Abstract
Glutamate promotes neuronal survival during brain development and destroys neurons after injuries in the mature brain. Glutamate antagonists are in human clinical trials aiming to demonstrate limitation of neuronal injury after head trauma, which consists of both rapid and slowly progressing neurodegeneration. Furthermore, glutamate antagonists are considered for neuroprotection in chronic neurodegenerative disorders with slowly progressing cell death only. Therefore, humans suffering from Huntington's disease, characterized by slowly progressing neurodegeneration of the basal ganglia, are subjected to trials with glutamate antagonists. Here we demonstrate that progressive neurodegeneration in the basal ganglia induced by the mitochondrial toxin 3-nitropropionate or in the hippocampus by traumatic brain injury is enhanced by N-methyl-d-aspartate antagonists but ameliorated by α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate antagonists. These observations reveal that N-methyl-d-aspartate antagonists may increase neurodestruction in mature brain undergoing slowly progressing neurodegeneration, whereas blockade of the action of glutamate at α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors may be neuroprotective.
Glutamate antagonists were demonstrated to be neuroprotective in stroke and head trauma in rodents and nonhuman primates (1, 2). Accordingly, the excitatory neurotransmitter glutamate has been pathogenetically linked to cell death in acute neurodegenerative disorders in humans such as stroke or traumatic brain injury (1). This inference prompted the assumption that glutamate antagonists must be neuroprotective in chronic neurodegenerative disorders in humans (3, 4). However, whether glutamate antagonists limit neurodegeneration in slowly progressing neurodegenerative disorders is not known. Nevertheless, clinical trials in humans suffering from Huntington's disease, Parkinson's disease, and severe dementia using glutamate N-methyl-d-aspartate (NMDA) antagonists are in progress (5).
Neuronal loss in the basal ganglia is the hallmark pathological feature of Huntington's disease and can be reproduced in rodents and primates by administration of the succinate dehydrogenase inhibitor 3-nitropropionate (3NP) (6). Intrastriatal administration of 3NP produces in rodents rapid neuronal death and dystonia resembling that seen in humans suffering from moldy sugarcane poisoning (7). Systemic administration of 3NP produces slowly progressing neuronal death in the striatum of rodents and primates with symptoms of chorea developing with a considerable delay (8). Similarly, traumatic head injury causes immediate death of neurons at impact in the cortex and slowly progressing neurodegeneration at sites distant to the impact such as hippocampus (9).
Here we demonstrate that slowly progressing neuronal death induced by systemic treatment with 3NP in the striatum or by traumatic brain injury in the hippocampus is enhanced by NMDA but ameliorated by α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) antagonists in mature rodent brain. Rapidly progressing neuronal death induced by intrastriatal treatment with 3NP or by traumatic brain injury in the cortex is decreased by NMDA antagonists.
Materials and Methods
3NP.
Systemic delivery.
Wistar rats (24–28 months old, 750–950 g) were implanted s.c. with osmotic minipumps under ether anesthesia. 3NP was infused at 12 or 24 mg/kg per d for 28 days. Dizocilpine (MK801; 0.3 mg/kg per d), memantine (24 mg/kg per d), 3-((±)-2-carboxypiperazin-4-yl)propyl-1-phosphonate (CPP; 24 mg/kg per d), 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (NBQX; 24 mg/kg per d), [1,2,3,4-tetrahydro-7-morpholinyl-2,3-dioxo-6-(trifluoromethyl)quinoxalin-1-yl]methylphosphonate (MPQX; 24 mg/kg per d), or vehicle were administered s.c. by means of osmotic minipumps either simultaneously with 3NP, 12 mg/kg per d or 24 mg/kg per d, or alone.
Intrastriatal microinjections.
Wistar rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and subjected to bilateral microinjection of 3NP, 100, 250, or 500 nmol, into the striatum at coordinates derived from the stereotaxic atlas of Swanson (10). The coordinates were: AP (anterior/posterior) 7.63; L (lateral) 2.0; V (ventral) 3.2. To assess effect of NMDA antagonists on neurodegeneration induced by 3NP, CPP, 250 nmol, was coadministered with 3NP, 250 nmol, into one striatum. The contralateral side received 3NP alone and served as control. Drugs were delivered into the striatum in a volume of 2 μl at a rate of 0.1 μl/min.
Neurologic assessment.
The following scoring system was used to grade neurologic impairment in rats subjected to treatment with 3NP: 0, no observable motor deficits; 1, reduction of spontaneous locomotor activity; 2, unsteady, wobbly gait, ataxia; and 3, severe depression of movement and loss of righting reflex. Scores were taken three times a week by a single observer under blinded conditions. Shown are mean ± SEM maximal scores registered during the entire observation period.
Morphology.
For morphological examination, rats were anesthetized with an overdose of pentobarbital and perfused with a fixative containing 1% paraformaldehyde and 1.5% glutaraldehyde in pyrophosphate buffer (for combined light and electron microscopy), or containing 10% acetic acid, 10% formaldehyde, 80% methanol (for paraffin embedding). Serial coronal sections of the whole brain were cut 10 μm thick, and every 20th section was mounted on a glass slide and stained with cresyl violet, or by Fink and Heimer technique (11). For electron microscopy striatal tissue was processed in osmium tetroxide, dehydrated in graded ethanols, cleared in toluene, embedded in araldite, and examined by transmission electron microscope.
Quantification of neuronal damage in the striatum.
