Abstract
Investigations using electrical impedance spectroscopy to measure the responses of fish embryos to the cryoprotective chemicals methanol and dimethyl sulfoxide (DMSO) were carried out. Zebrafish (Danio rerio) embryos were used as a model to study the newly proposed technique. The permittivity and conductivity changes of the embryos were measured continuously over a 20 min period in a customised embryo holding cell. The permittivity and conductivity spectra were obtained during embryo exposure to different concentrations of methanol (1.0M, 2.0M and 3.0M) and DMSO (0.5M, 1.0M and 2.0M) solutions. The results showed significant permittivity and conductivity changes after embryo exposure to methanol and DMSO at the optimum embryo loading level (6 embryos). Embryos in different concentrations of methanol and DMSO also resulted in quantitative responses shown in the permittivity and conductivity spectra. The results demonstrated that fish embryo membrane permeability to cryoprotective chemicals could be monitored in real-time. Measurement of permittivity at a lower frequency range (10 – 103 Hz) and conductivity at a higher frequency range (104 – 106 Hz) during fish embryo exposure to cryoprotective chemicals using impedance spectroscopy can be used as a new tool for the fast screening for most effective cryoprotective chemicals. The results from the present study also demonstrated the possibility of quantifying the level of cryoprotective chemicals penetrating the fish embryos.
Keywords: impedance spectroscopy, cryopreservation, embryos, membrane permeability, zebrafish (Danio rerio), permittivity, conductivity
1. Introduction
Electrical Impedance Spectroscopy (EIS) is a widely used technique for measuring electrochemical phenomenon on solid, porous, synthetic and biological materials. EIS measures the electrical properties of biological materials, i.e. the conductivity and permittivity as a function of applied alternative current (AC) frequency. For many years, the technique has been used to determine the impedance properties of normal and damaged biological tissues (Schwan, 1957; Ackmann and Seitz, 1984; Foster and Schwan, 1989; Gabriel et al, 1996). McRae et al (1999) and Paulsen et al (1999) demonstrating the application of the technique as a non-invasive method for measuring the damaged tissues in human tumours. Applications of the technique in membrane studies were also reported by Moreno and Valiente (1996) and Wiegand et al (2000) for non-invasive measurement of biological membranes during ionic transportation processes. Wiegand (2000) studied a supported bilayer focusing on their electrical properties using EIS. In this work, the electrical characteristics of the lipid bilayers were studied with a number of structural and environment variables. The technique was also used for the evaluation of the incorporation kinetics and selective conductivity of ion channels of membranes (Benavente and Ramos-Barrado, 1997).
Although considerable progress has been made in cryopreservation of embryos of mammalian species over the last two decades, successful cryopreservation of fish embryos has not been achieved and low fish embryo membrane permeability to water and cryoprotectants has been identified as one of the major obstacles to their successful cryopreservation (Zhang, 2004). Information on membrane permeability is vital to the successful cryopreservation of fish embryos. Obtaining accurate information on cryoprotectant penetration of fish embryos and selecting the most effective cryoprotective chemicals are important in designing optimum cryopreservation protocols.
At present, a conventional volumetric measurement based technique is used in fish embryo membrane permeability studies (Zhang and Rawson, 1996 and 1998). This method is inherently difficult with zebrafish embryos because a high percentage of the embryo volume is osmotically inactive (Zhang and Rawson 1998), and volumetric changes during exposure to both permeating and non-permeating solutes are therefore small (Hagedorn et al, 1997). Also the embryos are only approximately spherical which makes accurate estimations of volume changes difficult and the method is lengthy, reducing the capacity for multi-embryo measurement. Although some new techniques have been applied in fish embryo membrane permeability studies including nuclear magnetic resonance (NMR) spectroscopy (Hagedorn et al, 1996 and 1997), these methods do not provide real-time or quantitative information on cryoprotectant penetration into the embryos.
