Abstract
Autism spectrum disorders are characterized by cognitive control deficits as well as impairments in social interactions. However, the brain mechanisms mediating the interactive effects of these deficits have not been addressed. We employed event-related functional magnetic resonance imaging (fMRI) to examine the effects of processing directional information from faces on activity within brain regions mediating cognitive control. High-functioning individuals with autism and age-, gender-, and IQ-matched neurotypical individuals attended to the direction of a centrally-presented arrow or gaze stimulus with similar flanker stimuli oriented in the same (“congruent”) or opposite (“incongruent”) direction. The incongruent arrow condition was examined to assess functioning of brain regions mediating cognitive control in a context without social-cognitive demands, whereas the incongruent gaze condition assessed functioning of the same brain regions in a social-cognitive context. Consistent with prior studies, the incongruent arrow condition recruited activity in bilateral midfrontal gyrus, right inferior frontal gyrus, bilateral intraparietal sulcus, and the anterior cingulate relative to the congruent arrow condition in neurotypical participants. Notably, there were not diagnostic group differences in patterns of regional fMRI activation in response to the arrow condition. However, while viewing the incongruent gaze stimuli, although neurotypical participants recruited the same brain regions, participants with autism showed marked hypoactivation in these areas. These findings suggest that processing social-cognitive stimuli interferes with functioning of brain regions recruited during cognitive control tasks in autism. Implications for research into cognitive control deficits in autism are discussed.
Keywords: Autism, Functional Magnetic Resonance Imaging (fMRI), Cognitive Control, Executive Function, Attention, Social Cognition, Gaze
Introduction
Autism spectrum disorders are pervasive developmental disorders characterized by impairments in social interaction, impairments in communication, and restricted and repetitive behaviors and interests (American Psychiatric Association., 1994). The spectrum of social problems includes individuals who are completely uninterested in social interchange to those who are interested but incompetent at understanding the meaning of social interactions (Wing & Gould, 1979). Communication abnormalities range from a complete absence of speech, to echolalia, to speech that is deficient only in pragmatic understanding (Kjelgaard & Tager-Flusberg, 2001). Restricted repetitive and stereotyped behaviors and interests may be manifested as “lower order” behaviors, such as motor stereotypies, perseverative motor acts, or repetitive self-injurious behavior, or “higher order” behaviors such as compulsions, rituals, or insistence-on-sameness behaviors (Lewis & Bodfish, 1998).
Restricted repetitive and stereotyped behaviors and interests may reflect poor cognitive control abilities (i.e., so-called “executive” abilities) that interfere with behavioral control (Turner, 1997, 1999; Hughes, Russell, & Robbins, 1994; Ozonoff, Pennington, & Rogers, 1991; Lopez, Lincoln, Ozonoff, & Lai, 2005). The link between deficits in cognitive control and restricted repetitive behaviors in autism may reflect poor behavioral inhibition and/or generation of novel behavior, and is supported by their co-occurrence in other forms of psychopathology (Frith & Done, 1990). Associations have been reported between tasks designed to tap cognitive control abilities and specific classes of restricted repetitive behavior symptoms, and there is preliminary evidence that, whereas so-called “low level” repetitive motor behaviors are not specific to autism, “high level” repetitive behaviors appear to be characteristic and perhaps specific to this disorder (Frith, 1989; Wing & Gould, 1979; Turner, 1997, 1999).
Deficits in autism on neuropsychological tasks measuring executive functioning are well documented. Executive function subsumes the domains of working memory, inhibition, and mental flexibility, abilities that share the purpose of engaging, disengaging, and reengaging with the immediate milieu to guide actions (Lezak, 1995). A comprehensive review of the executive function literature in autism by Elisabeth Hill (2004) suggests that impairments in autism are most consistently found in the domain of planning (e.g., the Tower of Hanoi test), cognitive flexibility (e.g., the Wisconsin Card Sort test), and cognitive switching (e.g., the CANTAB ID/ED Shift task). The effect size of deficits has been estimated to be medium-to-large, more pronounced in autism than in other disorders characterized by disinhibition, and largest for differences on Wisconsin Card Sort Perseveration and Tower tasks than in other disorders characterized by disinhibition (Sergeant, Geurts, & Oosterlaan, 2002; Pennington & Ozonoff, 1996). However, not all studies reveal executive function deficit in autism: whereas there are consistent deficits in planning and flexibility, tests that assess inhibition, working memory, and attention do not consistently reveal impairments (Ozonoff & Jensen, 1999; Ozonoff & Strayer, 1997; Eskes, Bryson, & McCormick, 1990; Goldberg, Mostofsky, Cutting, Mahone, Astor, Denckla, & Landa, 2005).
In the current study, we examined cognitive control in high-functioning adults with autism. Cognitive control may be conceptualized as the allocation of top-down resources for task-relevant processes, and includes both behavioral control and performance monitoring components. Cognitive control is recruited under novel or complex conditions to optimize goal-directed behavior (Botvinick, Braver, Barch, Carter, & Cohen, 2001; MacDonald, Cohen, Stenger, & Carter, 2000; Barber & Carter, 2005). Although the constructs of “cognitive control” and “executive function” are not synonymous, these terms have been used interchangeably to describe maintenance of goal representations, updating goals, top-down guidance of information-processing, and performance monitoring. However, “executive function” is properly described in terms of its eventual outcome (i.e., deliberate and accurate problem-solving), and includes necessary sub-functions (Zelazo, Carter, Reznick, & Frye, 1997; Zelazo & Frye, 1998). For example, executive function is required both to understand a problem and attempt a solution, whereas cognitive control is the more specific component that mediates the latter process. In the present study, inhibition interference was examined to evaluate, in autism, brain regions mediating cognitive control in both social and non-social contexts.
Brain regions mediating cognitive control functions in neurotypical individuals include the lateral prefrontal cortex, the anterior cingulate cortex, the inferior frontal cortex (including the insula), the intraparietal sulcus, and the striatum. Lesions of the lateral prefrontal cortex have been linked to impaired performance on tasks of cognitive flexibility, stimulus categorization, and planning (Milner, 1963; Robinson, Heaton, Lehman, & Stilson, 1980; Shallice, 1982; Stuss, Levine, Alexander, Hong, Palumbo, Hamer, Murphy, & Izukawa, 2000), and functional neuroimaging studies suggest that the prefrontal cortex mediates cognitive set shifting and inhibitory control tasks (Rubia, Russell, Overmeyer, Brammer, Bullmore, Sharma, Simmons, Williams, Giampietro, Andrew, & Taylor, 2001; Konishi, Nakajima, Uchida, Kikyo, Kameyama, & Miyashita, 1999; Rogers, Andrews, Grasby, Brooks, & Robbins, 2000; Elliott, Baker, Rogers, O'Leary, Paykel, Frith, Dolan, & Sahakian, 1997). The functions of the anterior cingulate cortex are complex and wide-ranging, but include error detection, response monitoring, response evaluating, and correcting behaviors (MacDonald, Cohen, Stenger, & Carter, 2000), as well as integrating emotional and attentional processes (Fichtenholtz, Dean, Dillon, Yamasaki, McCarthy, & LaBar, 2004). The inferior frontal cortex appears to mediate response inhibition (Ramautar, Slagter, Kok, & Ridderinkhof, 2006), whereas parietal regions, both superior parietal cortex and temporal parietal junction, mediate visual and auditory spatial orienting and selective attention (Zimmer, Lewald, Erb, & Karnath, 2006). Finally, the striatum, consisting of the caudate nucleus and the putamen, has been implicated in planning and the execution of self-generated novel actions (Monchi, Petrides, Strafella, Worsley, & Doyon, 2006).
