Abstract
To dynamically investigate the long-term response of an ischemic lesion in rat brain to the administration of sildenafil, male Wistar rats subjected to embolic stroke were treated with sildenafil (n=11) or saline (n=10) at a dose of 10mg/Kg administered subcutaneously 24-hours after stroke and daily for an additional 6-days. Magnetic resonance images were acquired and functional performance was measured in all animals at 1-day, 2-days and weekly for 6-weeks post-stroke. All rats were sacrificed 6-weeks after stroke and endothelial barrier antigen immunostaining was employed for morphological analysis and quantification of cerebral vessels. Map-ISODATA was computed from T1, T2 and T1sat maps. ISODATA derived tissue signatures characterize the degree of ischemic injury. Based on the map-ISODATA calculated at 6-weeks, the ischemic lesion for each animal was divided into two specific regions, the ischemic boundary and ischemic core. The temporal profiles of cerebral blood flow (CBF) and tissue signature were retrospectively tracked in these two regions and were compared with histological evaluation and functional outcome. After 1-week of sildenafil treatment, the ischemic lesion exhibited two significantly different regions, with higher CBF level and correspondingly, lower tissue signature value in the boundary region than in the core region. Sildenafil treatment did not significantly reduce the lesion size, but did enhance angiogenesis. Functional performance was significantly increased after sildenafil treatment compared with the control group. Administration of sildenafil to rats with embolic stroke enhances angiogenesis and selectively increases the CBF level in the ischemic boundary, and improves neurological functional recovery compared to saline-treated rats.
Keywords: Sildenafil, angiogenesis, CBF, tissue signature, functional recovery, MRI
1. Introduction
As a vasodilator with good hemodynamic effects, sildenafil has been successfully used in the treatment of patients with pulmonary arterial hypertension (Sheth et al., 2005; Lopez-Guarch et al., 2004; Kataoka et al., 2004; Rosenkranz et al., 2004; Michelakis et al., 2003; Watanabe et al., 2002) and cardiovascular disease (Jackson et al., 2005; Cheitlin et al., 1999). By selectively inhibiting phosphodiesterase type-5 (PDE-5) and thus effectively reducing the breakdown of cGMP, sildenafil administration can markedly improve pulmonary and cardiac functional capacity, and hemodynamics (du Toit et al., 2005; Traverse et al., 2000).
Sildenafil also significantly increases cortical levels of cGMP in ischemic rat brain (Zhang et al., 2005; Zhang et al., 2002) and transiently elevates localized cerebral blood flow (CBF) in non-ischemic rat brain (Zhang et al., 2002). However, the long-term, especially, dynamic evolution of CBF in the ischemic brain after sildenafil intervention remains largely unexplored. In the present study, we therefore dynamically and non-invasively investigated the long-term (up to 6 weeks) response of ischemic rat brain to sildenafil treatment using magnetic resonance imaging (MRI). Our data indicate that treatment with sildenafil leads to a significantly increased CBF level in the ischemic boundary area at the late stage after stroke (6-week) compared to the control group.
2. Experimental Procedure
All experimental procedures have been approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Animal model and experimental groups
Male Wistar rats weighing 300 to 350g (12 to 16 weeks) were subjected to embolic stroke by placement of an embolus at the origin of the middle cerebral artery (MCA) (Zhang et al., 1997). Rats with stroke were randomly assigned to sildenafil-treated (n=11) and control (n=10) groups. Sildenafil (Viagra, Pfizer Inc) was administered at a dose of 10mg/Kg subcutaneously to rats in the treated group 24 hours after MCA occlusion and daily for an additional 6 days. Blood pressure was measured before and after sildenafil administration. The selected dose has previously proved effective for this model (Zhang et al., 2005). Rats in the control group were treated with the same volume of saline as in the treated group. All rats were sacrificed 6 weeks after stroke.
MRI measurements and data processing
MRI measurements were performed using a 7T, 20-cm-bore superconducting magnet (Magnex Scientific, Abingdon, U.K.) interfaced to a Bruker console (Bruker Company, Boston, U.S.). The detailed procedure for the experimental setup has been previously described (Li et al., 2006; Li et al., 2005). A complete set of MR images, including T1, T2, T1sat, and CBF was acquired for all animals at approximately 1 day, 2 days and weekly for 6 weeks after onset of stroke. The detailed MRI methods employed to measure T1, T2, T1sat and CBF images, and to obtain the corresponding maps have been described elsewhere (Jiang et al., 2005).