The complex nature of fish embryos requires the development of a new membrane permeability assessment method. An alternative approach is to measure the electrical impedance response of fish embryos during their exposure to cryoprotective chemicals that could provide important information on embryo membrane permeability. At an optimal frequency it would also be possible to correlate the changes of permittivity and conductivity values with embryo responses to different concentrations of cryoprotective chemicals, thereby providing quantitative information on cryoprotectant penetration of the embryos. The objectives of the present study were 1) to assess the sensitivity of the EIS technique in studying zebrafish embryo membrane permeability; 2) to investigate permittivity and conductivity spectra responses to different concentrations of the selected cryoprotective chemicals.
2. Materials and Methods
2.1. Instrumentation
Permittivity and conductivity spectra were recorded using Solartron SI1260 Impedance/Gain-Phase Analyser (Novocontrol Technologies, Germany) with the impedance measurement range from 10 mOhms – 100 Mohms (accuracy 0.1% and 0.1°), AC amplitude range 0.01V – 3.0 Vrms, a resolution in loss factor < 0.4 mrad and frequency from 10 μHz - 32 MHz. Six parameters including dielectric constant (permittivity), specific conductivity, serial/parallel impedance, capacity and tan (d), temperature and actual time are measured by the instrument in dependence of four free variables (frequency, temperature, dc-bias and time). In this study, the AC output voltage on the system was set at 1.0Vrms for current measurement across the frequency range between 10 Hz – 106 Hz with 12 logarithmic sweeping points per decade. An embryo holding cell was designed and made from a brass based and gold plated BDS 1200 sample cell (Novocontrol Technologies, Germany), which contains an embryo holding chamber and a cover lid. The holding cell is placed between the two parallel electrodes in the analyser. An embryo holding chamber was made with a diameter of 5.0 mm and 0.7 mm in depth which can hold up to 10 embryos (Figure 1). A thin Teflon isolative ring was placed on top of the embryo holding chamber for preventing unwanted contact between the chamber and cover lid. Geometry of the embryo holding chamber was specially designed for intact zebrafish embryos which are approximately 1.0 mm in diameter. When the embryos are loaded into the chamber, the outer membrane (chorion) of the embryo should be in contact with both sides of the holding cell that are linked to the system electrodes.
Figure 1.
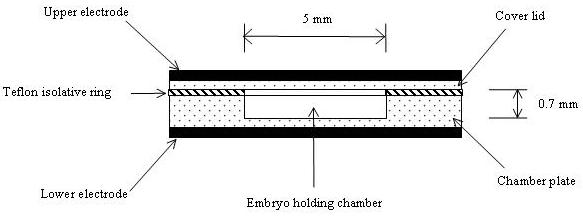
Schematic diagram of the embryo holding cell and connections with the system electrodes.
2.2. Zebrafish breeding and embryo collection
Adult zebrafish were held in a closed re-circulating system in 12 L tanks with 8 – 12 fish per tank. The temperature of the system water (deionised water containing 250 mg/L Ocean salt) was maintained at 28°C and the photo period was fixed at 14 h light: 10 h dark. Zebrafish were fed three times daily with TetraMin flake food (Tetra, Germany) and newly hatched brine shrimps. Embryos were collected from breeding trays each morning and maintained in embryo medium (60μg/ml ocean salt) at 28°C until 50% epiboly stage. Intact embryos at 50% epiboly stage were used in this study. The developmental stage of the embryos was examined using a laboratory microscope (Lecia, UK). All embryos used in the experiments were kept in embryo medium for at least 1 hour before use.
2.3. Studies of permittivity and conductivity spectra during embryos exposure to methanol and dimethyl sulfoxide
Embryo medium was prepared by dissolving 60 μg/ml ocean salt (ZM SYSTEMS Ltd, UK) in deionised water shortly before use. Two commonly used cryoprotective chemicals methanol (Sigma, UK) and dimethyl sulfoxide (DMSO, Sigma, UK) were selected for the experiments. Different concentrations of methanol at 1.0M, 2.0M and 3.0M and DMSO at 0.5M, 1.0M and 2.0M were made up with the embryo medium. For each experiment, six embryos were loaded into the holding cell with a pipette. Six embryos were used because this loading level was confirmed as the optimum loading level from our previous study (Zhang et al, 2004). The excess solutions were removed using tissue paper. For cryoprotectant treated embryos, the embryo medium in the holding cell was carefully removed with tissue paper and replaced with the medium containing cryoprotectant. The holding cell was then quickly covered with the lid and secured between the two electrodes. A continue sweep was set for 20 minutes at 1 minute intervals. All experiments were run under room temperature at 23 ± 1 °C. The holding cell was carefully cleaned and recalibrated with standard KCl solutions before each experiment. The permittivity data at each frequency step were normalised by subtracting t = 0 data from the subsequent data points. The absolute values of the subtracted data were used in the graphs to present the changing trends over time and between the different conditions at 5 minute after starting the experiment. The data normalisation is essential to extract the permittivity changing information from the experiment and minimise the impact of the polarisation effect on the electrodes. .