Current theory and research suggest that autism may be conceptualized as a disorder of the frontostriatal system (Bradshaw, 2001; Russell, 1997), and neuroimaging data provides direct validation of frontostriatal dysfunction in autism. Prefrontal hypoactivation in individuals with autism has been demonstrated using a number of paradigms, including “theory of mind” tasks (Happe, Ehlers, Fletcher, Frith, Johansson, Gillberg, Dolan, Frackowiak, & Frith, 1996; Baron-Cohen, Ring, Wheelwright, Bullmore, Brammer, Simmons, & Williams, 1999), receptive and expressive language tasks (Muller, Chugani, Behen, Rothermel, Muzik, Chakraborty, & Chugani, 1998), visual searches for embedded figures (Ring, Baron-Cohen, Wheelwright, Williams, Brammer, Andrew, & Bullmore, 1999), and selective attention (Belmonte & Yurgelun-Todd, 2003), target detection tasks (Gomot, Bernard, Davis, Belmonte, Ashwin, Bullmore, & Baron-Cohen, 2006), and a “go-no-go” task (Kana, Keller, Minshew, & Just, 2006). However, not all studies implicate hypoactivation of frontostriatal circuitry in autism. Schmitz and colleagues (2006) found that left-sided brain regions mediating inhibitory and set shifting behaviors were overactive in individuals with autism, whereas Just and colleagues (2006) found largely comparable cortical activations in individuals with autism during a planning task.
Autism is also characterized by profound deficits in social cognition, particularly within the domain of face processing. For example, individuals with autism demonstrate impaired face recognition and discrimination (Blair, Frith, Smith, Abell, & Cipolotti, 2002; Klin, Sparrow, de Bildt, Cicchetti, Cohen, & Volkmar, 1999; Boucher & Lewis, 1992; Robel, Ennouri, Piana, Vaivre-Douret, Perier, Flament, & Mouren-Simeoni, 2004; Behrmann, Avidan, Leonard, Kimchi, Luna, Humphreys, & Minshew, 2005; Tantam, Monaghan, Nicholson, & Stirling, 1989), deficits in social emotional judgments about faces (Adolphs, Sears, & Piven, 2001; Tantam, Monaghan, Nicholson, & Stirling, 1989; Weeks & Hobson, 1987), reduced emotion recognition and perception (e.g., Klin, Sparrow, de Bildt, Cicchetti, Cohen, & Volkmar, 1999; Gross, 2004), and abnormal eye scanpaths when viewing faces (Pelphrey, Sasson, Reznick, Paul, Goldman, & Piven, 2002; Klin, Jones, Schultz, Volkmar, & Cohen, 2002).
Despite evidence of deficits in cognitive control and processing faces in autism, reviewed above, there is little research to date addressing the interactive effects of deficits in these two domains. This is notable given the provocative evidence that performance on the Wisconsin Card Sort test in autism may be superior when the test is administered via computer than by an experimenter (Ozonoff, 1995), and that children with autism show greater deficits in visual orientation to social stimuli (name calling and hand clapping) than nonsocial stimuli (rattle, musical jack-in-the-box) (Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998). Moreover, no neuroimaging research to date has addressed the effects of processing social-cognitive information on the neural circuitry mediating cognitive control in autism.
In the present study, we sought to examine the neural correlates of one sub-domain of cognitive control, namely the inhibition of responding to interfering visual information, in response to stimuli with and without social-cognitive content. We employed a variant of the Attention Network Test (ANT, Fan & Posner, 2004; Fan, McCandliss, Sommer, Raz, & Posner, 2002), a well-replicated task originally designed to assess the integrity of attentional and executive networks. The so-called “executive attention” component of the ANT requires participants to indicate the orientation of a centrally-presented arrow in the presence of flanking congruent or incongruent arrows, with the latter condition imposing higher cognitive control demands. The incongruent condition has been shown to recruit the lateral prefrontal cortex, the anterior cingulate and the inferior frontal gyrus (MacDonald, Cohen, Stenger, & Carter, 2000; Fan, McCandliss, Fossella, Flombaum, & Posner, 2005; Bush, Luu, & Posner, 2000) and orienting to this task has been shown to activate parietal regions (both superior parietal cortex and temporal parietal junction (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005)). To examine whether the presence of social-cognitive stimuli adversely affects recruitment of this neural circuitry in autism, half of the trails employed left- and right-facing gaze stimuli instead of arrow stimuli. Although the ANT has not, to our knowledge, been used with gaze stimuli, it has been used with other, iconic stimuli with similar behavioral facilitation / conflict effects in neurotypical populations (Rueda, Fan, McCandliss, Halparin, Gruber, Lercari, & Posner, 2004).
Eye gaze is an important social perception cue and is a critical component of joint attention and social interaction, domains in which individuals with autism demonstrate marked impairments (e.g., Mundy, Sigman, Ungerer, & Sherman, 1986). In neurotypical individuals, observing averted gaze results in a reflexive shift of attention to the gazed-at location. Although individuals with autism demonstrate deficits in coordinating visual attention with others (i.e., joint attention) and understanding the mental states of others on the basis of information gathered from the eyes (e.g., Loveland & Landry, 1986; Mundy, Sigman, Ungerer, & Sherman, 1986; Baron-Cohen, 1995; Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001), such deficits in gaze processing do not appear to be based in eye gaze direction discrimination per se. Baron-Cohen (1995) demonstrated that such deficits appear to be characterized by impairments in using gaze to understand the mental states of others, and recent behavioral studies confirms that automatic attentional shifts in responses to static gaze direction are intact in individuals with autism (Kylliainen & Hietanen, 2004; Senju, Tojo, Dairoku, & Hasegawa, 2004; Ames & Jarrold, 2006; Bayliss & Tipper, 2005; Burgos, Kaplan, Foss-Feig, Kenworthy, Gilotty, Lee, Girton, Gaillard, & Vaidya, 2005). This pattern of findings suggests that autism is not characterized by deficits in reflexive attention orienting to gaze direction. Therefore, including an incongruent gaze condition in the present study would appear to be an appropriate method to examine inhibition interference in a social context in individuals with autism.
Based on prior research using variants of this paradigm (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005; Rueda, Fan, McCandliss, Halparin, Gruber, Lercari, & Posner, 2004), we hypothesized that in response to the incongruent arrow and gaze stimuli, neurotypical participants would show behavioral evidence of interference (i.e., decreased accuracy to incongruence) and would recruit mid- and inferior frontal cortex, the anterior cingulate gyrus, and the intraparietal sulcus. Because individuals with autism demonstrate intact behavioral performance on nonsocial tasks of interference inhibition (Ozonoff & Jensen, 1999; Eskes, Bryson, & McCormick, 1990) and automatic shift of attention in responses to static gaze direction (reviewed above), we predicted that both incongruent conditions (i.e., both arrows and gaze stimuli) would affect response accuracy in the autism group in a fashion similar to neurotypical participants and there would not be diagnostic group differences on behavioral performance on this task.
Based on brain imaging evidence, reviewed above, of reduced regional activation in individuals with autism during cognitive control tasks, we hypothesized that the autism group would demonstrate reduced activation in brain regions mediating inhibition interference (i.e., mid- and inferior frontal cortex, the anterior cingulate gyrus, and the intraparietal sulcus) in response to incongruent stimuli. Furthermore, because autism is characterized by deficits in processing information from faces, we hypothesized that hypoactivation of these brain regions would be particularly pronounced in response to the incongruent gaze stimuli.
Materials and methods
Participants
Seventeen right-handed participants with autism (1 female, 1 African American, 22.9 ± 5.2 years old) were recruited through the North Carolina Neurodevelopmental Disorders Research Center Subject Registry and the Treatment and Education of Autistic and Related Communication Handicapped Children (TEACCH) program in Chapel Hill, North Carolina, USA. Participants (and their guardians if younger than 18) consented to a protocol approved by the local Human Investigations Committees. Three met criteria for DSM-IV Asperger's Disorder and fourteen met criteria for DSM-IV High Functioning Autism. All participants with autism had Verbal IQ and Performance IQ ≥ 80 on the Weschler Abbreviated Scale of Intelligence (Weschler, 1999). Exclusion criteria included a prior history of gestational age <34 weeks, birth weight <2000 grams, intraventricular hemorrhage, history of known medical condition associated with autism including Fragile X syndrome, tuberous sclerosis, neurofibromatosis, phenylketouria, epilepsy and gross brain injury, or MRI contraindications.
