Abstract
Unprecedented agricultural intensification and increased crop yield will be necessary to feed the burgeoning world population, whose global food demand is projected to double in the next 50 years. Although grain production has doubled in the past four decades, largely because of the widespread use of synthetic nitrogenous fertilizers, pesticides, and irrigation promoted by the “Green Revolution,” this rate of increased agricultural output is unsustainable because of declining crop yields and environmental impacts of modern agricultural practices. The last 20 years have seen diminishing returns in crop yield in response to increased application of fertilizers, which cannot be completely explained by current ecological models. A common strategy to reduce dependence on nitrogenous fertilizers is the production of leguminous crops, which fix atmospheric nitrogen via symbiosis with nitrogen-fixing rhizobia bacteria, in rotation with nonleguminous crops. Here we show previously undescribed in vivo evidence that a subset of organochlorine pesticides, agrichemicals, and environmental contaminants induces a symbiotic phenotype of inhibited or delayed recruitment of rhizobia bacteria to host plant roots, fewer root nodules produced, lower rates of nitrogenase activity, and a reduction in overall plant yield at time of harvest. The environmental consequences of synthetic chemicals compromising symbiotic nitrogen fixation are increased dependence on synthetic nitrogenous fertilizer, reduced soil fertility, and unsustainable long-term crop yields.
Keywords: legume, nitrogen fixation, symbiosis, Sinorhizobium meliloti
In the past 40 years, synthetic nitrogen (N) fertilizer use has increased 7-fold, whereas pesticide use has increased 3-fold; if current practices continue unabated, application of both is expected to increase an additional 3-fold by 2050 (1–9). Paradoxically, as N fertilizer application has exponentially increased, yield potential of major staple food crops has become stagnant. To date, such diminishing returns have been explained by models showing the first addition of N fertilizer induces the largest gain in crop yield with efficiency declining at higher levels of application (10, 11). Additionally, agricultural intensification and continuous cropping can result in a loss of soil organic matter leaving soil less fertile, which then drives increased application of synthetic N fertilizer and pesticides.
Sustainable agriculture seeks to increase crop yields and nutrient-use efficiency while reducing the environmental costs associated with agricultural intensification. The goal of such strategies is to maximize the amount of crop output per unit of water, N fertilizer, and pesticide input. Certain farming practices have been found to increase efficiency and sustainability of crop production, including integrated pest management, improved drainage control, properly timed water and fertilizer application, and maximizing biological N fixation by incorporating legume crops and enriching soil with N-fixing rhizobia (Rhiz) bacteria (4, 10, 12–14). A well established practice for maintaining soil fertility has been the cultivation of legume crops, which replenish N in the soil via symbiosis with N-fixing Rhizobium bacteria that convert atmospheric N to ammonia and other sources utilizable by plants, in rotation with non-N-fixing crops (8, 15). The effectiveness of this strategy relies on maximizing symbiotic N fixation (SNF) and plant yield to resupply organic and inorganic N and nutrients to the soil. The vast majority of biologically fixed N is attributable to symbioses between leguminous plants (soybean, alfalfa, etc.) and species of Rhizobium bacteria; replacing this natural fertilizer source with synthetic N fertilizer would cost ≈$10 billion annually (16, 17).
Effective SNF can significantly reduce the need for synthetic N fertilizers. An estimated $50–90 million net return could be realized by rotating alfalfa and corn crops in the Midwestern U.S. (17). In Brazil, soybeans inoculated with Rhiz are responsible for $1.3 billion per year savings in production costs (18). In addition to the economic benefits, using SNF to reduce dependence on commercial N fertilizer has environmental benefits. An understanding of factors controlling SNF will help to maximize symbiotic effectiveness for agricultural sustainability. Therefore, it is important to determine how addition of synthetic chemicals to the soil environment may affect SNF.
SNF is both initiated and maintained by an active exchange of chemical signals between host plant and Rhiz soil bacteria. Each species of Rhiz interacts only with a particular subset of host plant species. For example, the soil bacterium Sinorhizobium meliloti will form a symbiotic partnership with its host plant, alfalfa, but not with other legumes such as soybean or clover (19, 20). To establish host specificity, alfalfa exudes a unique mixture of flavonoid (luteolin and apigenin) phytochemical signals into the soil, which serve dual functions, to recruit S. meliloti and to inhibit or antagonize nonfavorable species of Rhiz (21–23). Alfalfa phytochemical signals are specifically recognized by S. meliloti NodD receptors, which are transcriptional regulators that bind DNA response elements and induce transcription of rhizobial nodulation (nod) genes in a flavonoid-dependent manner. The end products of nod genes are Nod factors, which act as response signals sent by the S. meliloti back to the alfalfa host plant. S. meliloti Nod factors are recognized by specialized receptors in alfalfa roots, thus initiating development of root nodules where, in exchange for an energy source from the host plant, S. meliloti will fix atmospheric N to a usable fertilizer source for alfalfa (24). Therefore, symbiotic signaling is initiated by S. meliloti NodD receptors' specific recognition of alfalfa-produced phytochemicals. Nonhost plant phytochemicals, such as genistein produced by soybeans and chrysin produced by clover, inhibit symbiotic signaling between alfalfa and S. meliloti by interfering at the level of the rhizobial NodD receptors effectively blocking communication and disrupting initiation of symbiosis (21–23).