The volume of the striatum in rats subjected to systemic treatment with 3NP, glutamate antagonists, or vehicle was measured 3 days after termination of continuous administration of drugs, by using an image analysis system. To provide an estimate for the overall striatal neuronal loss over the treatment period of 28 days, numerical densities (Nv) of striatal neurons were determined by means of the stereologic disector (12, 13) and the total number of neurons remaining in the striatum were calculated. The volume of the striatum and striatal damage resulting from intrastriatal microinjections of 3NP was estimated volumetrically 4 h after administration by using image analysis. To provide an estimate for neuronal loss after microinjection of 3NP, CPP, or vehicle into the striatum, Nv of normal neurons were determined in striatum by means of the stereologic disector and the numbers of neurons lost in the striatum were calculated.
Traumatic Brain Injury.
Contusing device.
The contusing device consisted of a stainless steel tube, 40 cm in length, perforated at 1-cm intervals to prevent air compression in the tube. Fischer 344 rats, 230–270 g, were anesthetized with tribromoethanol, 260 mg/kg i.p. A craniotomy over the right hemisphere was made, the device guiding a falling weight onto the footplate resting on the surface of the dura was placed perpendicular to the surface of the skull, and a force of 380 g × cm produced by a 20-g weight was selected to produce brain contusion. A maximum of 2.5 mm depression of the brain surface was allowed to avoid mechanical puncture of the dura. The center of the footplate was stereotaxically positioned 1.5 mm posterior and 2.5 mm lateral to the bregma. The contusion was made to the cortex corresponding to areas Fr1, Fr2, HL, and FL (10). Sham controls were subjected to anesthesia and surgery without the injury. The rats underwent perfusion fixation 3 days after brain injury. The treatment regimen with CPP, three hourly doses of 30 mg/kg i.p., was chosen to assure that relevant concentrations in the brain to interact with NMDA receptors were present. The AMPA/kainate antagonist NBQX was used as a drug reference and was given following the same regimen as that of CPP. Treatment with CPP or NBQX was initiated 2 h before, 1, 4, 7, or 10 h after traumatic cortex injury. CPP and NBQX were administered in a volume of 0.5 ml/100 g of body weight.
Physiological monitoring.
To monitor arterial blood pressure and blood gases, catheters were placed in the femoral and tail arteries. Samples of arterial blood were collected 5 min before and 30 min, 1, 2, and 3 h after traumatic brain injury and the last administration of vehicle, CPP, or NBQX. The pH, arterial oxygen, carbon dioxide, glucose, and bicarbonate levels were determined by using automated diagnostic procedures. A rectal temperature probe was connected to a temperature controller coupled to a heating pad maintained at 37.5 ± 0.5°C throughout surgery. After surgery, animals were transferred to individual home cages that were kept on heated tables set at 36.5–37.5°C for up to 24 h. During the following 48 h animals were individually housed under standard environmentally controlled conditions (6 a.m. to 6 p.m.; 12-hr light/dark cycle; 24–25°C) and were permitted free access to food and water. Body weight was monitored by means of a Sartorius model U6100 balance.
Morphometric analysis in cortex and hippocampus.
The volume of the damage in the cortex was determined stereologically 3 days after traumatic injury by using an image analysis. The damage in the hippocampal CA3 subfield was determined stereologically at 13 different rostrocaudal levels extending from 9.67 to 12.21 mm (10) and throughout its mediolateral axis 3 days after traumatic injury. To quantitatively assess neuronal loss in the hippocampus, stereological disector technique was used to estimate Nv of pyramidal neurons, and the numbers of neurons remaining in the CA3 subfield were calculated. An unbiased counting frame (0.05 mm × 0.05 mm; disector height 0.01 mm) and a high-aperture objective (×100) were used for sampling. Normal neurons were identified by the presence of the typical nuclei with clear nucleoplasm and distinct nucleolus surrounded by cytoplasm containing Nissl substance. The border between CA2 and CA3 subfields was considered as the point where the looser arrangement of large pyramidal cells goes into densely packed pyramidal cells of the subfield CA3. An arbitrary line connecting the lateral ends of the dentate granule cell layers was considered a junction between subfields CA3 and CA4.
Statistics.
Data were analyzed statistically by means of ANOVA, Student's t test, Mann–Whitney u test, and χ2 test.
Results
Systemic Delivery of 3NP and Glutamate Antagonists.
To induce slowly progressing neuronal death in the striatum, 3NP, 12 and 24 mg/kg per d, was administered systemically to 24- to 28-month-old rats by means of osmotic minipumps over 28 days. Neurological scores assessed three times/week during treatment with 3NP revealed that 12 mg/kg per d caused little motor impairment (score 1.72 ± 0.23, n = 16). Morphometric analysis of the brains 3 days after termination of the treatment revealed that 3NP, 12 mg/kg per d, caused loss of 16% of neurons in the striatum (Table 1). 3NP, 24 mg/kg per d, produced severe motor disturbances such as unsteady gait, ataxia, and increase of muscle tone (score 2.41 ± 0.21, n = 19) as well as mortality. Morphometric analysis of the brains of rats subjected to treatment with 24 mg/kg per d showed loss of 59% of neurons in the striatum (Table 1).
Table 1.