Participants were paid for participating. Diagnoses were based on a history of clinical diagnosis of autism, parental interview (Autism Diagnostic Interview-Revised [ADI-R]; Lord, Rutter, & Le Couteur, 1994), and proband assessment (Autism Diagnostic Observation Schedule [ADOS]; Lord, Risi, Lambrecht, Cook, Leventhal, DiLavore, Pickles, & Rutter, 2000). Standard clinical ADI-R and ADOS algorithm cutoffs were employed. For the comparison group, 15 right-handed neurotypical participants (1 female, 24.6 ± 6.5 years) screened against major psychiatric illness, developmental disability, and neurological problems were recruited from the community.
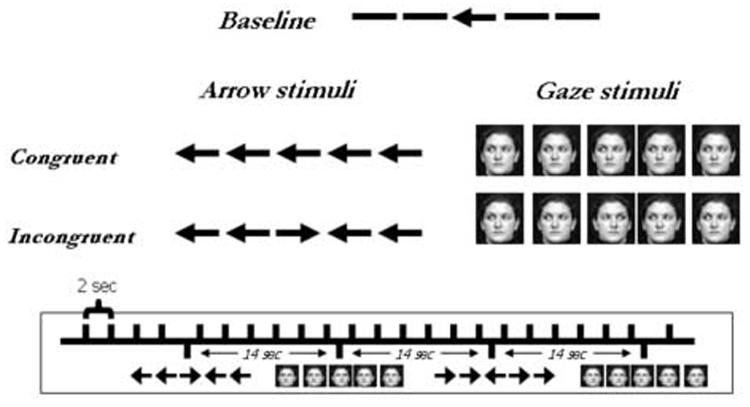
fMRI Task
Participants performed a reaction time flanker task modeled after the ANT task developed by Fan and colleagues (2002). The MRI session consisted of 10 task runs (3 min 54 sec each). Each run contained 116 stimuli presented centrally against a white background. Stimuli were presented every 2000 ms for 1700 ms or until the participant made a response, whichever came first. During the interval between trials, a fixation cross was presented. The baseline condition consisted of arrows flankered by horizontal lines. Figure 1 depicts the four experimental stimulus categories: (1) arrows flankered by same-direction arrows (‘congruent arrows’); (2) arrows flankered by opposite-direction arrows (‘incongruent arrows’); (3) gaze stimuli flankered by same-direction gaze stimuli (‘congruent gaze’); and (4) gaze stimuli flankered by opposite-direction gaze stimuli (‘incongruent gaze’). The single African American participant with autism viewed African American gaze stimuli rather than Caucasian gaze stimuli.
Figure 1.
Stimuli used in the present study. Participants viewed centrally-presented arrow or gaze stimuli that appeared for 1700 ms or until a button-press was made. Participants pressed one button to indicate that the central stimulus pointed to the left, and another to indicate that it pointed to the right.
Participants were required to indicate with a right-handed button press whether a central stimulus pointed to the left or right as quickly as possible without sacrificing accuracy. Rare stimuli were presented every seven trials (every 14 s) to adequately observe the hemodynamic response for each event. 72.2% of trials contained neutral arrows; 3.5% each contained congruent arrows, incongruent arrows, congruent faces, and incongruent faces. Finally, to diminish the predictability of the task, 13.8 % of the trials did not contain any targets but rather presented the fixation cross for the entire 2000 ms trial. In other words, the task was presented as an event-related design with 14.0 % of the trials containing rare events of interest embedded within the neutral arrow trials. All stimulus categories contained an equal number of left- and right-facing central stimuli, and all statistical analyses collapsed across left- and right-facing stimuli. Immediately prior to the scanning session, participants were trained on the task using a personal computer. All stimuli were presented using CIGAL presentation software (Voyvodic, 1996) on a Windows-compatible computer and displayed to participants through magnet-compatible goggles.
Imaging
Scanning was performed on a General Electric 4T LX NVi MRI scanner system equipped with 41 mT/m gradients (General Electric, Waukesha, Wisconsin, USA). A quadrature birdcage radio frequency (RF) head coil was used for transmit and receive. The participant's head was immobilized using a vacuum cushion. Sixty-eight high resolution images were acquired using a 3D fast SPGR pulse sequence (TR = 500 ms; TE = 20 ms; FOV = 24 cm; image matrix = 2562; voxel size = 0.9375 × 0.9375 × 1.9 mm) and used for coregistration with the functional data. These structural images were aligned in a near axial plane defined by the anterior and posterior commissures. Whole brain functional images were acquired using a gradient-recalled inward spiral pulse sequence (Glover & Law, 2001; Guo & Song, 2003) sensitive to blood oxygenation level dependent (BOLD) contrast (TR, 1500 ms; TE, 35 ms; FOV, 24 cm; image matrix, 642; α = 62°; voxel size, 3.75 × 3.75 × 3.8 mm; 34 axial slices). The functional images were aligned similarly to the structural images. A semi-automated high-order shimming program ensured global field homogeneity.
Imaging Data Analysis
Prior to statistical analysis, head motion was analyzed by center of mass measurements in three orthogonal planes. Data from three high-functioning autism participants were discarded due to excessive motion, resulting in fourteen autism participants with useable data. In addition, imaging epochs with mean intensities greater than three standard deviations of the average intensity in a run were excluded from analyses. Only epochs during which each participant gave a correct left-right response were included in analyses.
Image preprocessing was performed with custom programs and SPM modules (Wellcome Department of Cognitive Neurology, UK). Head motion was detected by center of mass measurements. No participant had greater than a 3-mm deviation in the center of mass in any dimension. Images were time-adjusted to compensate for the interleaved slice acquisition and then motion-corrected to compensate for small head movements. The realigned and motion-corrected images were then normalized to the Montréal Neurological Institute (MNI) template found in SPM99. These normalized functional data were then high-pass filtered and spatially smoothed with an 8 mm isotropic Gaussian kernel prior to statistical analysis. These normalized and smoothed data were used in the remaining analyses described below.
The primary analysis was a random-effects analysis of the differences between conditions (i.e., incongruent versus congruent arrow stimuli, incongruent versus congruent gaze stimuli) with respect to hemodynamic responses (HDRs). This random-effects analysis consisted of excising the epoch of image volumes beginning one image before (−1.5 s) and nine images after (+13.5 sec) the onset of each event from the continuous time series of volumes, regardless of response latency. Next, the average intensity of the HDR peak (i.e., the average magnitude values at 4.5 and 6.0 sec after stimulus onset) was derived, and a t-statistic was computed at each voxel to quantify the HDR differences between conditions. This process was performed separately for each participant. Next, the individual t-maps created in the preceding step were subjected to a random-effects analysis that assessed the significance of differences between conditions across participants within each diagnostic group. The threshold for significance was set at p < .05 (uncorrected) and a minimal spatial extent of six uninterpolated voxels.
To limit the possibility of Type I error due to multiple comparisons, we restricted our random effects analyses, described above, to only those voxels in which a significant HDR was evoked by any of the four different conditions. For this step, we thresholded our activation at a False Discovery Rate (FDR, Genovese, Lazar, & Nichols, 2002) of 0.01. The voxels with significant HDRs were identified by first averaging the single trial epochs for each participant separately for each condition, and the average BOLD-intensity signal values for each voxel within the averaged epochs were converted to percent signal change relative to the prestimulus baseline. Next, the time waveforms for each voxel were correlated with an empirical reference waveform and t-statistics were calculated for the correlation coefficients for each voxel. This procedure provided a whole-brain t-map in Montreal Neurological Institute (MNI) space for each condition. Next, the t-maps for each participant and for each condition were used to calculate an average t-map for all trial types across participants. Active voxels were then identified as those that surpassed the FDR threshold. Finally, the random-effects analysis difference t-maps were masked by the group average t-maps of the four different conditions. Thus, the differences in HDR amplitude between conditions were only evaluated for those voxels in which at least one condition evoked a significant HDR as defined above.