Temporal and chemical specificity of symbiotic signaling is crucial for coordinating the actions of alfalfa and S. meliloti necessary for SNF. In the dynamic soil environment, S. meliloti is exposed to a mixture of agonistic and antagonistic phytochemicals, and the degree to which its NodD receptors are able to interpret these signals to locate its symbiotic partner, alfalfa, will most likely determine the efficiency of SNF in that particular soil environment. If crucial alfalfa phytochemical signaling is disrupted, then SNF will also be compromised (21). The importance of temporal specificity, timing of S. meliloti recruitment to alfalfa, to SNF has been demonstrated by studies in which early onset nodulation and N fixation has resulted in enhanced alfalfa growth (25) and by studies showing that S. meliloti that are recruited to alfalfa roots faster have a marked competitive advantage over their slower counterparts (26). Therefore, natural or synthetic chemicals in the soil environment that disrupt symbiotic signaling would not only delay symbiotic initiation but also decrease symbiotic efficiency.
A plant's need for N increases progressively as the plant enters its exponential phase of growth and the demand for N outstrips the rate of supply from the soil (27). Crop legumes can fix 100–200 kg of N per hectare per year, but rates are often substantially lower, and over the past 25 years, soybeans and other legumes have shown a significant decline in N fixation (28, 29). To investigate what may be contributing to such a decline, we directly measured effects of exogenous factors, such as pesticides or environmental chemicals found in cattle feedlot effluent and irrigation water, on symbiotic signaling and SNF among legumes and Rhiz. In previous studies using in vitro reporter assays, we demonstrated that ≈30 different pesticides and environmental contaminants specifically disrupted crucial symbiotic signaling between flavonoid phytochemicals and S. meliloti NodD receptors (30, 31). Here, we show previously undescribed in vivo evidence that a subset of organochlorine pesticides and pollutants inhibit symbiotic signaling between alfalfa and S. meliloti, resulting in delayed symbiotic recruitment, reduced SNF, and a decline in alfalfa plant yield.
Results and Discussion
To determine the effects of different pesticides and environmental contaminants on in vivo N-fixing symbiosis, alfalfa seeds were inoculated with S. meliloti and then treated with a subset of chemicals previously shown to specifically inhibit phytochemical–NodD symbiotic signaling in vitro (30, 31). The following natural and synthetic chemicals were tested: (i) chrysin, a clover-derived phytochemical known to antagonize S. meliloti NodD activation in vitro (23); (ii) methyl parathion, an insecticide; (iii) dichlorodiphenyltrichloroethane (DDT), an insecticide; (iv) bisphenol A, a plasticizer and monomer used in the manufacture of polycarbonate plastic and a ubiquitous environmental contaminant; and (v) pentachlorophenol, an insecticide and wood preservative. Alfalfa seeds that were inoculated with S. meliloti and received no chemical treatment (no chemicals, +Rhiz) served as a positive control for SNF, whereas uninoculated alfalfa seeds [with chemicals (−Rhiz)] served as a negative control for effects of lack of SNF on nodule formation, N fixation activity, and plant yield.
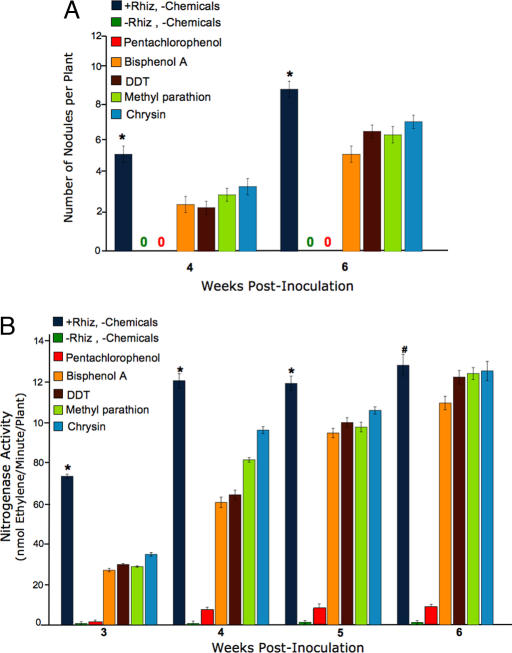
Alfalfa seeds were inoculated with S. meliloti and then received a one-time treatment with the chemicals listed above. To measure whether each chemical alone affected plant growth, treatment groups were established that were not inoculated with S. meliloti but did receive the same chemical treatments as described above. Five independent replicate populations per treatment group were then assayed at 2, 4, and 6 weeks after inoculation for the following parameters: number of plants, number of root nodules per plant, nitrogenase activity, and plant dry weight. The number of nodules per plant was significantly reduced in all chemical treatment groups compared with +Rhiz-positive control at 4 and 6 weeks after inoculation (Fig. 1A). In fact, the pentachlorophenol-treated plants exhibited no nodules throughout the course of experiments, the same results obtained from the uninoculated −Rhiz-negative control plants. These data indicate that a one-time treatment with some natural and synthetic environmental chemicals is sufficient to significantly inhibit nodule formation. Because nodule formation is necessary for symbiosis and the site of SNF, we then compared the N fixation capability of each treatment group.