Dose-response relationship of neurotoxic action of 3NP in the rat striatum after systemic administration over 28 days and effect of the NMDA antagonists CPP, MK801, and memantine, and the AMPA/kainate antagonists NBQX and MPQX on 3NP toxicity
| Treatment, mg/kg per d | Striatal volume, mm3 | Density of neurons in the striatum, NV; mean × 106/mm3 ± SEM | Neurons, mean ± SEM | % | n |
|---|---|---|---|---|---|
| Vehicle | 22.56 ± 0.93 | 0.141 ± 0.003 | 3,179,719 ± 148,757 | 100 | 13 |
| 3NP 12 + vehicle | 23.05 ± 0.74 | 0.116 ± 0.004214 | 2,682,490 ± 144,728213 | 84 | 15 |
| 3NP 24 + vehicle | 23.50 ± 0.61 | 0.057 ± 0.010219 | 1,301,823 ± 198,684219 | 41 | 8 |
| 3NP 12 + CPP 24 | 19.99 ± 0.75** | 0.084 ± 0.006*** | 1,665,659 ± 112,902*** | 52 | 9 |
| 3NP 12 + MK801 0.3 | 20.33 ± 0.91* | 0.088 ± 0.006*** | 1,766,705 ± 114,380*** | 56 | 6 |
| 3NP 12 + Memantine 24 | 20.42 ± 0.82* | 0.086 ± 0.007*** | 1,756,120 ± 115,768*** | 55 | 9 |
| Vehicle + CPP 24 | 22.35 ± 0.32 | 0.141 ± 0.005 | 3,156,938 ± 127,962 | 99 | 4 |
| Vehicle + MK801 0.3 | 22.50 ± 0.63 | 0.141 ± 0.003 | 3,176,258 ± 99,183 | 100 | 6 |
| Vehicle + Memantine 24 | 22.41 ± 0.41 | 0.140 ± 0.006 | 3,137,406 ± 135,672 | 99 | 4 |
| 3NP 12 + NBQX 24 | 22.61 ± 0.35 | 0.136 ± 0.004 | 3,080,253 ± 79,640* | 97 | 6 |
| 3NP 24 + NBQX 24 | 22.95 ± 0.23 | 0.130 ± 0.009 | 3,000,500 ± 217,863$$$ | 94 | 7 |
| Vehicle + NBQX 24 | 22.63 ± 0.74 | 0.140 ± 0.004 | 3,175,564 ± 104,348 | 100 | 6 |
| 3NP 12 + MPQX 24 | 22.51 ± 0.32 | 0.135 ± 0.004 | 3,036,040 ± 60,090* | 95 | 8 |
| 3NP 24 + MPQX 24 | 22.09 ± 0.39 | 0.121 ± 0.014 | 2,663,404 ± 277,681$$$ | 84 | 6 |
| Vehicle + MPQX 24 | 22.40 ± 0.30 | 0.141 ± 0.006 | 3,152,380 ± 125,392 | 99 | 8 |
Morphometric analyses revealed that striatal volume and Nv of striatal neurons were significantly reduced in rats subjected to parallel treatment with either 3NP, 12 mg/kg per d and CPP, or 3NP, 12 mg/kg per d and MK801, or 3NP, 12 mg/kg per d and memantine over 28 days. The total number of neurons lost in the striatum after combined treatment with 3NP and CPP, or 3NP and MK801, or 3NP and memantine was significantly higher than that in the striatum of rats subjected to 3NP and vehicle. NBQX and MPQX attenuated toxicity of 3NP decreasing loss of striatal volume and numerical density, and reducing loss of neurons in the striatum. The dose of either CPP, MK801, memantine, NBQX, or MPQX chosen for long-term treatment was the maximal tolerated dose that did not induce motor disturbances in rats.
, P < 0.05;
, P < 0.01;
, P < 0.001 vs. vehicle-treated rats; *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. rats treated with 3NP, 12 mg/kg per d, and vehicle; $$$, P < 0.001 vs. rats treated with 3NP, 24 mg/kg per d, and vehicle (Student's t test).
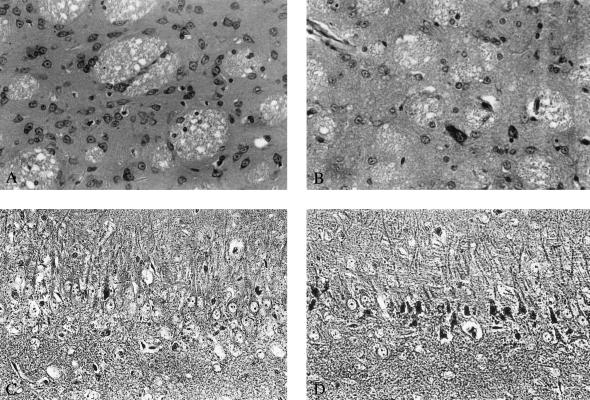
Morphological analysis of the neurodegenerative process induced by 3NP in rat striatum by means of light and electron microscopy performed 1 and 2 weeks after initiation and 3 days after termination of treatment with 3NP revealed that initial changes consisted of darkening of neuronal cytoplasm and the appearance of cytoplasmic vacuoles that seemed to derive from the endoplasmic reticulum (Fig. 1B). The chromatin and the nuclear membrane were preserved (Fig. 1B). At more advanced stages, nuclear chromatin formed clumps that attached to the nuclear membrane, the cytoplasm became darker, and the cells shrank (Fig. 1C). No ultrastructural changes were detected in the striatum of vehicle-treated rats (Fig. 1A).
Figure 1.
Electron microphotographs depicting a normal (A) and degenerating striatal neurons (B and C) in rats subjected to systemic treatment with 3NP, 12 mg/kg per d, over 7 days before death. The neuron in B has a darkened appearance, the cytoplasm displays fine vacuoles and widened cisternae of the endoplasmic reticulum. Cell organelles appear intact as do the cytoplasmic and nuclear membranes. Nuclear chromatin shows no clumps, nucleolus is intact. The neuron in C has a darker appearance and has shrunk down in size. Small vacuoles and prominent cisternae of the endoplasmic reticulum are also present. At this stage nuclear chromatin has started to form clumps that attach to the nuclear membrane that remains intact. Magnifications: ×3,600 in A and B and ×4,650 in C.