Average hemodynamic timecourses in active regions were derived by averaging BOLD activations in response to events of interest from voxels identified to be active by the random effects analysis described above. To assess for diagnostic group differences in conditions of interest, we computed Group X Region X Congruence repeated-measures ANOVAs separately for arrow and gaze trials with percent BOLD signal change within functionally-defined ROIs as dependent measures.
Results
Participant Characteristics
All analyses employed two-tailed statistical tests. As illustrated in Table 1, participant groups did not differ significantly with respect to age, education, verbal-, performance, or full-scale abbreviated IQ as assessed by the Weschler Abbreviated Scale of Intelligence (WASI, Weschler, 1999). Additionally, Wisconsin Card Sort Test-64 (WCST, Kongs, Thompson, Iverson, & Heaton, 2000) results indicated that autism participants demonstrated a trend towards completing fewer categories (M=2.5, SD=1.7) than neurotypical participants (M=3.3, SD=1.2), p < .10. . Finally, ADI-R and ADOS algorithm scores indicated mild- to- moderate levels of autism symptomatology.
Table 1.
Descriptive Statistics for the Autism and Neurotypical groups
| Autism (N=14, 1 ♀) |
Neurotypical (N=15, 1 ♀) |
|
|---|---|---|
| Age | 22.9 (5.2) | 23.2 (5.7) |
| Education | 12.79 (2.01) | 13.40 (2.95) |
| Abbreviated Verbal IQ | 105.1 (20.3) | 106.3 (16.2) |
| Abbreviated Performance IQ | 104.1 (17.8) | 103.7 (12.5) |
| Abbreviate Full Scale IQ | 105.0 (18.6) | 105.7 (15.0) |
| WCST-64 Categories* | 2.5 (1.7) | 3.3 (1.2) |
| WCST-64 Trials to first category | 23.5 (19.5) | 13.7 (4.8) |
| WCST-64 Perseverative Responses | 24.8 (15.6) | 17.7 (21.0) |
| WCST-64 Number of Errors | 38.57 (18.24) | 19.80 (10.46) |
| WCST-64 Number of Perseverative Errors | 32.71 (20.70) | 10.67 (5.61) |
| WCST-64 Failures to Maintain Set | 0.14 (0.53) | 0.40 (0.63) |
| ADI-R – Social | 20.29 (6.84) | |
| ADI-R – Verbal Communication | 16.07 (3.83) | |
| ADI-R – Repetitive Behavior | 5.50 (1.65) | |
| ADOS – Communication | 3.36 (1.95) | |
| ADOS – Social | 6.07 (2.62) | |
| ADOS – Imagination | 1.14 (0.66) |
p<.05
WCST-64: The Wisconsin Card Sorting Test-64 Card Version (Kongs, Thompson, Iverson, & Heaton, 2000); ADI-R: The Autism Diagnostic Interview-Revised (Lord, Rutter, & Le Couteur, 1994); ADOS: The Autism Diagnostic Observation Schedule (Lord, Risi, Lambrecht, Cook, Leventhal, DiLavore, Pickles, & Rutter, 2000).
Behavioral Performance
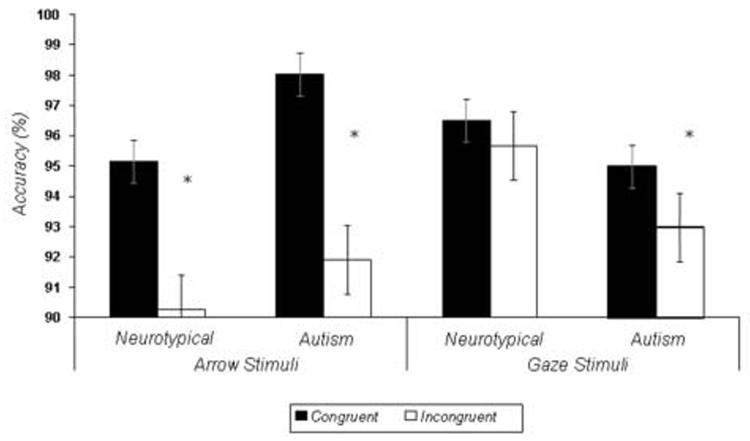
Figure 2 illustrates accuracy (percent correct) data in response to both arrow and gaze stimuli in both diagnostic groups. An omnibus 2 (Group: autism, neurotypical) X 2 (Stimulus Category: arrows, gazes) X 2 (Congruency: congruent, incongruent) repeated-measures ANOVA revealed a main effect of Congruency, multivariate F(1,27)=9.67, p <.005, reflecting that both groups were less accurate to incongruent than congruent trials, but no main effects of Stimulus Category or Group. A significant interaction of Congruency and Stimulus Category, multivariate F(1,27)=4.23, p < .05, was also found, reflecting a greater effect of congruency on arrow stimuli than gaze stimuli. All other interactions were not significant. Within groups t-tests indicated that the neurotypical group made more errors to incongruent trials when responding to arrow stimuli, t(13) = 3.15, p < .01, but not gaze stimuli, p > .20. The autism group, however, made more errors to incongruent trials when responding to both arrow stimuli, t(12) = 2.38, p < .04, and gaze stimuli, t(12) = 2.17, p < .05.
Figure 2.
Accuracy data for the fMRI task collected during the fMRI session. Errors bars represent group standard errors of the mean. * p < .05.
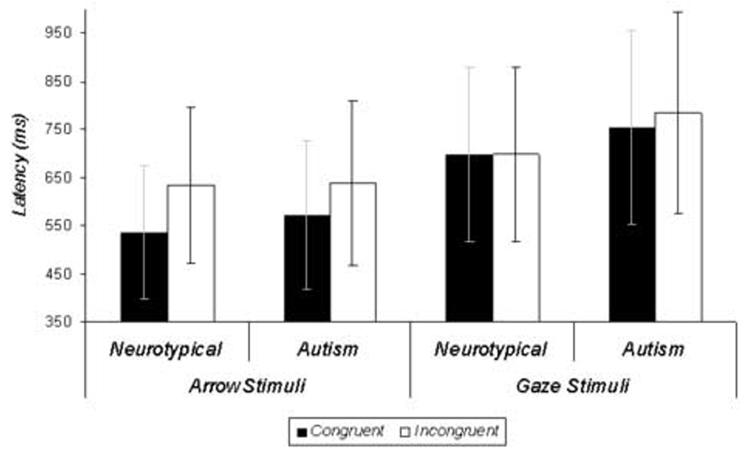
Figure 3 illustrates reaction time data in response to both arrow and gaze stimuli in both diagnostic groups. An omnibus 2 (Group: autism, neurotypical) X 2 (Stimulus Category: arrows, gazes) X 2 (Congruency: congruent, incongruent) repeated-measures ANOVA revealed no main effects or interactions (p's>.05). Within groups t-tests were not significant as well.
Figure 3.
Reaction time data for the fMRI task collected during the fMRI session. Errors bars represent group standard errors of the mean.
Imaging Data
Random-effects analysis of the differences between conditions with respect to hemodynamic responses (HDRs) included only epochs corresponding to correct responses (there were no differences overall with respect to accuracy between diagnostic groups (Neurotypical percentage mean (SD) = 94.4 (9.0), Autism mean percentage (SD) = 94.5 (10.0), p > .99).