Fig. 1.
Pesticides inhibit recruitment of bacteria to host plant, delay nodulation, and reduce nitrogenase activity. Alfalfa seeds were inoculated with S. meliloti and treated with various environmental chemicals (at 5 × 10−6 M) at day 0. Alfalfa was harvested at 2, 4, and 6 weeks after inoculation, and the following were assayed: (A) The number of root nodules per plant was determined for each treatment group. At 2 weeks after inoculation, no nodules were present; (0) indicates no nodules present at weeks 4 and 6 for these treatment groups. At 4 and 6 weeks after inoculation, all treatment groups had significantly fewer nodules (as indicated by ∗) compared with the +Rhiz-positive control. (B) Nitrogenase activity was measured by using an acetylene reduction assay (32, 33). Five replicates per treatment per time point were assayed, and average nanomolar ethylene produced per minute per plant was calculated for each treatment group. At 3, 4, and 5 weeks after inoculation, nitrogenase activity was significantly reduced in all treatment groups (as indicated by ∗) compared with the +Rhiz-positive control. At 6 weeks after inoculation, nitrogenase activity of bisphenol A and pentachlorohenol treatment groups was significantly reduced (as indicated by #) compared with the +Rhiz-positive control. All results shown are mean values ± SEM for a minimum of three independent experiments. A one-way ANOVA with a Bonferroni correction for multiple tests was used to compare parameters of treatment groups (significant if P < 0.05).
SNF capability is controlled by the rhizobial nitrogenase enzyme, which converts atmospheric N to NH3. Nitrogenase activity of the five replicate populations from each treatment group was measured at 3, 4, 5, and 6 weeks after inoculation using an acetylene reduction assay, as described (32, 33). Briefly, nitrogenase activity is defined as the rate at which acetylene is converted to ethylene, acetylene reduction, as measured by real-time gas chromatography. Nitrogenase activity was significantly reduced in all chemical treatment groups compared with the +Rhiz-positive control at 3, 4, and 5 weeks after inoculation (Fig. 1B). At week 6, the pentachlorophenol and bisphenol A treatment groups still exhibited significantly reduced nitrogen-ase activity compared with the +Rhiz group. At all time points measured, the −Rhiz uninoculated control group exhibited essentially no nitrogenase activity.
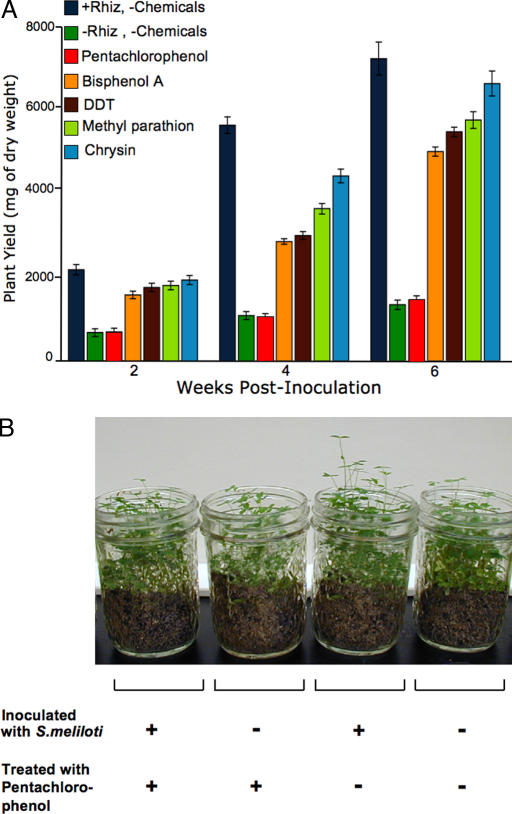
In the absence of synthetic N fertilizer, legumes rely on SNF for growth. To determine the effects of chemical treatment on overall plant yield, we compared both the number of seeds germinated and dry plant biomass for all treatment groups at 2, 4, and 6 weeks after inoculation. Plant yield, as determined by dry weight of roots and shoots from all plants in a treatment group, was significantly reduced for the pentachlorophenol treatment group compared with the +Rhiz-positive control group beginning at week 2 (Fig. 2A). At 4 weeks after inoculation, all chemical treatment groups exhibited significantly lower plant yields compared with +Rhiz-positive control. At 6 weeks after inoculation, plant yields for all chemical treatment groups, except chrysin, remained significantly lower than the +Rhiz-positive control group (Fig. 2A). Treatment with synthetic environmental chemicals resulted in significantly reduced overall plant yields, as calculated by per seedling plant biomass (Table 1). The marked reduction in plant yield seen in the chemical treatment groups was not attributable to seed toxicity or adverse affects on germination, for all but one chemical tested (Table 1). Only pentachlorophenol treatment (both +Rhiz and −Rhiz) significantly reduced germination of alfalfa seeds, resulting in fewer plants compared with all other groups, an effect shared with the −Rhiz-uninoculated control group (Fig. 2B and Table 1).