ISODATA (Iterative Self-Organizing Data Analysis Technique Algorithm) is a data processing technique based on cluster analysis in the feature space (Ball and Hall, 1967). By using the acquired MR weighted-images or fitted-maps, ISODATA can objectively identify normal and abnormal cerebral tissue and automatically demarcate the different ischemia-affected areas in the brain by assigning each segment a specific signature value according to the degree that this tissue segment deviates from normal tissue (Jacobs et al, 2001; Jacobs et al, 2000). Map-ISODATA derived tissue signature is highly correlated with alive neuron counting (R = −0.82) and damaged cell counting (R = 0.81) histologically (Li et al., 2005; Ding et al., 2006), indicating that the signature characterized by map-ISODATA reflects and quantitatively grades the degree of tissue damage in the ischemic area. The higher the tissue signature in the lesion area, the more severely affected is this area by ischemia. In this study, map-ISODATA was computed from T1, T2 and T1sat maps (Li et al., 2006; Li et al., 2005). Based on the map-ISODATA calculated at the last observation time point (6-week), the ischemic lesion area for each animal was divided into two specific regions, ischemic boundary and ischemic core. The ischemic boundary ROI (Region of Interest) was an area encompassed by two closed perimeters separated by a 4-pixel distance (Fig. 1a, transparent ROI). This boundary ROI was created by shrinking the rim of the ischemic lesion identified by ISODATA 4 pixels towards the ischemic core. The 4-pixel thickness of the boundary ROI was selected by comparison of MRI images and the corresponding coregistered immunostained tissue slices in the ischemic boundary area with angiogenesis. The angiogenesis area containing enlarged thin-walled vessels was marked on the tissue slice and the slice was coregistered to ISODATA. On the coregistered tissue slice (Fig. 1b), the angiogenesis area (Fig. 1b, yellow track) identified by histological evaluation was partially encompassed by the ischemic lesion area characterized by ISODATA (Fig. 1b, red track) within the ischemic boundary area. This overlapping area (Fig. 1b, green area) within the lesion determined the thickness of perimeter boundary ROI. The remaining lesion area located inside the boundary region was identified as the ischemic core region (Fig. 1a, solid ROI). CBF values and tissue signatures were measured in these two regions for each animal on a fixed coronal slice (bregma -0.8mm) at different time points. Data were normalized to the contralateral (non-ischemic) side for each animal and were averaged at the same time points for each experimental group.
Fig. 1.
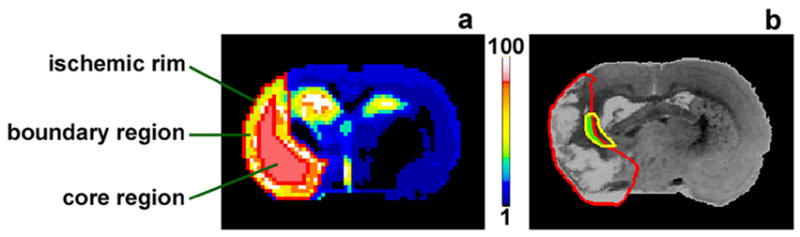
ISODATA (a) and coregistered EBA-immunostained tissue slice (b) of a rat brain (6-weeks after stroke) showing the two specific regions in the ischemic lesion area and the relationship between angiogenesis area and lesion boundary. The ischemic boundary region (a, transparent ROI) was created by shrinking the rim of ischemic lesion 4 pixels to the ischemic center. The remaining lesion area located inside the boundary region was identified as the ischemic core region (a, solid ROI). The angiogenesis area (b, yellow track) identified by immunohistological evaluation was partially covered by the ischemic lesion area characterized by ISODATA (b, red track). This overlapping area (b, green area) within the lesion was encompassed by the perimeter boundary ROI (comparing a with b).
Histopathologic evaluation
Immediately after the final MRI measurement at 6 weeks after stroke, animals were sacrificed and the brain tissue was prepared, as previously described (Li et al., 2006).
For morphological analysis and quantification of cerebral vessels, endothelial barrier antigen (EBA) immunohistological staining (Rosenstein et al., 1992) was employed. The angiogenesis area represented by enlarged thin-walled vessels along the ischemic lesion boundary was marked. The vessel density in this area and in the homologous area in the contralateral side of rat brain was digitized under a 40X objective (Olympus BX40), using a 3-CCD color video camera (Sony DXC-970MD) interfaced with MCID image analysis system (Imaging Research). A fixed reference coronal tissue section for each animal, which matched the MRI slice, was measured. Data are presented as the average vessel number per square millimeter.
Behavioral testing
All behavioral tests (at 1, 2, 7, 14, 21, 28, 35 and 42 days after onset of MCA occlusion) were performed blindly, without knowledge of the experimental groups and the corresponding MRI results.