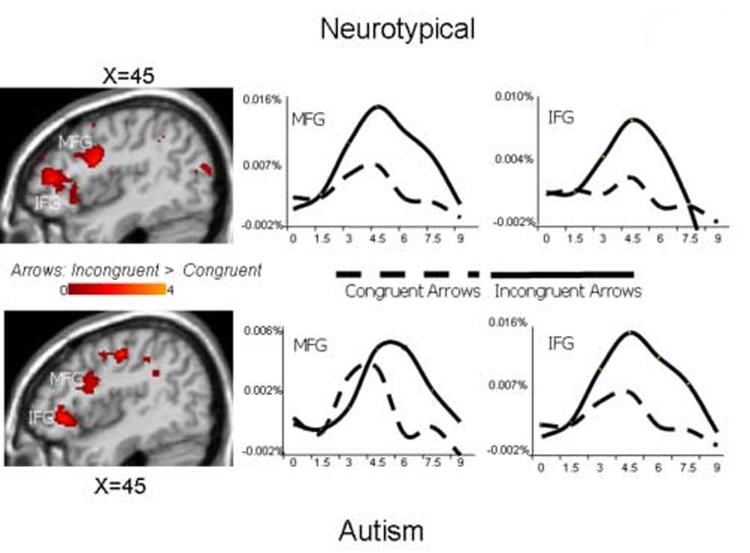
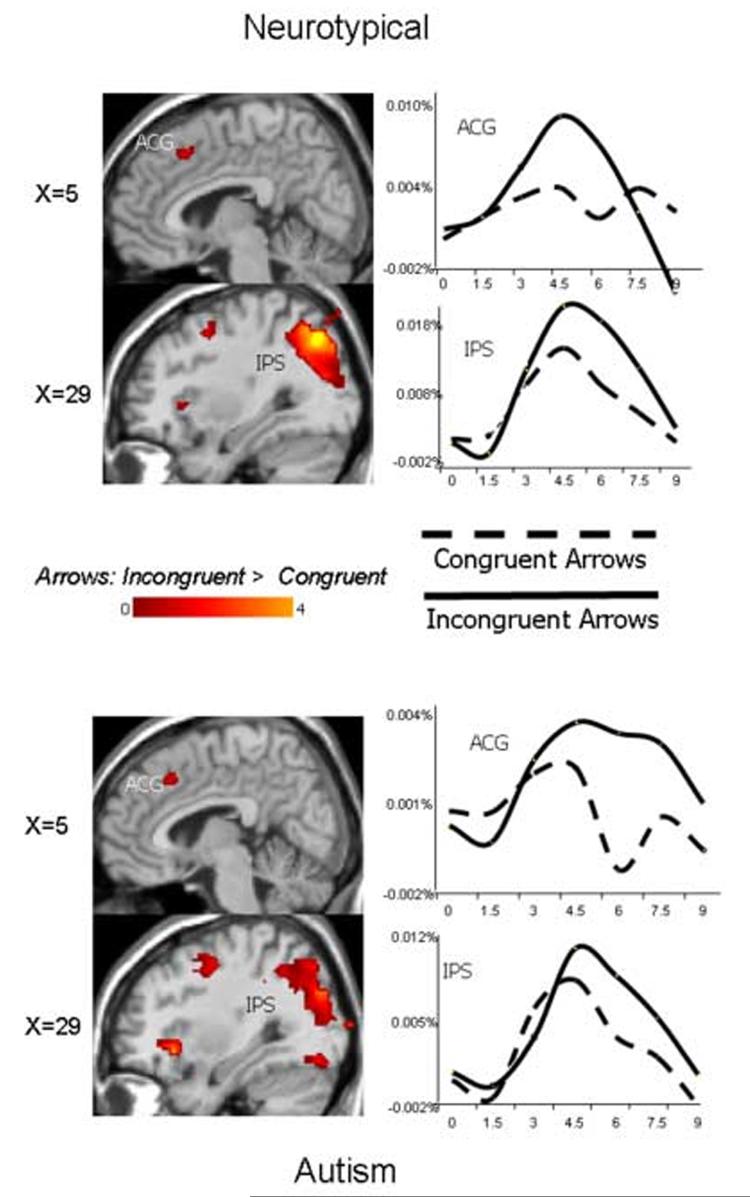
Figures 4 and 5 illustrate random effects difference contrast maps of responses to incongruent arrows and congruent arrows for neurotypical (top) and autism (bottom) participants as well as average BOLD HDRs from active regions of interest (i.e., mid- and inferior frontal cortex, the anterior cingulate gyrus, and the intraparietal sulcus) for these two conditions. The figures reveal that, in neurotypical participants, the incongruent arrow stimuli recruited a network shown to be associated with target detection, response selection, and action, relative to congruent trials. Common regions of significant signal increase included bilateral DLPFC, right inferior frontal/anterior insular cortex, anterior cingulate, and bilateral IPS. An examination of the corresponding random effects difference maps in the autism group revealed recruitment of a similar brain network in response to the incongruent relative to congruent arrow stimuli across these four brain regions. Average HDR's from voxels with significant differences between conditions illustrate that both groups recruited these regions to a greater extent in response to incongruent arrow stimuli relative to congruent arrow stimuli.
Figure 4.
Random effects contrast analyses comparing incongruent and congruent arrow trials and average hemodynamic response functions from voxels in the labeled regions that significantly differentiated conditions in neurotypical (top) and autism participants (bottom). Y-axis is percent signal change.
Figure 5.
Random effects contrast analyses comparing incongruent and congruent arrow trials and average hemodynamic response functions from voxels in the labeled regions that significantly differentiated conditions in neurotypical (top) and autism participants (bottom). Y-axis is percent signal change.
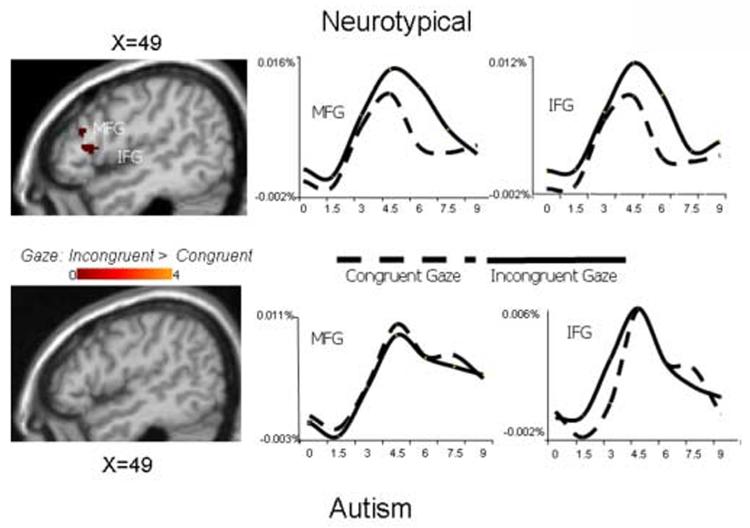
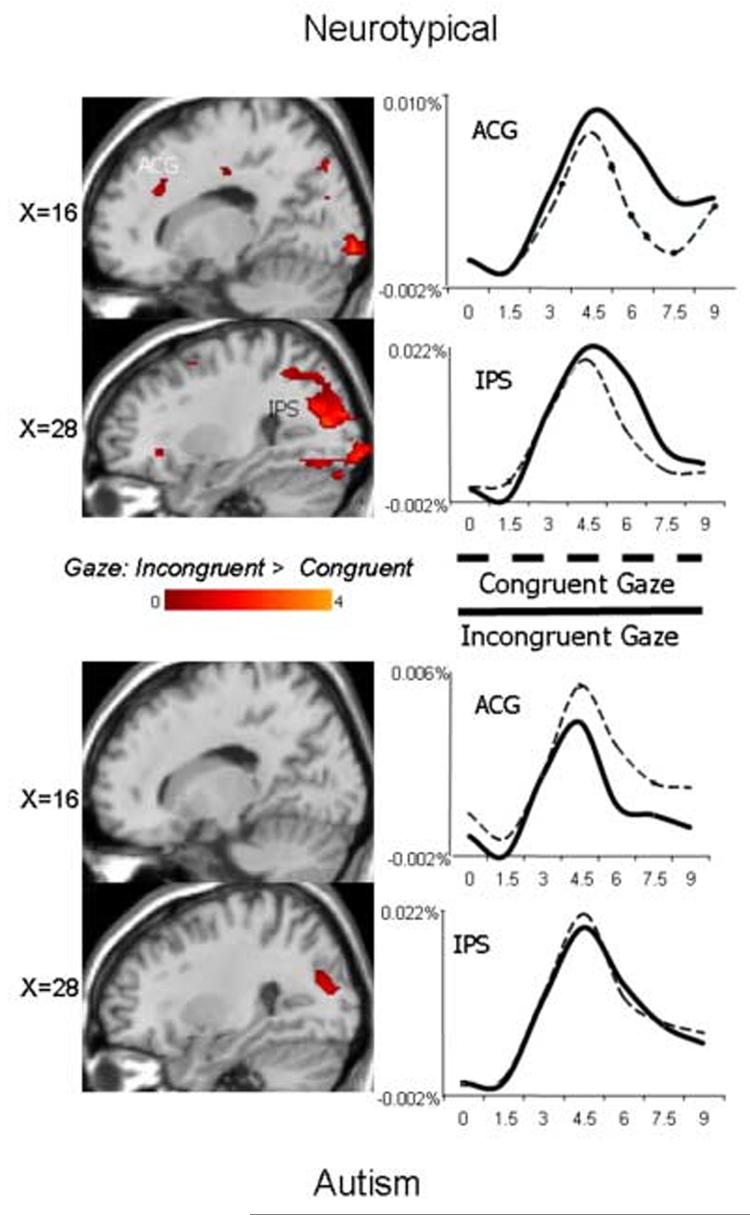
Figures 6 and 7 illustrate the results of similar analyses performed on incongruent and congruent gaze trials. In neurotypical participants, the incongruent gaze stimuli recruited the same network of brain regions as the incongruent arrow stimuli: bilateral DLPFC, right inferior frontal/anterior insular cortex, anterior cingulate, and bilateral IPS. The corresponding random effects difference map in the autism group, however, revealed no significant activation differences in response to the incongruent relative to congruent gaze stimuli in these four brain regions. Average HDR's illustrate that, whereas the neurotypical group recruited these regions to a greater extent to incongruent gaze stimuli relative to congruent gaze stimuli, the autism group did not. Each cluster of incongruent > congruent activation was localized and the anatomical label, Brodmann area, and Talairach coordinates are reported in Table 2, separated by stimulus (arrow and gaze) and group (autism, neurotypical).
Figure 6.
Random effects contrast analyses comparing incongruent and congruent gaze trials and average hemodynamic response functions from voxels in the labeled regions that significantly differentiated conditions in neurotypical (top) and autism participants (bottom). Y-axis is percent signal change.
Figure 7.
Random effects contrast analyses comparing incongruent and congruent gaze trials and average hemodynamic response functions from voxels in the labeled regions that significantly differentiated conditions in neurotypical (top) and autism participants (bottom). Y-axis is percent signal change.
Table 2.
Summary of Observed Regions of Incongruent > Congruent Activation for Arrow (top) and Gaze (bottom) Stimuli.
| Arrow Stimuli | ||||||
|---|---|---|---|---|---|---|
| X | Y | Z | Side | BA | Region | |
| Neurotypical Group | ||||||
| 47 | 30 | 13 | R | 46 | Inferior Frontal Gyrus | |
| 49 | 8 | 32 | R | 9 | Inferior Frontal Gyrus | |
| −43 | 10 | 33 | L | 9 | Middle Frontal Gyrus | |
| 35 | 1 | 53 | R | 6 | Middle Frontal Gyrus | |
| 3 | 21 | 44 | R | 8 | Cingulate gyrus | |
| 7 | 18 | 30 | R | 8 | Medial Frontal Gyrus | |