Fig. 2.
Pesticide treatment reduces plant yield. (A) Plant yield was measured as dry weight in milligrams of plants (roots and shoots) for five replicates per treatment per time point, with initial weight of seeds subtracted. At 2 weeks after inoculation, plant yields of the uninoculated negative control (−Rhiz) and pentachlorophenol treatment groups were significantly reduced compared with all other treatment groups and the +Rhiz-positive control. Plant yields were significantly reduced for all treatment groups compared with the +Rhiz-positive control at 4 weeks after inoculation. At 6 weeks after inoculation, plant yields of all treatment groups, except chrysin, were significantly reduced compared with the +Rhiz-positive control. (B) Pentachlorophenol treatment significantly reduced plant yield, both when plants were inoculated with S. melioti or left uninoculated, as compared with the +Rhiz-positive control. At all time points measured, plant yield of both pentachlorophenol treatment groups was statistically equivalent to the −Rhiz-negative control group, which was not inoculated with S. melioti. Plant yields of pentachlorophenol treatment groups and −Rhiz-negative control group were significantly less than +Rhiz-positive control at all time points measured. Treatment groups pictured are (from left to right) alfalfa inoculated with S. melioti and treated with pentachlorophenol, alfalfa that were not inoculated with S. melioti but were treated with pentachlorophenol, alfalfa inoculated with S. melioti and not treated with any chemicals (+Rhiz-positive control), and alfalfa that were not inoculated with S. melioti and not treated with any chemicals (−Rhiz-negative control). All plant biomass values are presented in Table 1.
Table 1.
Chemicals cause symbiotic phenotype of reduced S. meliloti recruitment and nodulation resulting in lower overall plant yields
| Treatment | No. of seedlings | No. of nodules* | Nitrogenase activity* | Total yield, mg* | Plant biomass per seedling, mg* |
|---|---|---|---|---|---|
| +Rhiz (+control) | 85 ± 6 | 510 ± 43 | 1.00 | 5,798 ± 202 | 68 ± 4 |
| +Rhiz, +chrysin | 83 ± 4 | 332 ± 42 | 0.89 | 4,547 ± 170 | 55 ± 3 |
| +Rhiz, +methyl parathion | 83 ± 6 | 290 ± 33 | 0.68 | 3,741 ± 131 | 45 ± 3 |
| +Rhiz, +DDT | 79 ± 6 | 213 ± 32 | 0.53 | 3,081 ± 97 | 39 ± 2 |
| +Rhiz, +bisphenol A | 82 ± 5 | 236 ± 41 | 0.50 | 2,932 ± 67 | 36 ± 2 |
| +Rhiz, +pentachlorophenol | 33 ± 4* | 0 | 0.06 | 1,073 ± 85 | 33 ± 1 |
| −Rhiz, +pentachlorophenol | 35 ± 2* | 0 | <0.01 | 1,092 ± 79 | 20 ± 1 |
| −Rhiz (−control) | 72 ± 2* | 0 | <0.01 | 1,110 ± 97 | 15 ± 1 |
The above parameters were measured at 4 weeks after inoculation. Results are mean values ± SEM for five replicate populations per treatment group. A one-way ANOVA with a Bonferroni correction for multiple tests was used to compare parameters among treatment groups (significant if P < 0.05, as indicated by ∗). Number of nodules, nitrogenase activity, total plant yield, and biomass per seedling were significantly reduced for all treatment groups compared with +Rhiz-positive control. To assess each chemical's effect on seed germination and plant growth, control groups were established that received chemical treatment but were left uninoculated (−Rhiz). With the exception of pentachlorophenol, chemicals did not inhibit seed germination and showed no significant reduction in number of seedlings as compared with +Rhiz-positive control. Number of seedlings was significantly reduced for both inoculated (+Rhiz) and uninoculated (−Rhiz) pentachlorophenol treatment groups and −Rhiz uninoculated negative control compared with the +Rhiz-positive control. At dosage tested, chemicals did not adversely affect bacterial growth (31).