Neurological severity score (Chen et al., 2001)
Neurological severity score (NSS) grades the composite neurological function of an animal in motor, sensory, reflex and balance tests. One point is rewarded for the inability to perform the task or for the lack of a tested reflex. Thus, the higher score, the more severe is the injury (normal score, 0; maximal deficit score, 18).
Foot-fault test (Zhang et al., 2005; Zhang et al., 2002)
A modified forelimb foot-fault placing test was used to examine forelimb function. Rats were set on an elevated hexagonal grid surface and were encouraged to traverse the surface. Inaccurately placing a forelimb, which included falling through one of the openings or slipping between the wires, was recoded as a foot-fault. The total number of steps that the rat used to cross the surface and the total number of foot-faults were counted. The percentage of forelimb foot-faults during a trial was then calculated.
Statistical analysis
Statistical comparisons of vessel density, lesion size, relative CBF and relative signature between two groups and between different regions were performed using repeated measures ANOVA (Analysis of Variance). Analysis began with testing the interaction between treatment group, region and time, followed by pair-wise comparisons if a significant interaction (p < 0.10) was detected. For behavioral testing data, the Generalized Estimating Equations (GEE) approach (Zeger and Liang, 1986) was used to analyze variance including the independent factor of treatment and dependent factor of time, because the data were not normal. Correlative analysis was performed between the percentage of foot-faults and relative CBF measured in the boundary region. The measurement results are summarized as mean ± SE. Statistical significance was inferred for p ≤ 0.05.
3. Results
Changes of lesion size and blood pressure
Ischemic lesion size was identified by ISODATA and was presented as the percentage of the ipsilateral hemisphere. The temporal profiles of lesion size for the treated and control groups along with the ischemic time are shown in Fig. 2a. While not significantly different throughout the experimental period, the mean values of lesion size were lower in the treated group than in the control group (average from 1 to 6 weeks: 41% vs. 49% of the ipsilateral hemisphere). The stable evolution of lesion size after 1-week time point for two groups allowed us to track the CBF and tissue signature in the fixed specific regions.
Fig. 2.
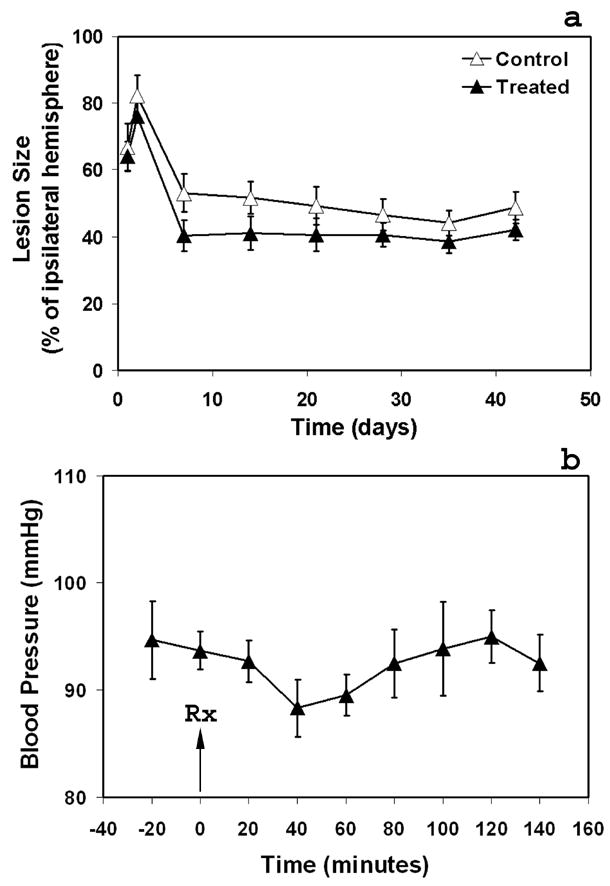
Temporal profiles of the ischemic lesion size for the treated and control groups (a) and blood pressure measurements (b). Although the mean values of lesion size were lower in the treated group than in the control group (average from 1 to 6 weeks: 41% vs. 49% of the ipsilateral hemisphere), there was no significant difference between the two groups throughout the experimental period. Sildenafil administered subcutaneously to rats at a dose of 10mg/Kg resulted in a transient but not a significant reduction of blood pressure compared with the pre-treatment level, with the lowest mean blood pressure exhibited 40 minutes after administration and the value gradually returning to the pre-treatment level 120 minutes after administration.
The blood pressure measurements of rats treated at 24 hours after stroke are given in Fig. 2b. Sildenafil administered subcutaneously to rats with embolic stroke at a dose of 10mg/Kg resulted in a transient but not a significant reduction of blood pressure compared with the pre-treatment level, with the lowest mean blood pressure exhibited 40 minutes after administration and the value gradually returning to baseline 120 minutes after administration.