To explore whether NMDA antagonists affect slowly progressing striatal neurodegeneration, noncompetitive NMDA antagonists, MK801, 0.3 mg/kg per d, and memantine, 24 mg/kg per d, or a competitive NMDA antagonist, CPP, 24 mg/kg per d, were administered s.c. by means of minipumps for 28 days to rats subjected to simultaneous treatment with 3NP, 12 mg/kg per d. Parallel treatment with MK801 or memantine and 3NP resulted in worsening of neurologic impairment (score 2.80 ± 0.13, n = 10 for 3NP + MK801 and 2.70 ± 0.3, n = 10 for 3NP + memantine vs. 1.72 ± 0.23, n = 16 for 3NP + vehicle; P < 0.01, Mann–Whitney u test) and increased mortality (90% and 90% vs. 37.5%; P < 0.01, χ2 test) as compared with treatment with 3NP and vehicle. Administration of CPP and 3NP, 12 mg/kg per d, also resulted in worsening of neurologic impairment (score 2.25 ± 0.16, n = 12; P < 0.05, Mann–Whitney u test). Treatment of rats with MK801, 0.3 mg/kg per d (n = 10), memantine, 24 mg/kg per d (n = 4), or CPP, 24 mg/kg per d (n = 5), over 28 days, did not cause neurological impairment or mortality. Treatment with 3NP, 12 mg/kg per d, and MK801, memantine, or CPP reduced striatal volume by 12%, 11.5%, and 13%, respectively and enhanced loss of neuronal density in the striatum by 24%, 26%, and 27.5%, respectively. A total of 497,229 striatal neurons degenerated after systemic administration of 3NP, 12 mg/kg per d and vehicle, as opposed to 1,413,014 neurons lost after treatment with 3NP and MK801, 1,423,599 neurons lost after treatment with 3NP and memantine, and 1,514,060 lost after treatment with 3NP and CPP. Treatment with MK801, 0.3 mg/kg per d, memantine, 24 mg/kg per d, or CPP, 24 mg/kg per d, alone did not affect survival of neurons in the striatum (Table 1).
To investigate involvement of non-NMDA receptors in pathogenesis of slowly progressing striatal neurodegeneration, competitive AMPA/kainate antagonists NBQX, 24 mg/kg per d, and MPQX, 24 mg/kg per d, were coadministered s.c. by means of minipumps for 28 days to rats subjected to treatment with 3NP, 12 mg/kg per d or 24 mg/kg per d. NBQX and MPQX prevented neuronal degeneration in the striatum induced by 3NP, 24 mg/kg per d, (Table 1) and led to an improvement of neurologic outcome (score 0.71 ± 0.29, n = 7 for 3NP + NBQX and 1.37 ± 0.32, n = 8 for 3NP + MPQX vs. 2.41 ± 0.21 for 3NP + vehicle, n = 19; P < 0.005 and P < 0.01, Mann–Whitney u test). Degeneration in the striatum induced by 3NP, 12 mg/kg per d, also was reduced by NBQX and MPQX (Table 1).
Intrastriatal 3NP and Glutamate Antagonists.
To determine whether NMDA antagonists affect rapidly progressing striatal neurodegeneration, CPP, 250 nmol, was coadministered with 3NP, 250 nmol, into the striatum, and the extent of the damage was assessed stereologically. 3NP caused widespread neurodegeneration in the striatum characterized ultrastructurally by swelling of dendrites, neuronal somata, and cytoplasmic organelles, early breakdown of cytoplasmic and nuclear membranes, and elimination of cell remnants by inflammatory cells. CPP prevented morphological sequelae and reduced the lesion volume induced by 3NP by 28% (Table 2). A total of 642,024 striatal neurons degenerated after intrastriatal injection of 3NP, 250 nmol, as opposed to 346,841 neurons degenerating after treatment with 3NP and CPP (Table 2).
Table 2.
Dose-response relationship of neurotoxic action of 3NP in the rat striatum after intrastriatal administration and protection against 3NP toxicity by the NMDA antagonist CPP
| Treatment, nmol | Striatal lesion volume, mm3 | % | Density of neurons within lesion, NV; mean × 106/mm3 ± SEM | % | Neuronal loss, mean ± SEM | % | n |
|---|---|---|---|---|---|---|---|
| Vehicle | 0.15 ± 0.02 | 0 | 0.135 ± 0.003 | 100 | 313 ± 41 | 0 | 6 |
| 3NP 100 | 1.95 ± 0.32 | 25 | 0.028 ± 0.008 | 21 | 217,553 ± 17,004 | 21 | 6 |
| 3NP 250 | 5.48 ± 0.52 | 71 | 0.023 ± 0.002 | 17 | 637,720 ± 56,849 | 62 | 6 |
| 3NP 500 | 7.73 ± 0.59 | 100 | 0.005 ± 0.001 | 4 | 1,024,391 ± 97,970 | 100 | 6 |
| CPP 250 (4 h) | 0.13 ± 0.03 | 0 | 0.135 ± 0.004 | 100 | 227 ± 39 | 0 | 6 |
| 3NP 250 (4 h) | 5.60 ± 0.38 | 100 | 0.023 ± 0.0025 | 17 | 642,024 ± 47,203 | 100 | 11 |
| 3NP + CPP 250 (4 h) | 4.01 ± 0.62* | 72 | 0.047 ± 0.0045*** | 35 | 346,841 ± 47,148*** | 54 | 11 |
| CPP 250 (7 d) | 0.14 ± 0.02 | 0 | 0.134 ± 0.003 | 100 | 237 ± 42 | 0 | 6 |
| 3NP 250 (7 d) | 5.84 ± 0.69 | 100 | 0.019 ± 0.0025 | 14 | 682,330 ± 86,547 | 100 | 5 |
| 3NP + CPP 250 (7 d) | 3.46 ± 0.72* | 59 | 0.037 ± 0.003** | 28 | 338,137 ± 72,747* | 50 | 5 |
Morphometric analyses revealed that the striatal volume in rats subjected to microinjections of vehicle, 3NP, or CPP did not significantly differ from each other. Densities of neurons in nonlesioned parts of the striatum also did not significantly differ from each other. *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. rats treated with 3NP, 250 nmol (Student's t test).