Pentachlorophenol effectively negated the benefits of SNF for plant biomass production over the 6-week growth period (Fig. 2B and Table 1), as seen when comparing S. meliloti inoculated vs. uninoculated pentachlorophenol treatment groups. With the exception of pentachlorophenol, none of the chemicals tested was toxic to alfalfa or S. meliloti (31), yet each significantly inhibited nodule formation and nitrogenase activity. Although chrysin-treated plants recovered nitrogenase activity comparable to +Rhiz-positive control plants by 6 weeks posttreatment, the synthetic pesticides and pollutants significantly inhibited nitrogenase activity throughout the course of the experiments. The greater persistence of inhibition by the synthetic chemicals may be because of S. meliloti bacteria more readily metabolizing the natural phytochemical chrysin than synthetic chlorinated chemicals (34).
At each time point measured, all parameters of SNF efficiency, including number of nodules, nitrogenase activity, and plant yields, were consistently highest for the +Rhiz-positive control group and lowest for the −Rhiz uninoculated negative control group. SNF efficiency of chemical treatment groups ranged from very low, the pentachlorophenol group was statistically equivalent to the −Rhiz uninoculated negative control, to midrange for the methyl parathion, DDT, and bisphenol A treatment groups, to nearly equivalent to +Rhiz-positive control levels for chrysin treatment group by week six. Synthetic chemicals, including methyl parathion, DDT, bisphenol A, and pentachlorophenol, which are present in the soil environment (Table 2), significantly inhibited in vivo establishment of symbiosis between S. meliloti and alfalfa, reduced SNF, and negatively impacted N fixation and plant biomass production.
Table 2.
Levels of environmental chemicals present in the soil environment
| Chemicals tested | Half-life in soil | Levels reported in cultivated soil, parts per billion | Refs. |
|---|---|---|---|
| Methyl parathion | 5–30 days | <1–44 | 49 |
| DDT* | 0.5–15 years | <1–150 | 50 |
| Bisphenol A | 1–10 days | <1–200 | 51, 52 |
| Pentachlorophenol† | 15–60 days | <1–700 | 53 |
*Although DDT is still in limited use in some countries, the U.S. banned DDT use in 1972 (50).
†Placed on restricted use by the U.S. in 1984. Pentachlorophenol is no longer available to the general public but is still used industrially as an insecticide and wood preservative for utility poles, railroad ties, and wharf pilings (53).
Conclusions
The results of this study demonstrate that one of the environmental impacts of pesticides and contaminants in the soil environment is disruption of chemical signaling between host plants and N-fixing Rhiz necessary for efficient SNF and optimal plant yield. Organochlorine pesticides and other environmental chemicals are applied at high rates to agricultural soil and can enter the soil environment as components of cattle feedlot effluent or water used for irrigation of crops (35, 36) at concentrations reaching ppm in soil and groundwater (37, 38). Sustainable agricultural practices seek to use enrichment crops and maximize SNF from legumes to overcome the diminishing returns of crop yields because of overfarming and poor soil quality (1, 5, 39). The benefits derived from SNF are crucial for meeting the world's food needs, even more so as crop yield increases from subsidies have begun to level off. Here we have shown that some agrichemicals, which are either applied to crops or found as contaminants in the soil, significantly disrupt SNF and subsequently lower plant yields.
Studies of chrysin, a clover-derived phytochemical and well characterized natural antagonist of alfalfa–S. meliloti signaling, have shown that the molecular mechanism of symbiotic inhibition is disruption of signaling between alfalfa-derived phytochemicals and S. meliloti NodD receptors that is necessary for initiation of nodulation and SNF (21, 22). Previously, we have presented data from in vitro assays showing a shared mechanism of action for chrysin, methyl parathion, DDT, bisphenol A, and pentachlorophenol (30, 31, 40). We showed not only that each of these chemicals competitively inhibited nod gene activation via NodD receptors in a dose-dependent manner, but also that each chemical's inhibitory effects on nod gene activation could be overcome by adding more flavonoid ligand, both hallmarks of competitive receptor–ligand interactions (30, 31, 40). Given the in vivo results described herein, which demonstrate that synthetic chemicals inhibit nodulation and SNF to a similar or stronger degree than chrysin, we propose that these pesticides are inhibiting symbiosis by exploiting preexisting antagonistic pathways established by chrysin and other competitive phytochemical signals. By mimicking and disrupting natural phytochemical signaling between legumes and Rhiz, these pesticides significantly delay the specific timing and initiation of symbiotic signaling crucial for efficient SNF. Pesticides may also disrupt symbiosis by altering the array of flavonoid phytochemicals a plant produces or by reducing the overall flavonoid secretion pattern, thereby disrupting plant–rhizobial signaling.
Metabolism of environmental chemicals by soil bacteria to more or less active compounds is an important consideration when studying effects on commercial crops that are treated with pesticides multiple times per year (41) and when considering the bioaccumulation and persistence of highly chlorinated pesticides (37). Although our data show that a one-time treatment with some pesticides delays or abolishes SNF, further studies are necessary to determine the effects of multiple pesticide treatments per harvest.
