999, 83:110-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishore K, Ghazvini S, Char DH, Kroll S, Selle J: p53 gene and cell cycling in uveal melanoma. Am J Ophthalmol 1996, 121:561-567 [DOI] [PubMed] [Google Scholar]
- 23.Boitnott JK, Maddrey WC: Alcoholic liver disease: I. Interrelationships among histologic features and the histologic effects of prednisolone therapy. Hepatology 1981, 1:599-612 [DOI] [PubMed] [Google Scholar]
- 24.Harbour JW, Char DH, Kroll S, Quivey JM, Castro J: Metastatic risk for distinct patterns of postirradiation local recurrence of posterior uveal melanoma. Ophthalmology 1997, 104:1785-1792 [DOI] [PubMed] [Google Scholar]
- 25.Prives C: Signaling to p53: breaking the MDM2–p53 circuit. Cell 1998, 95:5-8 [DOI] [PubMed] [Google Scholar]
- 26.Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, III, Johnson BE, Skolnick MH: A cell cycle regulator potentially involved in genesis of many tumor types. Science 1994, 264:436-440 [DOI] [PubMed] [Google Scholar]
- 27.Merbs SL, Sidransky D: Analysis of p16 (CDKN2/MTS-1/INK4A) alterations in primary sporadic uveal melanoma. Invest Ophthalmol Vis Sci 1999, 40:779-783 [PubMed] [Google Scholar]
- 28.Mooy CM, De Jong PT: Prognostic parameters in uveal melanoma: a review. Surv Ophthalmol 1996, 41:215-228 [DOI] [PubMed] [Google Scholar]
- 29.Coupland SE, Bechrakis N, Schuler A, Anagnostopoulos I, Hummel M, Bornfeld N, Stein H: Expression patterns of cyclin D1 and related proteins regulating G1-S phase transition in uveal melanoma and retinoblastoma. Br J Ophthalmol 1998, 82:961-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Roger I, Kim SH, Griffiths B, Sewing A, Land H: Cyclins D1 and D2 mediate myc-induced proliferation via sequestration of p27(Kip1) and p21(Cip1). EMBO J 1999, 18:5310-5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateyak MK, Obaya AJ, Sedivy JM: c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol 1999, 19:4672-4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, Reed S, Sicinski P, Bartek J, Eilers M: Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J 1999, 18:5321-5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chana JS, Cree IA, Foss AJ, Hungerford JL, Wilson GD: The prognostic significance of c-myc oncogene expression in uveal melanoma. Melanoma Res 1998, 8:139-144 [DOI] [PubMed] [Google Scholar]
- 34.Royds JA, Sharrard RM, Parsons MA, Lawry J, Rees R, Cottam D, Wagner B, Rennie IG: C-myc oncogene expression in ocular melanomas. Graefes Arch Clin Exp Ophthalmol 1992, 230:366-371 [DOI] [PubMed] [Google Scholar]
- 35.Harris CC, Hollstein M: Clinical implications of the p53 tumor-suppressor gene. N Engl J Med 1993, 329:1318-1327 [DOI] [PubMed] [Google Scholar]
- 36.Florenes VA, Oyjord T, Holm R, Skrede M, Borresen AL, Nesland JM, Fodstad O: TP53 allele loss, mutations and expression in malignant melanoma. Br J Cancer 1994, 69:253-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haupt Y, Maya R, Kazaz A, Oren M: Mdm2 promotes the rapid degradation of p53. Nature 1997, 387:296-299 [DOI] [PubMed] [Google Scholar]
- 38.Florenes VA, Maelandsmo GM, Forus A, Andreassen A, Myklebost O, Fodstad O: MDM2 gene amplification and transcript levels in human sarcomas: relationship to TP53 gene status. J Natl Cancer Inst 1994, 86:1297-1302 [DOI] [PubMed] [Google Scholar]
- 39.Coupland SE, Anastassiou G, Stang A, Schilling H, Anagnaostopoulos I, Bornfeld N, Stein H: The prognostic value of cyclin D1, p53, and MDM2 protein expression in uveal melanoma. J Pathol 2000, 191:120-126 [DOI] [PubMed] [Google Scholar]
- 40.Landers JE, Cassel SL, George DL: Translational enhancement of mdm2 oncogene expression in human tumor cells containing a stabilized wild-type p53 protein. Cancer Res 1997, 57:3562-3568 [PubMed] [Google Scholar]
- 41.Gross A, McDonnell JM, Korsmeyer SJ: BCL-2 family members and the mitochondria in apoptosis. Genes Dev 1999, 13:1899-1911 [DOI] [PubMed] [Google Scholar]
- 42.Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T: Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 1997, 385:637-640 [DOI] [PubMed] [Google Scholar]
- 43.Basu A, Haldar S: The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod 1998, 4:1099-1109 [DOI] [PubMed] [Google Scholar]
- 44.Jay V, Yi Q, Hunter WS, Zielenska M: Expression of bcl-2 in uveal malignant melanoma. Arch Pathol Lab Med 1996, 120:497-498 [PubMed] [Google Scholar]
- 45.Radhi JM: Malignant melanoma arising from nevi, p53, p16, and Bcl-2: expression in benign versus malignant components. J Cutan Med Surg 1999, 3:293-297 [DOI] [PubMed] [Google Scholar]
- 46.Harbour JW, Dean DC: Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol 2000, 2:E65-E67 [DOI] [PubMed] [Google Scholar]