Traumatic Brain Injury and Glutamate Antagonists.
In rats subjected to traumatic brain injury, rapidly developing neurodegeneration occurs in the cortex adjacent to the site of injury, whereas slowly progressing degeneration occurs in the hippocampal CA3 subfield distant to the site of injury. To evaluate whether NMDA antagonists differentially affect neurodegeneration triggered by traumatic brain injury, rats were subjected to cortex trauma, and the NMDA antagonist CPP, 30 mg/kg, was administered at either 2, 1, or 0 h before trauma, 1–3 h, 4–6 h, 7–9, or 10–12 h after trauma, and the extent of cortical as well as hippocampal degeneration was evaluated 3 days after trauma.
When treatment with CPP was initiated at 2 h before trauma, the volume of the damage in the parietal cortex was reduced by 37% (7.17 ± 0.78 mm3, n = 8 vs. 11.26 ± 1.18 mm3, n = 14 in vehicle-treated rats; P < 0.05, Student's t test). When treatment was delayed by 1 h or more, no significant difference in the volume of cortical damage was noted between CPP- and vehicle-treated rats (the volume of the cortex damage varied between 10.62 and 11.78 mm3). Damage in the CA3 subfield was decreased by treatment with CPP commencing 2 h before trauma, but was enhanced by treatment commencing 1, 4, and 7 h after traumatic injury to the cortex (Table 3). A total of 37,583 pyramidal neurons degenerated after traumatic brain injury in the CA3 subfield of vehicle-treated rats, as opposed to 57,067 neurons degenerating after treatment with CPP commencing 1 h, 53,715 when treatment started at 4 h, and 49,454 when treatment was initiated 7 h after trauma. Treatment with CPP, initiated as late as 10 h after trauma, did not affect the damage in the hippocampal CA3 subfield (Figs. 2 and 3). CPP, 3 × 30 mg/kg i.p., did not induce neuronal death in the hippocampus of rats subjected to sham surgery or in the hippocampus on the site contralateral to traumatic injury (Table 3). The AMPA/kainate antagonist NBQX reduced the volume of the damage in the parietal cortex on antecedent treatment by 39% (6.84 ± 0.89 mm3, n = 8; P < 0.05, Student's t test), but had no effect on the volume of cortical damage on delayed treatment. NBQX also prevented hippocampal neurodegeneration when administered 2 h before, as well as 1, 4, or 7 h after traumatic injury (Table 3). NBQX had no protective effect when treatment was initiated at 10 h (Table 3). Only 16,715 pyramidal neurons degenerated after traumatic brain injury in the CA3 subfield of NBQX-treated rats when treatment was initiated 1 h, 22,759 when treatment was started 4 h, and 26,661 when treatment was initiated 7 h after trauma. NBQX did not induce neuronal death in the hippocampus of rats subjected to sham surgery or in the hippocampus on the site contralateral to traumatic injury (Table 3). Monitoring of blood pressure (systolic and diastolic), arterial blood gases (pH, PaO2, PaCO2, bicarbonate), and body temperature in rats subjected to traumatic brain injury showed no evidence of cardiorespiratory compromise or hypothermia.
Table 3.
Effect of the NMDA antagonist CPP and the AMPA/kainate antagonist NBQX on neurodegeneration in the hippocampal CA3 subfield after traumatic brain injury
| Treatment, time (h) | Density of pyramidal cells in the hippocampal CA3 subfield, Nv, ipsilateral; mean × 106/mm3 ± SEM | Density of pyramidal cells in the hippocampal CA3 subfield, Nv, contralateral; mean × 106/mm3 ± SEM | Neurons, mean ± SEM | % | n |
|---|---|---|---|---|---|
| Sham | 0.1899 ± 0.0019 | 0.1891 ± 0.0020 | 123,435 ± 1,235 | 100 | 14 |
| Vehicle | 0.1343 ± 0.0024 | 0.1899 ± 0.0018 | 85,852 ± 1,534 | 70 | 14 |
| CPP, −2, −1, 0 | 0.1579 ± 0.0059 | 0.1887 ± 0.0016 | 102,635 ± 3,835219 | 83 | 8 |
| CPP, 1, 2, 3 | 0.1088 ± 0.0144 | 0.1883 ± 0.0023 | 66,368 ± 8,784*** | 54 | 8 |
| CPP, 4, 5, 6 | 0.1162 ± 0.0051 | 0.1879 ± 0.0018 | 69,720 ± 3,061*** | 56 | 9 |
| CPP, 7, 8, 9 | 0.1233 ± 0.0033 | 0.1839 ± 0.0016 | 73,981 ± 1,980** | 60 | 10 |
| CPP, 10, 11, 12 | 0.1313 ± 0.0068 | 0.1876 ± 0.0013 | 84,032 ± 4,352 | 68 | 6 |
| NBQX, −2, −1, 0 | 0.1871 ± 0.0043 | 0.1875 ± 0.0017 | 115,992 ± 2,666219 | 94 | 8 |
| NBQX, 1, 2, 3 | 0.1694 ± 0.0035 | 0.1883 ± 0.0019 | 106,720 ± 2,205219 | 86 | 8 |
| NBQX, 4, 5, 6 | 0.1624 ± 0.0018 | 0.1891 ± 0.0025 | 100,676 ± 1,116219 | 82 | 8 |
| NBQX, 7, 8, 9 | 0.1561 ± 0.0016 | 0.1877 ± 0.0019 | 96,774 ± 1,001219 | 78 | 7 |
| NBQX, 10, 11, 12 | 0.1412 ± 0.0021 | 0.1882 ± 0.0018 | 86,153 ± 1,281 | 70 | 8 |
| Sham + CPP | 0.1885 ± 0.0027 | 0.1892 ± 0.0013 | 120,650 ± 1,742 | 98 | 8 |
| Sham + NBQX | 0.1888 ± 0.0034 | 0.1885 ± 0.0023 | 120,045 ± 2,169 | 97 | 8 |
Treatment with CPP, 3 × 30 mg/kg, and NBQX, 3 × 30 mg/kg, was initiated 2 h before or 1, 4, 7, or 10 h after traumatic cortex injury. To quantitatively assess neuronal loss in the hippocampal CA3 subfield in Fischer 344 rats 3 days after injury, a stereological disector technique was used to estimate Nv. Morphometric analysis revealed that the volume of the hippocampal CA3 subfield in rats subjected to traumatic brain injury and treatment with CPP or NBQX did not significantly differ from that in rats subjected to brain trauma and vehicle and ranged from 0.61 to 0.64 mm3. The differences between vehicle- and drug-treated rats in the numbers of neurons remaining in the hippocampus after injury were analysed statistically by means of Student's t test. **,
, P < 0.01; ***,
, P < 0.001 vs. vehicle-treated rats. Stars indicate significant increase in neurodegeneration, while crosses indicate neuroprotection. Monitoring of blood pressure and arterial blood gases in rats subjected to head trauma and systemic treatment with either CPP (n = 8) or NBQX (n = 12) showed no evidence of cardiorespiratory compromise.