Vessel density and cerebral blood flow
Fig. 3 shows EBA-immunoreactive cerebral vessels from a treated animal (Fig. 3a–3b), vessel density (Fig. 3c) evaluated by histological assessment and relative CBF (Fig. 3d) measured by MRI in the lesion boundary area 6-weeks after stroke. Compared with the control group, treatment with sildenafil significantly increased cerebral vessel number in the lesion boundary area (Fig. 3c, p < 0.05). Correspondingly, relative CBF in the lesion boundary area was also significantly improved compared with the control group (Fig. 3d, p < 0.05). These data indicate that sildenafil significantly enhances angiogenesis and increases CBF in the ischemic boundary region.
Fig. 3.
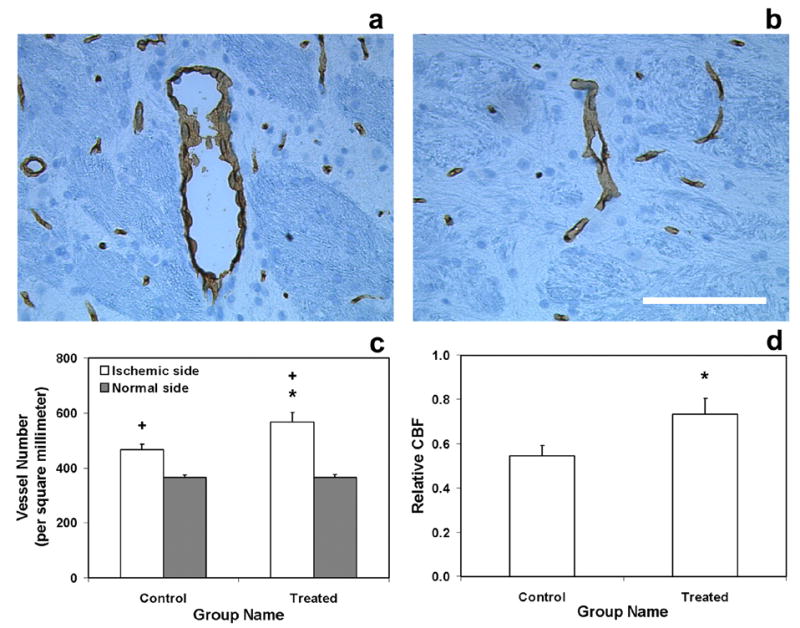
Immunoreactive cerebral vessels from a treated animal (a, ipsilateral side; b, contralateral side. Bar = 100μm), vessel density (c) and relative CBF (d) in lesion boundary area 6-weeks after stroke. Treatment with sildenafil significantly increased cerebral vessel number in the lesion boundary area compared with the control group (c). Relative CBF in the lesion boundary area was also significantly improved (d) compared with the control group. Significance of difference: * = p < 0.05, comparing treated and control groups in the ischemic boundary region; + = p < 0.05, comparing ischemic boundary region with the homologous area in the contralateral (normal) side of rat brain in treated group and control group, respectively.
Evolution of cerebral blood flow and tissue signature in different regions
As shown in Fig. 4, ischemic boundary and ischemic core areas were significantly distinct regions identified by both CBF and tissue signature values for the treated group (Fig. 4a and 4c, p < 0.05). In contrast, for the control group, these two regions did not differ in both CBF and tissue signature values until 6-weeks after stroke (Fig. 4b and 4d, p < 0.05). Treatment with sildenafil resulted in significantly higher CBF and correspondingly, significantly lower tissue signature values in the ischemic boundary region than in the ischemic core region (Fig. 4a and 4c).
Fig. 4.
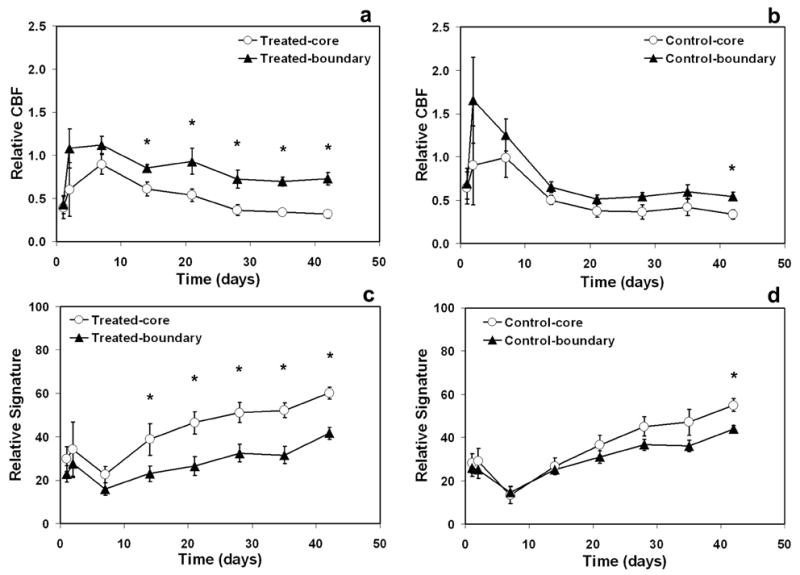
Evolution of CBF (a–b) and tissue signature (c–d) in different regions. Ischemic boundary and ischemic core areas were identified as two significantly different regions by both CBF (a) and tissue signature values (c) for the treated group after the 1-week time point. These two regions, however, for the control group did not differ in both CBF (b) and tissue signature values (d) until 6-weeks. Significance of difference: * = p < 0.05, comparing boundary and core regions at the same time points.