| 33 | −64 | 45 | R | 7 | Superior Parietal Lobule | |
| −29 | −59 | 45 | L | 7 | Superior Parietal Lobule | |
| Autism Group | ||||||
| 42 | 28 | 23 | R | 46 | Middle Fontal Gyrus | |
| 40 | 24 | 0 | R | 47 | Inferior Frontal Gyrus | |
| 50 | 7 | 27 | R | 9 | Inferior Frontal Gyrus | |
| −31 | 26 | 5 | L | 45 | Inferior Frontal Gyrus | |
| 10 | 22 | 41 | R | 32 | Cingulate Gyrus | |
| 29 | −71 | 42 | R | 19 | Precuneus | |
| −23 | −61 | 43 | L | 7 | Superior Parietal Lobule | |
| 31 | −78 | −2 | R | 18 | Inferior Occipital Gyrus | |
| 36 | −39 | 42 | R | 40 | Inferior Parietal Lobule | |
| −33 | −65 | −3 | L | 19 | Fusiform Gyrus | |
| Gaze Stimuli | ||||||
|---|---|---|---|---|---|---|
| X | Y | Z | Side | BA | Region | |
| Neurotypical Group | ||||||
| 45 | 16 | 12 | R | 44 | Inferior Frontal Gyrus | |
| 54 | 25 | 25 | R | 46 | Middle Frontal Gyrus | |
| −38 | 27 | 21 | L | 46 | Middle Frontal Gyrus | |
| 17 | −18 | 43 | R | 24 | Cingulate Gyrus | |
| 21 | −98 | 7 | R | 18 | Middle Occipital Gyrus | |
| −23 | 80 | 0 | R | 18 | Middle Occipital Gyrus | |
| −29 | −84 | 17 | L | 19 | Middle Occipital Gyrus | |
| 26 | 27 | 0 | R | 13 | Insula | |
| Autism Group | ||||||
| 31 | −79 | 25 | R | 19 | Superior Occipital Gyrus | |
| −29 | −85 | −2 | L | 18 | Middle Occipital Gyrus | |
X, Y, and Z refer to the stereotaxic coordinates of the center of the ROI activation. R: right hemisphere; L: left hemisphere; BA: Broadman's Area.
To evaluate diagnostic group differences in functional activity within regions of the cognitive control brain network (i.e., bilateral DLPFC, right inferior frontal/anterior insular cortex, anterior cingulate, and bilateral IPS), average HDR's (expressed as percent signal change relative to baseline) in these regions were used as dependent measures in Group (Autism, Neurotypical) X Region (bilateral DLPFC, right inferior frontal/anterior insular cortex, anterior cingulate, and bilateral IPS) X Congruence (Incongruent, Congruent) repeated-measures ANOVAs separately for arrow and gaze trials. These values were derived by averaging activation values at the peak of the HDR (4.5-6.0 seconds) for voxels identified to have greater activation in random effects contrast analyses, described earlier, in response to incongruent relative to congruent trials. Because there was no congruency effect for the gaze conditions in regions of interest in the autism group, voxels showing a congruency effect in the neurotypical group were queried in the autism group to generate average HDR's for the gaze conditions.
For the arrow trials, there were main effects of Congruence (multivariate F[1,27]=4.31, p < .05) and Region (multivariate F[1,27]=13.79, p < .0001), but no main effect of Group or interactions with Group, p's>.05. For the gaze trials, there was a main effect of Region (multivariate F[1,27]=12.05, p < .0001), a main effect of Group, (F[1,27]=4.42, p < .05), and, critically, a Congruence X Group interaction (multivariate F[1,27]=4.67, p < .05). This latter interaction reflects the fact that increased activation in these brain regions in response to incongruent relative to congruent gaze trials was significantly greater for the neurotypical group than the autism group. This interaction was not significant for the arrows trials, signifying that increased regional brain activation to incongruent relative to congruent trials differed between diagnostic groups for only the gaze conditions.
Given that accuracy data from the autism group revealed an incongruency effect in response to gaze stimuli, we assessed whether the autism group demonstrated relatively greater activation than the neurotypical group in any regions in response to incongruent gaze stimuli. Because of the post-hoc nature of this exploratory analysis, we examined the whole brain. Similar to other analyses, the threshold for significance was set at p < .05 (uncorrected) and a minimal spatial extent of six uninterpolated voxels. This analysis revealed that there were no regions demonstrating greater activations in the autism group in response to the gaze stimuli.