Figure 2.
Light microphotographs demonstrating the effect of the NMDA antagonist CPP on slowly progressing neurodegeneration induced in the striatum by systemic administration of 3NP and in the hippocampus by traumatic brain injury. (A) Morphology of striatum after treatment with 3NP, 12 mg/kg per d over 28 days, 3 days after termination of treatment. No obvious neurodegeneration can be detected in the striatum; the spiny neurons have normal appearance. (B) Profound neuronal loss and predominance of glia in the striatum 3 days after termination of treatment with 3NP, 12 mg/kg per d, and CPP, 24 mg/kg per d over 28 days. Large-size striatal neurons are relatively preserved. (C) Hippocampal pathology in the CA3 subfield 3 days after traumatic brain injury. Dark argyrophylic profiles indicate ongoing degeneration in pyramidal layer. Intact pyramidal cells with prominent nuclei are also present. (D) The effect of treatment with CPP, 3 × 30 mg/kg given i.p. 1, 2, and 3 h after trauma is shown. Widespread degeneration of pyramidal neurons predominates. Magnifications: A and B, ×60 (cresyl violet stain); C and D, ×80 (Fink and Heimer stain).
Figure 3.
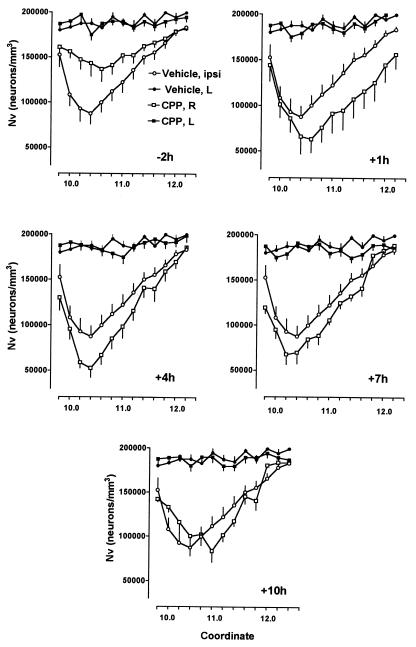
Effect of CPP on morphology of the hippocampal CA3 subfield in rats subjected to traumatic cortex injury. CPP was administered i.p. at a dose of 30 mg/kg at 2, 1, and 0 h before, 1, 2, and 3 h, 4, 5, and 6 h, 7, 8, and 9 h, or 10, 11, and 12 h after traumatic injury. Nvs of pyramidal cells in the hippocampal CA3 subfield were estimated between rostro-caudal coordinates 9.61 and 12.21 according to the stereotaxic atlas of Swanson (10) using the stereologic disector method (12, 13). Traumatic brain injury in vehicle-treated rats resulted in significant reduction of neuronal densities in the ipsilateral (right, R) hippocampal CA3 subfield and had no effect on densities of pyramidal cells on contralateral side (left, L). Two-way ANOVA revealed that treatment with CPP commencing 2 h before traumatic injury prevented reduction of numerical density of pyramidal cells in the ipsilateral CA3 subfield [F-2h(1,12) = 6.97, P < 0.001, n = 8] as compared with vehicle-treated rats (n = 14). Treatment with CPP starting at 1 h [F+1h(1,12) = 5.56, P < 0.001, n = 8], 4 h [F4h(1,12) = 3.43, P < 0.001, n = 9], or 7 h [F7h(1,12) = 1.31, P < 0.005, n = 10] after traumatic injury significantly enhanced decrease of numerical density of pyramidal cells in the ipsilateral CA3 subfield, whereas treatment commencing after 10 h was ineffective [F10h(1,12) = 0.06, NS, n = 6].
Discussion
NMDA antagonists MK801, memantine, and CPP exacerbated slowly progressing neurodegeneration in the striatum produced by 3NP. In contrast, neurons that degenerated rapidly after microinjection of 3NP into the striatum responded favorably to treatment with the NMDA antagonist CPP. The exacerbation is unlikely to depend on compromise of oxidative phosphorylation by NMDA antagonists, because NMDA antagonists did not block activity of succinate dehydrogenase in neuronal cultures (14). The NMDA antagonist CPP also exacerbated slowly progressing neurodegeneration in the hippocampus triggered by traumatic cortex injury when administered between 1 and 7 h after injury. In contrast, neurons that degenerated rapidly after exposure to traumatic injury to the cortex, responded favorably to antecedent treatment with CPP. Thus, slowly progressing neurodegeneration triggered by two different mechanisms was enhanced by NMDA antagonists.