No significant difference of CBF and tissue signature in the ischemic core region between the two groups was found. Significant difference of CBF in the ischemic boundary region between the two groups was detected at the 3-week (p < 0.02) and 6-week (p < 0.05) time points. However, no significant difference of tissue signature in the ischemic boundary region between the two groups was detected, although the mean values of tissue signature were lower in the treated group than in the control group (from 2 to 6 weeks: 23 vs. 25; 26 vs. 31; 32 vs. 37; 31 vs. 36; and 41 vs. 44).
Neurological functional outcome and correlation analysis
Functional outcome is shown in Fig. 5. Treatment with sildenafil significantly improved performance on NSS (Fig. 5a) and on foot-fault test (Fig. 5b) compared with control group (p < 0.05).
Fig. 5.
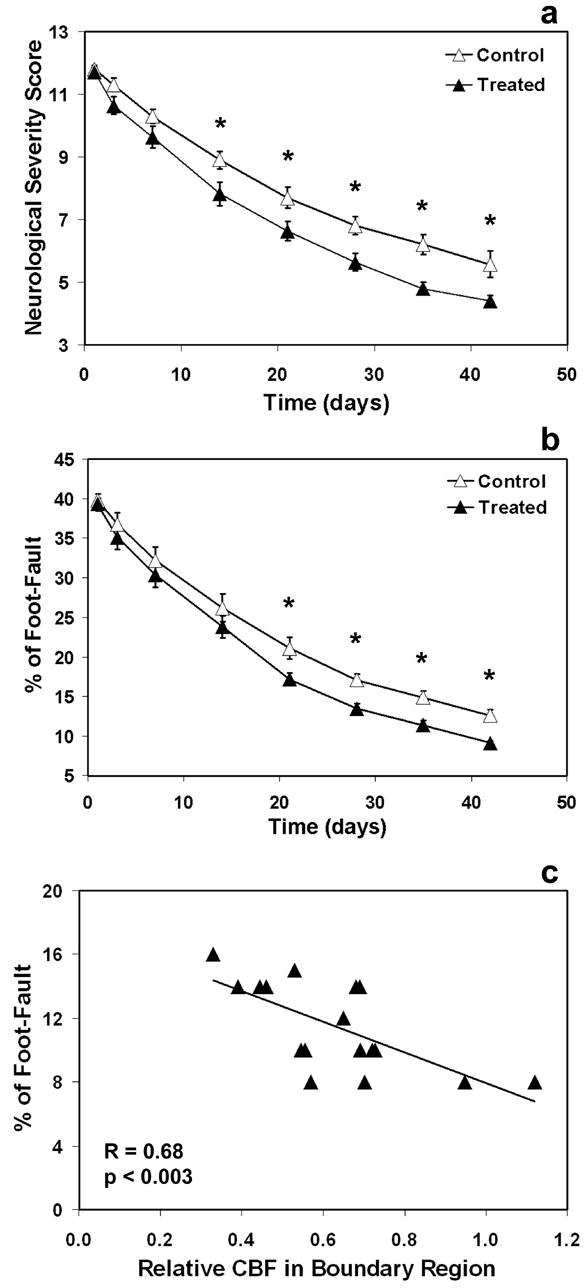
Neurological functional outcome (a–b) and correlation analysis (c). Treatment with sildenafil significantly improved performance on NSS (a) and on foot-fault test (b) from 2-weeks and 3-weeks after stroke, respectively, until the end of observation compared with control group. Significance of difference: * = p < 0.05, comparing treated and control groups at the same time points. Higher CBF level in the boundary region correlates to lower percentage of foot-faults (c).