3. Results and Discussion
3.1. Changes of permittivity and conductive during embryo exposure to methanol and DMSO over time
The normalised permittivity spectra of 6 embryos loaded in embryo medium, 2.0 M methanol, and 2.0 M DMSO over 20 minutes are presented in Figures 2, 3 and 4 respectively. In the embryo medium experiment (Fig. 2), a clear increasing trend in the normalised permittivity spectra within 10 minutes at the frequency range between 10 – 102 Hz was obtained. At 10 Hz, the values were increased from 772.5 (standard deviation: 38.47) to 3349.3 (standard deviation: 207.99) and from 616.4 (standard deviation: 30.7) to 1687.11 (standard deviation: 104.77) for 102 Hz. A relatively small increase to unchanged values was followed 103 Hz - 106 Hz. The normalised permittivity values remained relatively steady after 10 minutes of the experiment. The same changing patterns were also recorded in both methanol and DMSO experiments. In the methanol experiment, the normalised permittivity were increased from 10.28 (standard deviation: 1.34) to 23.72 (standard deviation: 2.61) at 10 Hz (Fig 3) and 29.83 (standard deviation: 2.42) to 63.9 (standard deviation: 7.03) for DMSO (Fig 4). However, the increasing scale in the DMSO was higher than the methanol experiment.
Figure 2.
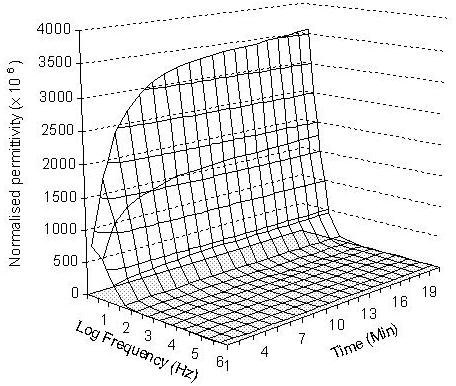
Three-dimensional presentation of the normalised permittivity on the measured 6 embryos over 20 minutes at the given frequency range from 10 to 106 Hz in embryo medium.
Figure 3.
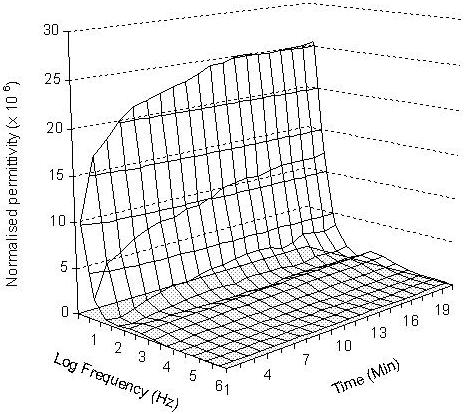
Three-dimensional presentation of the normalised permittivity on the measured 6 embryos over 20 minutes at the given frequency range from 10 to 106 Hz in 2.0 M methanol.
Figure 4.
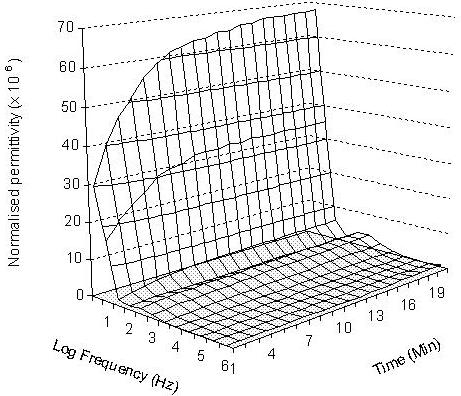
Three-dimensional presentation of the normalised permittivity on the measured 6 embryos over 20 minutes at the given frequency range from 10 to 106 Hz in 2.0 M DMSO.