The AMPA antagonists NBQX and MPQX prevented both rapid and slowly progressing neuronal death induced by 3NP or traumatic brain injury. These observations suggest that glutamate differentially determines the fate of neurons endangered to die slowly after brain injuries. It may save neurons by activating NMDA receptors or destine neurons to die by activating AMPA/kainate receptors.
The opposite response to NMDA antagonists of rapid and slowly progressing neurodegeneration raises questions as to the mechanisms involved. High levels of energy compromise or profound elevation of extracellular glutamate concentrations after traumatic injury lead to depolarization of neuronal membranes, relief of the voltage-dependent Mg2+ block of NMDA receptor channels, and NMDA receptor-mediated excitotoxicity (15). Under such conditions NMDA antagonists are neuroprotective. In contrast, slowly progressing neurodegeneration induced by low level of energy compromise or traumatic injury at sites distant to the impact has been suggested to evolve in a programmed fashion that involves activation of caspases (16). Low-intensity stimulation of NMDA receptors increases intracellular Ca2+ concentration and protects cells from caspase-mediated death (17). In murine cortical neurons, elevation of intracellular Ca2+ protects against ischemic apoptosis.§ Thus, decrease of intracellular Ca2+ concentration caused by blockade of Ca2+-permeable NMDA channels may be one mechanism contributing to enhancement of slowly progressing neuronal death by NMDA antagonists.
Stimulation of NMDA but not AMPA/kainate receptors has been shown to enhance mRNA levels of neurotrophins in the injured brain (18, 19). Synthesis of neurotrophins increases in the brain after exposure to neurotoxins (20) and in the hippocampus after traumatic brain injury (21). Thus, it appears feasible that prevention of neurotrophin synthesis by NMDA antagonists may be deleterious for neuronal survival following injuries. Neurotrophins such as brain-derived neurotrophic factor (BDNF), NT3, or NT4/5 possess glutamate-like depolarizing properties in mature neurons (22). Neurotrophins enhance NMDA-mediated excitotoxic degeneration in murine neurons (23) and may protect either against degeneration induced by serum deprivation in vitro (23), against hippocampal damage induced by ischemia or spinal cord damage induced by traumatic injury (24, 25), or against damage of substantia nigra produced by mitochondrial toxins (26). It can be speculated that glutamate acting via NMDA receptors may serve a trophic function resembling that of neurotrophins in mature brain.
Antagonists of neurotransmitter receptors trigger in vivo compensatory elevation of synthesis and/or release of agonists from presynaptic endings (27). It can be hypothesized that when NMDA receptors are selectively blocked by antagonists such as MK801, memantine, or CPP, glutamate will activate AMPA/kainate receptors, for as long as imbalance in the NMDA system continues, and will promote neuronal death. Selective blockade of AMPA/kainate receptors by antagonists such as NBQX or MPQX will divert action of glutamate toward NMDA-dependent mechanisms and promote survival (13).
Alternatively, NMDA and AMPA receptor antagonists may differently influence activity of neuronal populations by decreasing the input to or output from GABAergic interneurons and enhancing input to or output from cholinergic interneurons to promote or prevent neuronal death (28, 29).
The apparently paradoxical promotion of neurodestruction by NMDA antagonists on one hand and neuroprotective action of AMPA/kainate antagonists on the other in slowly progressing neurodegeneration in the striatum subjected to systemic 3NP and in the hippocampus subjected to traumatic injury suggest that such mechanisms may be operative in vivo.
Our findings have implications for clinical trials with NMDA antagonists, which were initiated in humans based on neuroprotection that has been demonstrated in rodent models of stroke and trauma on antecedent treatment. In patients undergoing cardiopulmonary bypass and subjected to antecedent treatment with the NMDA antagonist remacemide starting at 4 days before surgery, a beneficial effect on neuropsychomotor performance has been documented (30). These observations are in agreement with our findings showing that in rats subjected to antecedent treatment with CPP neuronal loss in the hippocampus after traumatic brain injury can be mitigated. It is also in agreement with the observation that MK801 and memantine prevent learning deficits in rats caused by acute damage of the hippocampus triggered by the NMDA agonist quinolinate (31). In no other clinical trial, including those in stroke and head or spinal cord trauma, in which delayed treatment regimens with NMDA antagonists were chosen, could neuroprotective effects be confirmed so far (1) and in fact, in some trials, increased mortality prompted termination (32, 33). In chronic neurodegenerative disorders, such as Huntington's or Parkinson's disease, symptomatological improvement with NMDA antagonists has been documented, but increases of survival or slowing of disease progression have not been reported so far (2). Symptomatological improvement seen in chronic neurodegenerative disorders with NMDA antagonists can be explained by their interaction with glutamate receptors in the basal ganglia that are involved in transmitting the symptoms of the disease to respective motor centers and not necessarily by prevention of degeneration of striatal or nigral neurons (34, 35).
In light of our current findings, caution should be exercised when using NMDA antagonists as monotherapy in humans suffering from brain injuries or progressive neurodegenerative disorders with unknown mechanism of neuronal death. If our observations are valid for humans, our conclusions may explain why clinical trials have failed to identify neuroprotective effects of NMDA antagonists.
Acknowledgments
This work was supported by Grant 01K095151 from the Bundesministerium für Bildung und Forschung (BMBF).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate
- CPP
3-((±)-2-carboxypiperazin-4-yl)propyl-1-phosphonate
- MK801
dizocilpine
- MPQX
[1,2,3,4-tetrahydro-7-morpholinyl-2,3-dioxo-6-(trifluoromethyl)quinoxalin-1-yl]methylphosphonate
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline
- NMDA
N-methyl-d-aspartate
- 3NP
3-nitropropionate
- Nv
numerical density
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220412197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220412197
Babcock, D. J., Gottron, F. J. & Choi, D. W. (1999) Soc. Neurosci. Abstr. 25, 2103.