NSS is a complex composite of many neurological tests that are likely not directly related to a single physiological parameter, such as CBF. The correlation between the percentage of foot-faults and relative CBF measured in the boundary region is given in Fig. 5c (R=0.68, p<0.003). Higher CBF level corresponds to lower percentage of foot-faults, indicating that angiogenesis in the boundary region may contribute to the recovery of forelimb function.
4. Discussion
The evolution of an ischemic lesion in response to the sildenafil treatment was investigated. Our study showed that 1-week treatment with sildenafil altered the ischemic boundary region, as characterized by both CBF and tissue signature. After treatment, the ischemic lesion exhibited two significantly different regions, with higher CBF level and correspondingly, lower tissue signature value in the boundary region than in the core region. These two regions for the non-treated group, however, did not differ in both CBF and tissue signature values until 6-weeks after stroke. Sildenafil treatment did not significantly reduce the ischemic lesion size but did enhance angiogenesis and improve functional recovery, consistent with previous studies (Zhang et al., 2005; Zhang et al., 2002).
Map-ISODATA accurately identifies the ischemic lesion size and characterizes the ischemic injury (Li et al., 2006; Li et al., 2005). We therefore used ISODATA to divide the ischemic lesion into boundary and core regions and to identify the corresponding ischemic injury (using tissue signature) in these regions. By using the boundary and core ROIs identified at the 6-week time point, the evolution of ischemic tissue in the two specific regions could be properly tracked since there was little or no change of lesion size for each individual animal studied after the 1-week time point (Fig. 2a). Our investigation demonstrated that angiogenesis (Fig. 1b, yellow track) was present 6-weeks after stroke, indicating that the abnormal tissue identified by ISODATA in the ischemic boundary area undergoes vascular remodeling. One week after the treatment with sildenafil, a 4-pixel ischemic boundary region, which incorporated the angiogenic remodeling area, was a significantly distinct region from the remaining ischemic core region as identified by both tissue signature (Fig. 4c) and CBF (Fig. 4a).
Our regional measurements showed that treatment with sildenafil selectively increased CBF in the ischemic boundary region compared with the ischemic core region (Fig. 4a) and led to a significantly elevated CBF level in the ischemic boundary region at a late stage after stroke compared to the control group (Fig. 3d). The ischemic regions with significantly different CBF levels (Fig. 4a) exhibited significantly different tissue signature values (Fig. 4c). Higher CBF corresponded to lower tissue signature. Neither CBF nor tissue signature values in the ischemic core region were influenced by sildenafil treatment. These data confirm the observations that the ischemic boundary is an area which can be affected by the treatment (Li et al., 2005) and that the status of the ischemic tissue closely depends on the CBF level (Li et al., 2006).
The ischemic core with lower CBF than the surrounding ischemic tissue is an irreversibly damaged area by the stroke (Knight et al., 1994; Jiang et al., 1993), while the ischemic boundary adjacent to the non-ischemic tissue is a potentially salvageable area (Li et al., 2005). Various post-embolization events related to the repair of ischemic brain take place in the ischemic boundary area. For instance, synthesis of vascular endothelial growth factor (VEGF), which mediates angiogenesis (Yancopoulos et al., 2000), increases in the ischemic boundary region after treatment (Zhang et al., 2003; Chen et al., 2003a; Chen et al., 2003b). Angiogenesis, which is essential for repair of the injured cerebral tissue, occurs in the ischemic boundary area (Zhang et al., 2005; Zhang et al., 2003; Chen et al., 2003a; Chen et al., 2003b), as does synaptogenesis, which promotes recovery of neurological deficits after stroke (Zhang et al., 2005; Chen et al., 2003b).
Enhanced angiogenesis after treatment, as indicated by significantly increased vessel number compared with non-treated group (Fig. 3c), is likely a major contributor to the elevation of CBF in the ischemic boundary region. Angiogenesis is present in both treated and non-treated animals, but it was significantly elevated in sildenafil-treated animals compared to non-treated animals (Fig. 3c). While the CBF values in boundary and core regions were significantly different for both groups at 6-weeks (Fig. 4a–4b), the CBF level in the boundary region in the treated group was significantly higher than in the control group (Fig. 3d), which is consistent with enhanced angiogenesis after treatment (Fig. 3c).