Relations with ADI-R/ADOS scores
Pearson correlations conducted within the autism group between algorithmic domains of the ADI and ADOS-G and average congruent minus incongruent HDR differences at the peak of the HDR response for both incongruent arrow and gaze stimuli revealed no significant correlations (all p's > .20).
Discussion
The current investigation sought to characterize the neural circuitry mediating one component of cognitive control, the inhibition of interference, in neurotypical and high-functioning individuals with autism in the context of both non-social (arrow) and social-cognitive (gaze) stimuli. Our most novel finding was that, in response to incongruent non-social and social stimuli, neurotypical participants recruited a cognitive control brain network consisting of bilateral dorsolateral prefrontal cortex, right inferior frontal/anterior insular cortex, anterior cingulate, and bilateral intraparietal sulcus. Participants with autism demonstrated comparable activations in this brain network in response to the incongruent arrow stimuli. However, this cognitive control network was not recruited in response to incongruent social stimuli in the autism group. These brain imaging results suggest that social stimuli interfere with recruitment of brain regions mediating cognitive control in individuals with autism.
Our findings from neurotypical participants in response to the arrows trials are consistent with previous neuroimaging data using this interference inhibition task (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005). The recruitment of the DLPFC in response to incongruent arrow stimuli is particularly noteworthy given that this regions is critical for cognitive flexibility and control (e.g., Shafritz, Kartheiser, & Belger, 2005; Elliott, 2003). The finding that the autism group engaged this cognitive control brain network, and the DLPFC in particular, in the non-social task conditions was unexpected in light of previous evidence of reduced DLPFC activation in autism during other executive tasks (Ring, Baron-Cohen, Wheelwright, Williams, Brammer, Andrew, & Bullmore, 1999; Muller, Chugani, Behen, Rothermel, Muzik, Chakraborty, & Chugani, 1998; Gomot, Bernard, Davis, Belmonte, Ashwin, Bullmore, & Baron-Cohen, 2005) as well as evidence of anomalous error-related negativity (ERN) amplitudes in response to these stimuli (Henderson, Schwartz, Mundy, Burnette, Sutton, Zahka, & Pradella, 2006). Additionally, Schmitz and colleagues (2006) recently reported no differences in right hemisphere structures but increased brain activation in left inferior frontal and parietal regions in autism during executive tasks. It is likely that differences with respect to specific task demands across studies are responsible for disparate results, and more research is needed to delineate the nature of the neural correlates of executive function deficits in autism.
In the arrows conditions, both participant groups exhibited the classic pattern of decreased accuracy in response to incongruent relative to congruent stimuli. It is somewhat surprising that the autism group demonstrated comparable accuracy performance on this portion of the task in light of neuropsychological data demonstrating impaired performance on tasks of executive function in autism such as the Wisconsin Card Sort (Pennington & Ozonoff, 1996) and the CANTAB ID/ED Shift task (Ozonoff, Cook, Coon, Dawson, Joseph, Klin, McMahon, Minshew, Munson, Pennington, Rogers, Spence, Tager-Flusberg, Volkmar, & Wrathall, 2004). However, the ease of this portion of the task (i.e., both groups achieved over 90% accuracy on all trials) that cognitive control deficits in the autism group were not observed in accuracy profiles.
In the gaze conditions, the autism group demonstrated behavioral evidence of cognitive interference (i.e., less accuracy to incongruent relative to congruent stimuli), although the neurotypical group did not. It is possible that the neurotypical group automatically attended to the central faces, to the exclusion of the flanker faces, and thus received a relative accuracy benefit when viewing incongruent gaze stimuli. The autism group, however, appears not to have received such a benefit, and persisted in demonstrating cognitive interference from the flanker gaze stimuli. Alternatively, the neurotypical group may have engaged a more automatic, feature-based approach to classifying the faces as left- or right-facing. However, the autism group, perhaps due to less expertise with faces, may have engaged a more holistic approach, resulting in conflict from the peripheral face stimuli (although such a conjecture is inconsistent with evidence of weak central coherence in autism (e.g., Happe & Frith, 1996)). Future studies should examine this hypothesis by systematically varying the saliency of gaze features to assess the effects on induced behavioral conflict.
Reaction time data revealed no congruence effects, diagnostic group differences, or group interactions, suggesting that the accuracy patterns do not reflect a differential reaction time-accuracy trade-off in the autism group. It is also particularly noteworthy that the social nature of the stimuli impacted regional brain activation patterns but not behavioral performance in the autism group, suggesting that imaging data reveal subtle deficits not observable in overt performance in this context.
We did not find significant correlations between the magnitude of activation in executive brain regions and ADI or ADOS-G algorithm scores, likely due in part to our relatively small sample size and restricted range of symptoms in our high-functioning sample. Future studies should more systematically assess links between regional brain hypoactivation and restricted and repetitive behaviors in autism with measurement tools designed to tap this construct specifically.
Although our findings are broadly consistent with the frontostriatal hypothesis of autism (for a review, see Bradshaw, 2001), we note that the conflict conditions in the present study were not designed to recruit striatal activations because the probabilities of different responses (i.e., left or right) did not differ between task conditions. Studies that employ tasks with rare response sets, such as target detection tasks (Shafritz, Kartheiser, & Belger, 2005) are necessary to examine links between striatal activation and executive deficits in autism. Additionally, the non-social and social stimuli used in this study were not equated for stimulus complexity, and there is evidence that the visuo-spatial information processing abilities of individuals with autism are intact with simple stimuli but impaired with complex stimuli (Bertone, Mottron, Jelenic, & Faubert, 2005). Although random effects difference maps should yield differences only due to stimulus incongruency, this potential confound should be addressed in future studies.
In summary, we found functional brain imaging evidence of unimpaired activation of a cognitive control brain network in high-functioning individuals with autism while processing non-social stimuli requiring inhibition of interfering information. However, when processing social stimuli requiring inhibition of interference, the autism group demonstrated hypoactivation in key regions of this brain network. These imaging findings suggest that processing social-cognitive stimuli interferes with recruitment of brain regions mediating cognitive control in individuals with autism. These findings are consistent with reports of better Wisconsin Card Sort test performance in individuals with autism when the test is administered via computer than by an experimenter (Ozonoff, 1995) and behavioral reports of the moderating effects of social stimuli in other neurocognitive domains (Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998). These results suggest that social impairments in autism may adversely interact with executive function deficits, and that studies of social and executive abilities in autism should address that these domains are likely not isolated but rather influence each other.