References
- 1.Lee J-M, Zipfel G J, Choi D W. Nature (London) 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 2.Parsons C, Danysz W, Quack G. Drug News Perspect. 1998;11:423–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 3.Lipton S A, Rosenberg P A. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 4.Lancelot E, Beal M F. Prog Brain Res. 1998;116:331–347. doi: 10.1016/s0079-6123(08)60446-x. [DOI] [PubMed] [Google Scholar]
- 5.Herrling P L. Excitatory Amino Acids: Clinical Results with Antagonists. New York: Academic; 1997. [Google Scholar]
- 6.Brouillet E, Conde F, Beal M F, Hantraye P. Prog Neurobiol. 1999;59:427–468. doi: 10.1016/s0301-0082(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 7.Xingjie L, Xueyun L, Wenjuan H. Biomed Environ Sci. 1992;5:161–177. [Google Scholar]
- 8.Brouillet E, Hantraye P, Ferrante R J, Dolan R, Leroy-Willig A, Kowall N W, Beal M F. Proc Natl Acad Sci USA. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin S A, Gibson T, Callihan C T, Sullivan P G, Palmer E, Scheff S W. J Neurotrauma. 1997;14:385–398. doi: 10.1089/neu.1997.14.385. [DOI] [PubMed] [Google Scholar]
- 10.Swanson L W. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 11.Fink R P, Heimer L. Brain Res. 1967;4:369–374. doi: 10.1016/0006-8993(67)90166-7. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Orive L M, Weibel E R. Am J Physiol. 1990;258:L148–L156. doi: 10.1152/ajplung.1990.258.4.L148. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova T I, Stefovska V, Turski L, Olney J W. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 14.Zeevalk G D, Derr-Yellin E, Nicklas W J. J Neurochem. 1995;64:455–458. doi: 10.1046/j.1471-4159.1995.64010455.x. [DOI] [PubMed] [Google Scholar]
- 15.Nicotera P, Leist M, Manzo L. Trends Pharmacol Sci. 1999;20:46–51. doi: 10.1016/s0165-6147(99)01304-8. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh T K, Saatman K E, Raghupathi R, Graham D I, Smith D H, Lee V M, Trojanowski J Q. Neuropathol Appl Neurobiol. 1998;24:251–267. doi: 10.1046/j.1365-2990.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 17.Yano S, Tokumitsu H, Soderling T R. Nature (London) 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 18.Comelli M C, Seren M S, Guidolin D, Manev R M, Favaron M, Rimland J M, Canella R, Negro A, Manev H. NeuroReport. 1992;3:473–476. doi: 10.1097/00001756-199206000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Rocamora N, Massieu L, Boddeke H W, Palacios J M, Mengod G. Mol Brain Res. 1994;26:89–98. doi: 10.1016/0169-328x(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 20.French S J, Humby T, Horner C H, Sofroniew M V, Rattray M. Mol Brain Res. 1999;67:124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 21.Hicks R R, Martin V B, Zhang L, Seroogy K B. Exp Neurol. 1999;160:469–478. doi: 10.1006/exnr.1999.7216. [DOI] [PubMed] [Google Scholar]
- 22.Kafitz K W, Rose C R, Thoenen H, Konnerth A. Nature (London) 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- 23.Koh J Y, Gwag B J, Lobner D, Choi D W. Science. 1999;268:573–575. doi: 10.1126/science.7725105. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Pardridge W M. Proc Natl Acad Sci USA. 1999;96:254–259. doi: 10.1073/pnas.96.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma H S, Nyberg F, Westman J, Alm P, Gordh T, Lindholm D. Amino Acids. 1998;14:121–129. doi: 10.1007/BF01345252. [DOI] [PubMed] [Google Scholar]
- 26.Frim D M, Uhler T A, Galpern W R, Beal M F, Breakefield X O, Isacson O. Proc Natl Acad Sci USA. 1994;91:5104–5108. doi: 10.1073/pnas.91.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloom F E. In: The Pharmacological Basis of Therapeutics. Goodman Gilman A, Rall T W, Nies A S, Taylor P, editors. New York: Pergamon; 1990. pp. 244–268. [Google Scholar]
- 28.Olney J W, Labruyere J, Price M T. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 29.Olney J W, Labruyere J, Wang G, Wozniak D F, Price M T, Sesma M A. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 30.Arrowsmith J E, Harrison M J, Newman S P, Stygall J, Timberlake N, Pugsley W B. Stroke. 1998;29:2357–2362. doi: 10.1161/01.str.29.11.2357. [DOI] [PubMed] [Google Scholar]
- 31.Misztal M, Frankiewicz T, Parsons C G, Danysz W. Eur J Pharmacol. 1996;296:1–8. doi: 10.1016/0014-2999(95)00682-6. [DOI] [PubMed] [Google Scholar]
- 32.Davis S M, Albers G W, Diener H C, Lees K R, Norris J. Lancet. 1997;349:32. doi: 10.1016/s0140-6736(05)62166-6. [DOI] [PubMed] [Google Scholar]
- 33.McBurney R N. Int Rev Neurobiol. 1997;40:173–195. doi: 10.1016/s0074-7742(08)60720-5. [DOI] [PubMed] [Google Scholar]
- 34.Klockgether T, Turski L. Trends Neurol Sci. 1989;12:285–286. doi: 10.1016/0166-2236(89)90007-6. [DOI] [PubMed] [Google Scholar]
- 35.Klockgether T, Turski L. Ann Neurol. 1993;34:585–593. doi: 10.1002/ana.410340413. [DOI] [PubMed] [Google Scholar]