For methanol and DMSO treated embryos, the increases in the normalised permittivity within the first 10 minutes might caused by the water, ion and cryoprotective chemical exchanges from both sides of the membrane, then the system gradually reaches an equilibrium stage which was reflected by the measured steady permittivity between 10 and 20 minutes. The differences in the normalised permittivity spectra between the untreated embryos (in embryo medium) and treated embryos (in both 2.0 M methanol and 2.0 M DMSO) reflected different levels of ion strength in embryo medium and cryoprotectant solutions, and embryo membrane permeability to the cryoprotective chemicals. The untreated embryos show the largest normalised permittivity increases in the first 10 minutes in comparison to methanol and DMSO treated embryos. This is mainly caused by the presence of stronger ionic properties in both intra-embryonic fluid and the extra-embryo media in the untreated embryos than the 2.0 M methanol and DMSO treated embryos. The normalised permittivity values measured from 2.0 M methanol exposure experiment were lower than those obtained with 2.0 M DMSO, which could be explained by the movements of more methanol molecules than DMSO to the intra-embryonic fluid of the treated embryos. This demonstrated that zebrafish embryo chorion is more permeable to methanol than DMSO. The normalised permittivity spectrum shows that more methanol molecules penetrated into the embryos and changed the ionic properties of the intra-embryonic fluid. These results are in agreement with the results obtained by Zhang and Rawson (1996) using a conventional real-time video microscopy technique. Despa (1995) also demonstrated that the value of the dielectric permittivity at low frequencies decreased (increased in normalised permittivity) gradually with the increase of membrane permeability for ions, while the electrical permittivity at high frequencies were unchanged. The effect was considered especially important for analysing of the dielectric spectrum of a tissue or membrane that has undergone the influence of various chemical agents.
Biological membranes play an important role in establishing the resting and active electric properties through regulation of the movement of ions between the extracellular and intracellular environments (Alcaraz, et al., 2001). During cryoprotectant treatment, embryos shrank initially due to water losses of the intra-embryo fluid , and then re-expanded indicating cryoprotectant penetration (Mazur, 2004). The embryo membrane permeability to different cryoprotective chemicals was reflected by different embryo shrink-swell profiles in different cryoprotective chemical solutions (Zhang and Rawson, 1996). The results from the present study demonstrate that the processes of osmosis and cryoprotectant penetration were measured in the permittivity spectra. The permittivity spectra also reflected the differences in embryo membrane permeability to different cryoprotective chemicals. The results are in good agreement with the studies of Asami (1977) and Despa (1995) which demonstrated that the electric permittivity at low frequencies decreases gradually with the increase in membrane permeability to ions.
3.2. Changes of permittivity and conductivity during embryo exposure to different concentrations of methanol and DMSO
The embryo permittivity responses to the treatment of different concentrations of methanol and DMSO at 5 min of the experiments were presented in Figures 5 and 6. In general, the permittivity spectra of embryos in methanol were approximately 10 fold lower than the permittivity measured from the untreated embryos in the low frequency range (10 – 103, Fig. 5), demonstrating the direct impact of methanol on permittivity. The spectra measured from the methanol treated embryos showed that at the frequency range 10 – 103 Hz permittivity values decreased with decreasing concentrations of methanol. Similar trends were also recorded for embryos treated by 0.5 M, 1.0 M and 2.0 M DMSO solutions (Figure 6). The results showed that the amount of methanol and DMSO penetration into the embryos also depended on the concentrations of the applied cryoprotectants. Embryos exposed to higher concentrations of both methanol and DMSO had larger permittivity value changes at the frequency 10 - 103 Hz. The results also showed that methanol treated embryos had larger permittivity changes than DMSO. The permittivity spectra responses to different concentrations of methanol and DMSO demonstrated that the changes of the intra-embryonic fluid caused by the penetrated methanol or DMSO can be measured by the EIS technique and it is clearly sensitive to commonly used concentration levels of cryoprotectants in cryobiological studies.
Figure 5.