cGMP is the second messenger of NO and a principal mediator of smooth muscle relaxation and vasodilatation (Cheitlin et al., 1999). Levels of cGMP in vascular smooth muscle are tightly regulated by several cyclic nucleotide phosphodiesterase enzymes (PDEs) that catalyze cGMP degradation and terminate this second messenger signal (Traverse et al., 2000). Sildenafil is a potent and selective PDE-5 inhibitor that increases cGMP levels in the systemic circulation by reducing the breakdown of this nucleotide (Cheitlin et al., 1999; Kloner et al., 2000). Sildenafil is a valuable vasodilator with good hemodynamic effects in patients with pulmonary arterial hypertension and cardiovascular disease (Jackson et al., 2005; Lopez-Guarch et al., 2004; Michelakis et al., 2003; Cheitlin et al., 1999). Sildenafil also significantly increases cortical levels of cGMP after 1-week treatment in this experimental stroke model (Zhang et al., 2005), and the elevated cGMP levels cause dilation of cerebral vessels (Sobey and Quan, 1999). Thus, the increased cGMP levels in the ischemic rat brain may promote perfusion via dilatation of cerebral vessels and consequently improve CBF in the entire ischemic boundary region soon after the 1-week sildenafil treatment (Fig. 4a).
Restoration of cerebral circulation is important for functional recovery after a stroke (Zhang et al., 2000). Our data show that administration of sildenafil to a rat within 1-week after onset of stroke divides the ischemic lesion into two distinct regions, with significantly higher CBF levels in the ischemic boundary region than in the ischemic core region. This situation persisted throughout the observation period and was associated with significant improvement of neurological functional outcome evaluated by neurological severity score (Fig. 5a) and foot-fault test (Fig. 5b).
In summary, administration of sildenafil to rats with embolic stroke enhances angiogenesis, selectively increases CBF in the ischemic boundary region compared to the ischemic core region and leads to a significantly elevated CBF level in the ischemic boundary region at the late stage after stroke compared to the control group, all of which may contribute to the improvement of neurological functional recovery.
Acknowledgments
This work was supported by NINDS grants PO1 NS23393, PO1 NS42345, RO1 NS48349, RO1 NS38292, RO1 NS43324 and HL64766.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ball GH, Hall DJ. A clustering technique for summarizing multivariate data. Behav Sci. 1967;12:153–155. doi: 10.1002/bs.3830120210. [DOI] [PubMed] [Google Scholar]
- Cheitlin MD, Hutter AM, Brindis RG, Ganz P, Kaul S, Russell RO, Zuaman RM. Use of sildenafil (Viagra) in patients with cardiovascular disease. Circulation. 1999;99:168–177. doi: 10.1161/01.cir.99.1.168. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang ZG, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003a;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003b;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Soltanian–Zadeh H, Li Q, Whitton PA, Ewing JR, Chopp M. Characterization of cerebral tissue by MRI map ISODATA in embolic stroke in rat. Brain Res. 2006;1084:202–209. doi: 10.1016/j.brainres.2006.02.054. [DOI] [PubMed] [Google Scholar]
- du Toit EF, Rossouw E, Salie R, Opie LH, Lochner A. Effect of sildenafil on reperfusion function, infarct size, and cyclic nucleotide levels in the isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:23–31. doi: 10.1007/s10557-005-6894-2. [DOI] [PubMed] [Google Scholar]
- Jackson G, Keltai M, Csanady M, Edes I, Bellamy GR, Widimsky P, Lisa L, Gillies H. Hemodynamic effecte of sildenafil citrate and isosorbide mononitrate in men with coronary artery disease and erectile dysfunction. J Sex Med. 2005;2:407–414. doi: 10.1111/j.1743-6109.2005.20359.x. [DOI] [PubMed] [Google Scholar]
- Jacobs MA, Knight RA, Soltanian-Zadeh H, Zhang ZG, Goussev AV, Peck DI, Windham JP, Chopp M. Unsupervised segmentation of multiparameter MRI in experimental cerebral ischemia with comparison to T2, diffusion, and ADC MRI parameters and histopathological validation. J Magn Reson Imaging. 2000;11:425–437. doi: 10.1002/(sici)1522-2586(200004)11:4<425::aid-jmri11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jacobs MA, Zhang ZG, Knight RA, Soltanian-Zadeh H, Goussev AV, Peck DI, Chopp M. A model for multiparametric MRI tissue characterization in experimental cerebral ischemia with histological validation in rat: part 1. Stroke. 2001;32:943–949. doi: 10.1161/01.str.32.4.943. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Chopp M, Helpern JA, Ordidge RJ, Garcia JH, Marchese RA, Qing ZX, Knight RA. Temporal evolution and spatial distribution of the diffusion constant of water in rat brain after transient middle cerebral artery occlusion. J of Neurol Sci. 1993;120:123–130. doi: 10.1016/0022-510x(93)90262-w. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Ding GL, Zhang L, Ewing JR, Wang L, Zhang RL, Li L, Lu M, Meng H, Arbab AS, Hu J, Li QJ, Pourabdollah Nejad DS, Athiraman H, Chopp M. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. NeuroImage. 2005;28:698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Kataoka M, Satoh T, Manabe T, Anzai T, Yoshikawa T, Mitamura H, Ogawa S. Marked improvement with sildenafil in a patient with primary pulmonary hypertension- unresponsive to epoprostenol. Intern Med. 2004;43:945–950. doi: 10.2169/internalmedicine.43.945. [DOI] [PubMed] [Google Scholar]
- Kloner RA. Sex and the patient with cardiovascular risk factors: Focus on sildenafil. Am J Med. 2000;109:13S–21S. doi: 10.1016/s0002-9343(00)00656-2. [DOI] [PubMed] [Google Scholar]
- Knight RA, Dereski M, Helpern JA, Ordidge RJ, Chopp M. Magnetic resonance imaging assessment of evolving focal cerebral ischemia: Comparison with histopathology in rats. Stroke. 1994;25:1252–1262. doi: 10.1161/01.str.25.6.1252. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Ding GL, Zhang L, Zhang ZG, Ewing JR, Knight RA, Soltanian-Zadeh H, Chopp M. Map-ISODATA demarcates regional response to combination rt-PA and 7E3 F(ab’)2 treatment of embolic stroke in the rat. J Magn Reson Imaging. 2005;21:726–734. doi: 10.1002/jmri.20318. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Zhang L, Ding GL, Wang L, Zhang RL, Zhang ZG, Li QJ, Ewing JR, Kapke A, Lu M, Chopp M. Ischemic Cerebral Tissue Response to Subventricular Zone Cell Transplantation Measured by Iterative Self-Organizing Data Analysis Technique Algorithm. J Cerebr Blood F Met. 2006 doi: 10.1038/sj.jcbfm.9600288. in press. [DOI] [PubMed] [Google Scholar]
- Lopez-Guarch CJ, Subias PE, de Meneses RT, Jimenez JFD, Perez DS, Martin MTV, Sanchez MAG, de la Calzada CS. Efficacy of oral sildenafil as rescue therapy in patients with severe pulmonary arterial hypertension chronically treated with prostacyclin. Long-term results. Rev Esp Cardiol. 2004;57:946–951. [PubMed] [Google Scholar]
- Michelakis ED, Tymchak W, Noga M, Webster L, Wu XC, Lien D, Wang SH. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–2069. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S, Caglayan E, Diet F, Karasch T, Weihrauch J, Wassermann K, Erdmann E. Long-term effects of sildenafil in a patient with scleroderma-associated pulmonary hypertension and Raynaud’s syndrome. Dtsch Med Wochenschr. 2004;129:1736–1740. doi: 10.1055/s-2004-829025. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Krum JM, Sternberger LA, Pulley MT, Strnberger NH. Immunocytochemical expression of the endothelial barrier antigen (EBA) during brain angiogenesis. Brain Res Dev Brain Res. 1992;66:47–54. doi: 10.1016/0165-3806(92)90138-m. [DOI] [PubMed] [Google Scholar]
- Sheth A, Park JE, Ong YE, Ho TB, Madden BP. Early haemodynamic benefit of sildenafil in patients with coexisting chronic thromboembolic pulmonary hypertension and left ventricular dysfunction. Vascul Pharmacol. 2005;42:41–45. doi: 10.1016/j.vph.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Quan L. Impaired cerebral vasodilator responses to NO and PDE V inhibition after subarachnoid hemorrhage. Am J Physiol. 1999;277:H1718–1724. doi: 10.1152/ajpheart.1999.277.5.H1718. [DOI] [PubMed] [Google Scholar]
- Traverse JH, Chen YJ, Du R, Bache RJ. Cyclic Nucleotide phosphodiesterase type 5 activity limits blood flow to hypoperfused myocardium during exercise. Circulation. 2000;102:2997–3002. doi: 10.1161/01.cir.102.24.2997. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Ohashi K, Takeuchi K, Yamashita K, Yokoyama T, Tran QK, Satoh H, Terada H, Ohashi H, Hayashi H. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther. 2002;71:398–402. doi: 10.1067/mcp.2002.123554. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Weigand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- Zhang L, Zhang RL, Wang Y, Zhang C, Zhang ZG, Meng H, Chopp M. Functional recovery in aged and young rates after embolic stroke-Treatment with a phosphodiesterase type 5 inhibitor. Stroke. 2005;36:847–852. doi: 10.1161/01.STR.0000158923.19956.73. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Wang Y, Zhang L, Zhang ZG, Tsang W, Lu M, Zhang LJ, Chopp M. Sildenafil (Viagra) induces Neurogenesis and promotes functiuonal recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang RL, Davies K, Powers C, van Bruggen N, Chopp M. VEGF enchances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]