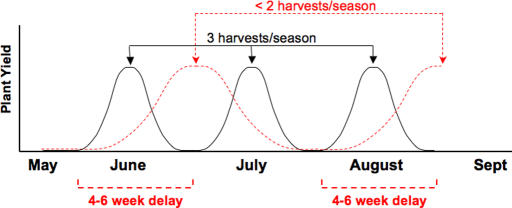
Because most commercial crops are treated with a mixture of pesticides multiple times during each growing season, and the average time until alfalfa harvest ranges from 28–35 days to obtain most protein per acre (42, 45), we propose that the significant degree and duration of pesticide-induced SNF inhibition demonstrated in this study is ecologically relevant and translates to an estimated one-third loss of plant yield per growing season according to our model (Fig. 3). Sustainable agricultural practices may be negatively impacted by pesticides, which, when applied to legume crops, disrupt SNF, decrease plant yield, and render legume crop rotations less effective for maintaining soil fertility. Pentachlorophenol, which is used as a pesticide, herbicide, antifungal agent, and wood preservative, is among the most ubiquitous chlorinated compounds found contaminating groundwater (43). With the exception of pentachlorophenol, all other chemicals tested in our study did not inhibit seed germination or general plant homeostasis. Although not affecting growth or expression of general housekeeping genes in bacteria or plants (44), the other organochlorine pesticides tested significantly disrupted the crucial timing of symbiotic initiation and caused a significant reduction in SNF.
Fig. 3.
Model of pesticide inhibition of symbiotic recruitment resulting in reduced harvest yields. Pesticides and environmental chemicals delay recruitment of S. melioti to host plants by 4–6 weeks after treatment. Alfalfa is harvested approximately every 30 days, which yields an average of three harvests per growing season (45). The environmental chemicals methyl parathion, DDT, bisphenol A, and pentachlorophenol inhibited symbiotic recruitment and delayed nodulation, resulting in suboptimal N fixation and reduced plant yields for 4–6 weeks after treatment. The resulting reduction in number of plants and/or prolonged time until plant yield maxima is reached may prolong time until harvest, resulting in fewer total harvests per season; a 4- to 6-week delay would result in an estimated loss of one harvest per season and a one-third reduction in overall alfalfa crop yield. The solid line indicates alfalfa treated with noninhibitory pesticides and/or not exposed to inhibitory environmental chemicals. The dashed red line indicates alfalfa treated with pesticides and/or exposed to inhibitory environmental chemicals that disrupt or delay symbiotic recruitment (i.e., methyl parathion, DDT, bisphenol A, pentachlorophenol, etc.).
Given that long-term agricultural studies have shown SNF is markedly lower in legume crops that are treated with N fertilizer and pesticides compared with untreated legumes (8), the observed reduction in N fixation and plant yield in pesticide treated vs. untreated alfalfa in this study is of important practical significance for sustainable agriculture. We propose that one explanation of the disparity in N fixation in treated vs. untreated legumes is that some pesticides disrupt the natural phytochemical signaling of legumes and thus disrupt the chemical communication between plant and Rhiz bacteria that is necessary for optimal SNF. We have shown that environmental chemicals impact SNF, a process that is crucial for meeting the world's food demands as crop yields from subsidies begin to level off. Therefore, in the future, in addition to planting enrichment crops such as legumes that are able to replenish the soil with N as a result of symbiosis, another way to combat the “diminishing returns” seen with conventional farming and promote sustainable agricultural practices may be to test and select pesticides that do not disrupt SNF.
Materials and Methods
Chemicals and Reagents.
The following chemicals were purchased: methyl parathion and pentachlorophenol (>99% pure) from AccuStandard (New Haven, CT), DDT (99% pure) from Aldrich (Milwaukee, WI), bisphenol A (98% pure) from Sigma (St. Louis, MO), and chrysin (>99% pure) from INDOFINE (Belle Mead, NJ). All chemicals were obtained neat and dissolved in DMSO. S. meliloti strain 1021 (46) was kindly provided by S. R. Long (Stanford University, Palo Alto, CA).
Inoculation, Treatment, and Growth of Plants.
Alfalfa (Medicago sativa) var. Iroquois seeds were surface-sterilized and inoculated with S. meliloti strain 1021 (46). Five replicate populations were prepared for each time point tested (2, 4, and 6 weeks after inoculation) for each treatment group. Each replicate contained 1.0 g of surface-sterilized alfalfa (M. sativa) var. Iroquois seeds (≈200 seeds by weight) suspended in either 25 ml of sterile water (−Rhiz, uninoculated negative control and chemical treatment-only groups) or dilute bacterial inoculate (all other treatment groups) prepared from overnight cultures of S. meliloti strain 1021 (46) that had been centrifuged and resuspended to a dilution of 106 cells/ml in sterile water. Sets of uninoculated and inoculated seed cultures then received a one-time treatment with either no chemicals (+Rhiz, positive control) or chrysin, methyl parathion, DDT, bisphenol A, or pentachlorophenol at 5 × 10−5 M concentration. Plants were incubated at 21°C in glass containers (240-ml volume) containing 20 g of sterilized vermiculite, and soil moisture content was maintained at 60% of water-holding capacity using sterile water. Plants were exposed to a 12-h photoperiod with cool white fluorescent lights at 110.4 μmol m−2·sec−1 and watered with Jensen liquid media (25, 47). Five replicates from each treatment group were harvested at 2, 4, and 6 weeks after inoculation, and the number of plants, number of nodules, and dry biomass were determined for each replicate at each time point.