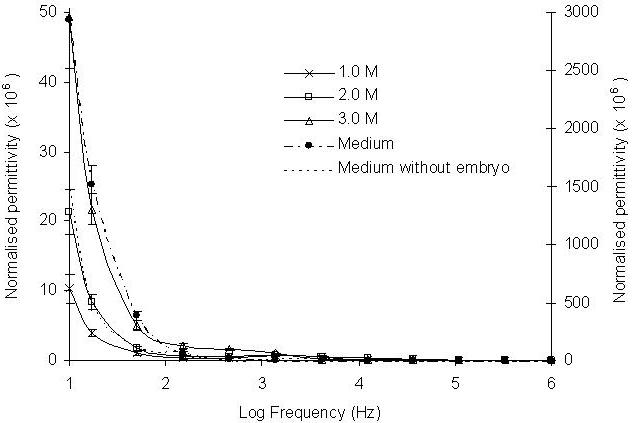
Effect of different concentrations of methanol on normalised permittivity change after 5 minutes exposure to concentrations of 1.0, 2.0 and 3.0 M (left-hand axis). Six embryos were exposed to the methanol in the frequency range between 10 - 106 Hz. The presented permittivity spectra were an average of ten runs. The reference spectra of the medium with or without embryos are presented using right-hand axis.
Figure 6.
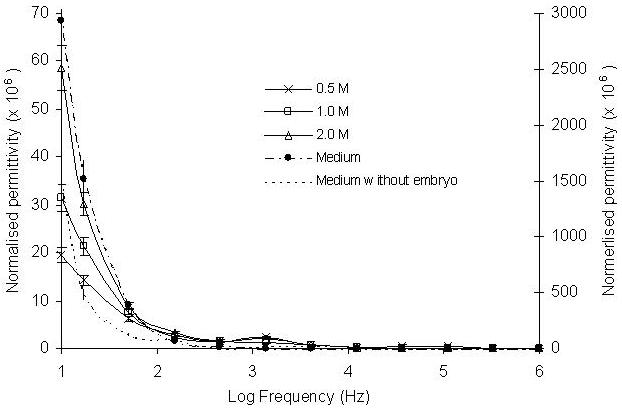
Effect of different concentrations of DMSO on normalised permittivity change after 5 minutes exposure to concentrations of 0.5, 1.0 and 2.0 M. Six embryos were exposed to the methanol in the frequency range between 10 - 106 Hz. The presented permittivity spectra were an average of ten runs. The reference spectra of the medium with or without embryos are presented using right-hand axis.
When embryos were treated with cryoprotectants, the properties of the bathing embryo medium were also changed due to exosmosis of the intra-embryonic water. These changes were revealed by the recorded conductivity spectra at the frequency range between 104 – 106 Hz for both methanol and DMSO treated embryos (Figures 7 and 8). Conductivity values decreased with increasing concentrations of methanol and DMSO treated embryos indicating changes of ionic levels of the bathing embryo medium.
Figure 7.
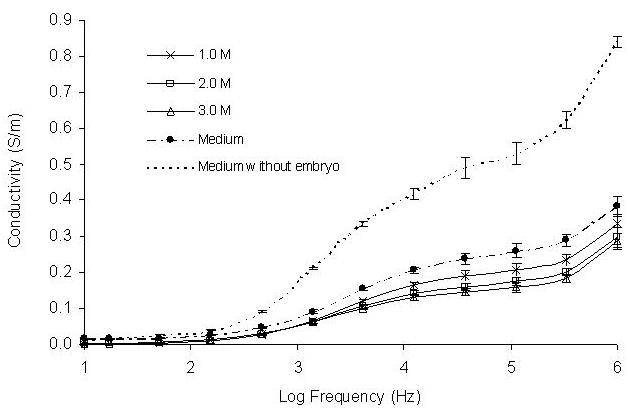
Effect of different concentrations of methanol on conductivity change after 5 minutes exposure to concentrations of 1.0, 2.0 and 3.0 M. Six embryos were exposed to the methanol in the frequency range between 10 - 106 Hz. The presented conductivity spectra were an average of ten runs.
Figure 8.