Acknowledgements
The authors would like to thank Syam Gadde, Chris Petty, and Cy Kim for assistance with image analysis and MRI technologists Jordan Tozer, Susan Music, and Natalie Goutkin for assistance with data acquisition. We wish to thank Dr. Grace Baranek, Marisa Houser, Raechel Kiska, and Dr. Allen Song for assistance with several aspects of this research. This research was supported by the North Carolina Studies to Advance Autism Research and Treatment Center, Grant 1 U54 MH66418 from the National Institutes of Health. Gabriel Dichter was supported by Postdoctoral Research in Neurodevelopmental Disorders, NICHD T32-HD40127.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13(2):232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Ames CS, Jarrold C. The Problem with Using Eye-Gaze to Infer Desire: A Deficit of Cue Inference in Children with Autism Spectrum Disorder? J Autism Dev Disord. 2006 doi: 10.1007/s10803-006-0309-5. [DOI] [PubMed] [Google Scholar]
- Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb Cortex. 2005;15(7):899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11(6):1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Bayliss AP, Tipper SP. Gaze and arrow cueing of attention reveals individual differences along the autism spectrum as a function of target context. Br J Psychol. 2005;96(Pt 1):95–114. doi: 10.1348/000712604X15626. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, et al. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2005 doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003;17(3):651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128(Pt 10):2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Frith U, Smith N, Abell F, Cipolotti L. Fractionation of visual memory: agency detection and its impairment in autism. Neuropsychologia. 2002;40(1):108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic children. J Child Psychol Psychiatry. 1992;33(5):843–859. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL. Developmental disorders of the frontostriatal system: neuropsychological, neuropsychiatric, and evolutionary perspectives. Psychology Press Ltd.; Taylor & Francis Inc; East Sussex, Philadelphia, Pa.: 2001. [Google Scholar]
- Burgos E, Kaplan LA, Foss-Feig J, Kenworthy L, Gilotty L, Lee PS, et al. poster presented at the Society for Neuroscience. Washington, DC: 2005. Atypical neural basis of inhibitory control in the context of emotion in childhood Autism. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Elliott R. Executive functions and their disorders. Br Med Bull. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O'Leary DA, Paykel ES, Frith CD, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27(4):931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- Eskes GA, Bryson SE, McCormick TA. Comprehension of concrete and abstract words in autistic children. J Autism Dev Disord. 1990;20(1):61–73. doi: 10.1007/BF02206857. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, Posner M. Human attentional networks. Psychiatr Prax. 2004;31(Suppl 2):S210–214. doi: 10.1055/s-2004-828484. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Res Cogn Brain Res. 2004;20(1):67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Frith C, Done DJ. Stereotyped behavior in madness and in health. In: Cooper SJ, Dourish CT, editors. Neurobiology of stereotyped behavior. Clarendon Press; Oxford: 1990. [Google Scholar]
- Frith U. Autism: Explaining the enigma. Blackwell; Oxford: 1989. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, Denckla MB, et al. Subtle executive impairment in children with autism and children with ADHD. J Autism Dev Disord. 2005;35(3):279–293. doi: 10.1007/s10803-005-3291-4. [DOI] [PubMed] [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, et al. Change detection in children with autism: An auditory event-related fMRI study. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, et al. Change detection in children with autism: An auditory event-related fMRI study. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Gross TF. The perception of four basic emotions in human and nonhuman faces by children with autism and other developmental disabilities. J Abnorm Child Psychol. 2004;32(5):469–480. doi: 10.1023/b:jacp.0000037777.17698.01. [DOI] [PubMed] [Google Scholar]
- Guo H, Song AW. Single-shot spiral image acquisition with embedded z-shimming for susceptibility signal recovery. J Magn Reson Imaging. 2003;18(3):389–395. doi: 10.1002/jmri.10355. [DOI] [PubMed] [Google Scholar]
- Happe F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8(1):197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Happe F, Frith U. The neuropsychology of autism. Brain. 1996;119(Pt 4):1377–1400. doi: 10.1093/brain/119.4.1377. [DOI] [PubMed] [Google Scholar]
- Henderson H, Schwartz C, Mundy P, Burnette C, Sutton S, Zahka N, et al. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain Cogn. 2006;61(1):96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32(4):477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and Anatomical Cortical Underconnectivity in Autism: Evidence from an fMRI Study of an Executive Function Task and Corpus Callosum Morphometry. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory Control in High-Functioning Autism: Decreased Activation and Underconnectivity in Inhibition Networks. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An Investigation of Language Impairment in Autism: Implications for Genetic Subgroups. Lang Cogn Process. 2001;16(23):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29(6):499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test—64 Card Computerized Version. Psychological Assessment Resources; Odessa, FL: 2000. [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen JK. Attention orienting by another's gaze direction in children with autism. J Child Psychol Psychiatry. 2004;45(3):435–444. doi: 10.1111/j.1469-7610.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Mental Retardation & Developmental Disabilities Research Reviews. 1998;(4):80–89. [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. Oxford University Press; New York: 1995. [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the Relationship between Executive Functions and Restricted, Repetitive Symptoms of Autistic Disorder. J Autism Dev Disord. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview - Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Loveland KA, Landry SH. Joint attention and language in autism and developmental language delay. J Autism Dev Disord. 1986;16(3):335–349. doi: 10.1007/BF01531663. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of brain lesions on card sorting. Arch Neurol. 1963;9:90–100. [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59(2):257–264. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Muller RA, Chugani DC, Behen ME, Rothermel RD, Muzik O, Chakraborty PK, et al. Impairment of dentato-thalamo-cortical pathway in autistic men: language activation data from positron emission tomography. Neurosci Lett. 1998;245(1):1–4. doi: 10.1016/s0304-3940(98)00151-7. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: the contribution of non-verbal communication measures. J Child Psychol Psychiatry. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Reliability and validity of the Wisconsin Card Sorting Test in studies of autism. Neuropsychology. 1995;9(4):491–500. [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, et al. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism network. J Autism Dev Disord. 2004;34(2):139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Brief report: specific executive function profiles in three neurodevelopmental disorders. J Autism Dev Disord. 1999;29(2):171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J Child Psychol Psychiatry. 1991;32(7):1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Inhibitory function in nonretarded children with autism. J Autism Dev Disord. 1997;27(1):59–77. doi: 10.1023/a:1025821222046. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32(4):249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Slagter HA, Kok A, Ridderinkhof KR. Probability effects in the stop-signal paradigm: The insula and the significance of failed inhibition. Brain Res. 2006 doi: 10.1016/j.brainres.2006.02.091. [DOI] [PubMed] [Google Scholar]
- Ring HA, Baron-Cohen S, Wheelwright S, Williams SC, Brammer M, Andrew C, et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain. 1999;122(Pt 7):1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Robel L, Ennouri K, Piana H, Vaivre-Douret L, Perier A, Flament MF, et al. Discrimination of face identities and expressions in children with autism: same or different? Eur Child Adolesc Psychiatry. 2004;13(4):227–233. doi: 10.1007/s00787-004-0409-8. [DOI] [PubMed] [Google Scholar]
- Robinson AL, Heaton RK, Lehman RA, Stilson DW. The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions. J Consult Clin Psychol. 1980;48(5):605–614. doi: 10.1037//0022-006x.48.5.605. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13(2):250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Russell J. Autism as an executive disorder. Oxford University Press; Oxford ; New York: 1997. [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006;59(1):7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. J Child Psychol Psychiatry. 2004;45(3):445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav Brain Res. 2002;130(12):3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Kartheiser P, Belger A. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. Neuroimage. 2005;25(2):600–606. doi: 10.1016/j.neuroimage.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, et al. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38(4):388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children's ability to interpret faces: a research note. J Child Psychol Psychiatry. 1989;30(4):623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Turner M. Towards an Executive Dysfunction Account of Repetitive Behaviour in Autism. In: Russell J, editor. Autism as an executive disorder. Oxford University Press; Oxford ; New York: 1997. pp. 57–100. [Google Scholar]
- Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40(6):839–849. [PubMed] [Google Scholar]
- Voyvodic JT. Real-time fMRI paradigm control software for integrating stimulus presentation, Behavioral and physiological monitoring, and statistical analysis (pp. 1835) Proc. Soc. Mag. Reson. Med., 15th Annual Meeting. 1996 [Google Scholar]
- Weeks SJ, Hobson RP. The salience of facial expression for autistic children. J Child Psychol Psychiatry. 1987;28(1):137–151. doi: 10.1111/j.1469-7610.1987.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Weschler D. Weschler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J Autism Dev Disord. 1979;9(1):11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Carter A, Reznick JS, Frye D. Early development of executive function: a problem-solving framework. Review of General Psychology. 1997;(1):198–226. [Google Scholar]
- Zelazo PD, Frye D. Cognitive Complexity and Control II: The development of executive function in chidhood. Current Directions in Psychological Science. 1998;7(4):121–126. [Google Scholar]
- Zimmer U, Lewald J, Erb M, Karnath HO. Processing of auditory spatial cues in human cortex: an fMRI study. Neuropsychologia. 2006;44(3):454–461. doi: 10.1016/j.neuropsychologia.2005.05.021. [DOI] [PubMed] [Google Scholar]