Acetylene Reduction Measurements.
At 2, 3, 4, 5, and 6 weeks after inoculation, nitrogenase activities were determined for five replicate containers from each treatment group by using an acetylene reduction method as described (32, 33). Briefly, containers were sealed, and 10% of the headspace volume was replaced with atomic absorption-grade acetylene. Ethylene concentration in jar headspace was analyzed immediately after adding acetylene and subsequently at regular intervals for 4 h, by using a Shimadzu GC-14A gas chromatograph set up for direct headspace sampling (48) and equipped with a Valco E60 gas sampling valve, a 2-m stainless-steel column packed with Porapak N resin (Supelco, Bellefonte, PA), and a flame ionization detector. Rates of acetylene reduction were determined by monitoring the increase in the concentration of the reduction product, ethylene, in the headspace over time. Peak areas were quantified by using Shimadzu Class VP software. For statistical analysis, rates were averaged for five replicates per treatment per time point.
Acknowledgments
We thank members of the J.A.M. and J.G. laboratories for suggestions and support and the United States Department of Agriculture for support.
Abbreviations
- N
nitrogen
- SNF
symbiotic N fixation
- Rhiz
rhizobia
- DDT
dichlorodiphenyltrichloroethane
- +Rhiz
no chemicals
- −Rhiz
with chemicals.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG. Ecol Appl. 1997;7:737–750. [Google Scholar]
- 2.van Breemen N. Nature. 2002;415:381–382. doi: 10.1038/415381a. [DOI] [PubMed] [Google Scholar]
- 3.Tilman D. Proc Natl Acad Sci USA. 1999;96:5995–6000. doi: 10.1073/pnas.96.11.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruttan VW. Proc Natl Acad Sci USA. 1999;96:5960–5967. doi: 10.1073/pnas.96.11.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matson PA, Parton WJ, Power AG, Swift MJ. Science. 1997;277:504–509. doi: 10.1126/science.277.5325.504. [DOI] [PubMed] [Google Scholar]
- 6.Nambiar KKM. Soil Fertility and Crop Productivity Under Long-Term Fertilizer Use in India. New Delhi, India: Indian Council of Agricultural Research; 1994. [Google Scholar]
- 7.Cassmann KS, Steiner R, Johnston AE. Agricultural Sustainability: Economic, Environmental and Statistical Considerations. Chichester, UK: Wiley; 1995. [Google Scholar]
- 8.Drinkwater LE, Wagoner P, Sarrantonio M. Nature. 1998;396:262–265. [Google Scholar]
- 9.Tilman D, Fargione J, Wolff B, D'Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 10.Frink CR, Waggoner PE, Ausubel JH. Proc Natl Acad Sci USA. 1999;96:1175–1180. doi: 10.1073/pnas.96.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 12.Reid WV, Mooney HA, Cropper A, Capistrano D, Carpenter SR, Chopra K, Dasgupta P, Dietz T, Duralappah K, Hassan R, et al. Millennium Ecosystem Assessment: Ecosystems and Human Well-Being: Synthesis. Washington, DC: Island; 2005. [Google Scholar]
- 13.Matson PA, Naylor R, Ortiz-Monasterio II. Science. 1998;280:112–115. doi: 10.1126/science.280.5360.112. [DOI] [PubMed] [Google Scholar]
- 14.Cassman KG. Proc Natl Acad Sci USA. 1999;96:5952–5959. doi: 10.1073/pnas.96.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havlin JL, Beaton JD, Tisdale SL, Nelson WL. Soil Fertility and Fertilizers: An Introduction to Nutrient Management. Saddle River, NJ: Prentice–Hall; 1999. [Google Scholar]
- 16.Graham PH, Vance CP. Plant Physiol. 2003;131:872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson TA, Russelle MP. J Soil Water Cons. 1991;46:229–235. [Google Scholar]
- 18.Coutinho HL, DeOliveira VM, Moreira FMS. In: Applied Microbial Systematics. Goodfellow M, editor. Dordrecht, The Netherlands: Kluwer; 2000. pp. 107–134. [Google Scholar]
- 19.Peck MC, Fisher RF, Long SR. J Bacteriol. 2006;188:5417–5427. doi: 10.1128/JB.00376-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters NK, Frost JW, Long SR. Science. 1986;233:977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- 21.Peters NK, Long SR. Plant Physiol. 1988;88:396–400. doi: 10.1104/pp.88.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Firmin JL, Wilson KE, Rossen L, Johnston AWB. Nature. 1986;324:90–93. [Google Scholar]