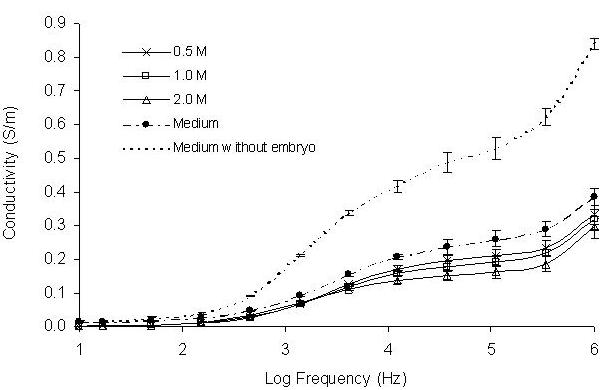
Effect of different concentrations of DMSO on conductivity change after 5 minutes exposure to concentrations of 1.0, 2.0 and 3.0 M. Six embryos were exposed to the methanol in the frequency range between 10 - 106 Hz. The presented conductivity spectra were an average of ten runs.
The electrical behaviour of biological tissues reveals a high frequency dependence of the dielectric parameters due to the various relaxation phenomena that occur when the current passes through the tissue (Schwan, 1988). When the frequency increases, the conductivity rises. The increase in conductivity is associated with a decrease in permittivity (Rigaud, B. et al, 1996). The changing trends and relationship between the measured conductivity and permittivity are clearly shown with both methanol and DMSO treated embryos. At low frequencies, the charge time is sufficiently low to allow the charging and discharging of the membrane to take place in a single period, which induces high permittivity. When the frequencies are increased, the membrane capacitive reactance decreases, which induces an increase in the current flow passing through the intercellular medium and, therefore, an increase in the conductivity. The increase in frequency also prevents the membrane from being completely charged during a complete cycle, thus causing a decrease in permittivity (Rigaud, B. et al, 1996).
4. Conclusions
To our knowledge, the EIS technique has never been explored and reported for fish embryo membrane permeability studies. The results presented in this study indicated that the EIS technique is clearly sensitive to methanol and DMSO penetration during the treatment of cryoprotective chemicals. The permittivity and conductivity changes appeared to be frequency dependent. In the lower frequency range (10 – 104 Hz), permittivity values increases with the increasing concentrations of cryoprotectants, which can be explained as the increasing of cryoprotective chemical compounds in the intra-embryonic fluid. The conductivity spectrum, however, has shown a clear decreasing trend with increasing concentrations of the cryoprotective chemicals at the higher frequencies 104 – 106 Hz, resulting from the reduced cryoprotective chemical concentration and the fractional increase of intra-embryonic water in the bathing embryo medium.
The changing trends of normalised permittivity over time at the frequency range 10 – 104 Hz agree well with the conventional video microscopic technique (Zhang and Rawson, 1996) which indicated that the significant penetration of the cryoprotective chemicals into the embryos occurs after 3 – 10 minutes of exposure to methanol and DMSO. However, EIS method is faster and can monitor changes in both the intra-embryonic fluid and the extra-embryonic bathing medium at the same time.
The results obtained from the present study on permittivity and conductivity changes are encouraging for the development of an EIS technique for use in embryo membrane permeability studies. The technique is especially useful for the selection of the suitable cryoprotective chemicals and may also allow quantitative measurements in embryo membrane permeability studies.
Acknowledgements
This research was funded by the Welcome Trust (GR069889RP).
References
- Ackman JJ, Seitz MA. Method of complex impedance measurements in biologic tissue. Crit Rev. Biomed. Eng. 1984;11:281–311. [PubMed] [Google Scholar]
- Alcaraz A, Ramirez P, Manzanares JA, mafe S. Conductivity and capacitive properties of bipolar membrane junction studied by AC impedance spectroscopy. J. Phys. Chem. B. 2001;105:11669–11677. [Google Scholar]
- Asami K. Dielectric behaviour of yeast cell suspensions: effects of some chemical agents and physical treatments on the plasma membrane and the cytoplasm. Bull. Inst. Chem. Res. 1977;55:283–295. [Google Scholar]
- Benavente J, Ramos-Barrado JR, Heredia A. A study of the electrical behaviour of isolated tomato cuticular membranes and cutin by impedance spectroscopy measurements. Colloids Sarfaces A: Physicochem. Eng. Aspect. 1997;I40:333–338. [Google Scholar]
- Despa Sanda. The influence of membrane permeability for irons on cell behaviour in an electric alternating field. Phys. Med. and Biol. 1995;40:1399–1409. doi: 10.1088/0031-9155/40/9/001. [DOI] [PubMed] [Google Scholar]
- Foster KR, Schwan HP. Dielectric properties of tissues and biological materials: a critical review. Crit Rev. Biomed. Eng. 1989;17:25–104. [PubMed] [Google Scholar]
- Gaberial S, Lau RW, Gaberial C. The dielectric properties of biological tissues: measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. and Biol. 1996;41:2251–2296. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Hsu E, Pilatus U, Wildy DE, Rall WF, Blakband SJ. Magnetic resonance microscopy and spectroscopy reveal kinetics of cryoprotectant permeation in multicompartmental biological system. Proc. Nat. Acad. Sci. 1996;93:7454–7459. doi: 10.1073/pnas.93.15.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M, Hsu E, Kleinhans FW, Wildt DE. New approaches for studying the permeability of fish embryos: Toward successful cryopreservation. Cryobiology. 1997;34:335–347. doi: 10.1006/cryo.1997.2014. [DOI] [PubMed] [Google Scholar]
- Mazur P. Principles of cryobiology. In: Fuller B, Lane N, Benson E, editors. Life in the Frozen State. CRC Press; 2004. pp. 415–435. [Google Scholar]
- McRae DA, Esrick MA, Mueller SC. Changes in the non-invasive, in vivo electrical impedance of three xenografts during the necrotic cell-response sequence. Int. J. Radi. Onco.y Biol. Phys. 1999;43:849–857. doi: 10.1016/s0360-3016(98)00487-8. [DOI] [PubMed] [Google Scholar]
- Moreno JP, Valiente ME, Rigaud B, Felice CJ, Chauveau N, Marsili PM. Bioelectrical impedance techniques in medicine. Crit Rev. Biomed. Eng. 1996;24:221–677. [PubMed] [Google Scholar]
- Paaulsen KD, Osterman KS, Hoopes PJ. In vivo electrical impedance spectroscopic monitoring of the progression of radiation-induced tissue injury. Radiat. Res. 1999;152:41–50. [PubMed] [Google Scholar]
- Rigaud B, Morucci JP, Xhauveau N. Biorlrctrical impedance techniques in medicine, Part I: Bioimpedance measurement. Crit Rev. Biomed. Eng. 1996;24:257–351. [PubMed] [Google Scholar]
- Schwan HP. Electrical properties of tissues and cell suspensions. Phys. Med. and Bio. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- Schwan HP. Biological effectsof non-ironizing raditions: Cellular properties and interactions. Ann. Biomed. Eng. 1988;16:245–263. doi: 10.1007/BF02368002. [DOI] [PubMed] [Google Scholar]
- Wiegand G. Fundamental principles of the electric properties of supported lipid memnranes investigated advanced methods of impedance spectroscopy. Germany: Shaker Verlag GmbH; 2000. pp. 1434–4556. ISBN. [Google Scholar]
- Zhang T, Bao Q-Y, Wang RY, Rawson DW. Development of a new rapid measurement technique for fish embryo membrane permeability studies using impedance spectroscopy. Cryobiol. 2004;49:318. doi: 10.1016/j.theriogenology.2006.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TT, Rawson DM. Feasibility studies on vitrification of intact zebrafish (Brachydanio rerio) embryos. Cryobiol. 1996;33:1–13. [Google Scholar]
- Zhang TT, Rawson DM. Permeability of dechorionated one-cell and six-somite stage zebrafish (Brachydanio rerio) embryos to water and methanol. Cryobiol. 1998;37:13–21. doi: 10.1006/cryo.1998.2093. [DOI] [PubMed] [Google Scholar]
- Zhang TT. Cryopreservation of gametes and embryos of aquatic species. In: Fuller BJ, Lane N, Benson EE, editors. life in the Frozen State. CRC Press; 2004. pp. 415–435. [Google Scholar]