- 23.Djordjevic MA, Redmond JW, Batley M, Rolfe BG. EMBO J. 1987;6:1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch AM, Bauer WD, Bird DM, Cullimore J, Tyler B, Yoder JI. Ecology. 2003;84:858–868. [Google Scholar]
- 25.Castillo M, Flores M, Mavingui P, Martinez-Romero E, Palacios R, Hernandez G. Appl Environ Microbiol. 1999;65:2716–2722. doi: 10.1128/aem.65.6.2716-2722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lupwayi NZ, Stephens PM, Noonan MJ. Symbiosis. 1996;21:233–248. [Google Scholar]
- 27.Unkovich MJ, Pate JS. Field Crops Res. 2000;65:211–228. [Google Scholar]
- 28.Graham PH, Vance CP. Field Crops Res. 2000;65:93–106. [Google Scholar]
- 29.Van Kessel C, Hartley C. Field Crops Res. 2000;65:165–181. [Google Scholar]
- 30.Fox JE, Starcevic M, Kow KY, Burow ME, McLachlan JA. Nature. 2001;413:128–129. doi: 10.1038/35093163. [DOI] [PubMed] [Google Scholar]
- 31.Fox JE, Starcevic M, Jones PE, Burow ME, McLachlan JA. Environ Health Perspect. 2004;112:672–677. doi: 10.1289/ehp.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardy RWF, Holsten RD, Jackson EK, Burns RC. Plant Physiol. 1968;43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weaver RW, Danso SKA. In: Methods of Soil Analysis. Part 2. Weaver RW, editor. Vol 5. Madison, WI: Soil Science Society of America; 1994. pp. 1019–1028. [Google Scholar]
- 34.D'Angelo EM, Reddy KR. Soil Sci Soc Am J. 2000;64:933–943. [Google Scholar]
- 35.Squillace PJ, Scott JC, Moran MJ, Nolan BT, Kolpin DW. Environ Sci Technol. 2002;36:1923–1930. doi: 10.1021/es015591n. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen JA, Yeager MA, Suffet IH. J Agric Food Chem. 2003;51:1360–1372. doi: 10.1021/jf025953q. [DOI] [PubMed] [Google Scholar]
- 37.Aigner EJ, Leone AD, Falconer RL. Environ Sci Technol. 1998;32:1162–1168. [Google Scholar]
- 38.Racke KD. Pure Appl Chem. 2003;75:1905–1916. [Google Scholar]
- 39.United Nations Food and Agriculture Organization. Land Quality Indicators and Their Use in Sustainable Agriculture and Rural Development. Rome: United Nations Food and Agriculture Organization; 1997. [Google Scholar]
- 40.Fox JE. Int Comp Biol. 2005;45:179–188. doi: 10.1093/icb/45.1.179. [DOI] [PubMed] [Google Scholar]
- 41.Gunier RB, Harnly ME, Reynolds P, Hertz A, Von Behren J. Environ Health Perspect. 2001;109:1071–1078. doi: 10.1289/ehp.011091071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rommann L. Alfalfa Production and Pest Management in Oklahoma. Tulsa, OK: Oklahoma Cooperative Extension Service, Oklahoma State University; 2003. [Google Scholar]
- 43.Kao CM, Chai CT, Liu JK, Yeh TY, Chen KF, Chen SC. Water Res. 2004;38:663–672. doi: 10.1016/j.watres.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 44.Fox JE, Starcevic M, Jones PE, Burow ME, McLachlan JA. Environ Health Perspect. 2004;112:648–653. doi: 10.1289/ehp.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Undersander D, Becker R, Cosgrove D, Cullen E, Doll J, Grau C, Kelling K, Rice ME, Schmitt M, Sheaffer C, et al. Alfalfa Management Guide. Madison, WI: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America; 2004. pp. 48–50. [Google Scholar]
- 46.Mulligan JT, Long SR. Proc Natl Acad Sci USA. 1985;82:6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon R, Oconnell M, Labes M, Puhler A. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- 48.Breitenbeck GA. Soil Sci Soc Am J. 1990;54:1794–1797. [Google Scholar]
- 49.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Methyl Parathion. Atlanta: US Department of Health and Human Services, Public Health Service; 2001. [PubMed] [Google Scholar]
- 50.Agency for Toxic Substances and Disease Registry. Toxicological Profile for DDT, DDE, and DDD. Atlanta: US Department of Health and Human Services, Public Health Service; 2002. [PubMed] [Google Scholar]
- 51.Fromme H, Kuchler T, Otto T, Pilz K, Muller J, Wenzel A. Water Res. 2002;36:1429–1438. doi: 10.1016/s0043-1354(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 52.Kang JH, Kondo F, Katayama Y. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Pentachlorophenol. Atlanta: US Department of Health and Human Services, Public Health Service; 2001. [Google Scholar]