Summary
Eukaryotic cells from fungal hyphae to neurites that grow by polarized extension must coordinate cell growth and cell orientation to enable them to exhibit growth tropisms and to respond to relevant environmental cues. Such cells generally maintain a tip-high Ca2+ cytoplasmic gradient, which is correlated with their ability to exhibit polarized tip growth and to respond to growth-directing extracellular signals 1, 2, 3, 4, 5. In yeast and other fungi, the polarisome, exocyst, Arp2/3, and Spitzenkörper protein complexes collectively orchestrate tip growth and cell polarity, but it is not clear whether these molecular complexes also regulate cell orientation or whether they are influenced by cytoplasmic Ca2+ gradients. Hyphae of the human pathogenic fungus Candida albicans reorient their growth axis in response to underlying surface topography (thigmotropism) [6] and imposed electric fields (galvanotropism) [7]. The establishment and maintenance of directional growth in relation to these environmental cues was Ca2+ dependent. Tropisms were attenuated in media containing low Ca2+, or calcium-channel blockers, and in mutants where calcium channels or elements of the calcium signaling pathway were deleted. Therefore galvanotropism and thigmotropism may both be mediated by localized Ca2+ influx at sites of polarized growth via Ca2+ channels that are activated by appropriate environmental signals.
Keywords: MICROBIO, SIGNALING
Results and Discussion
Calcium Channels in Candida albicans
Calcium channels have not been identified previously in C. albicans, but high-affinity and low-affinity calcium-uptake systems (HACS and LACS) have been described and partially characterized in S. cerevisiae 8, 9. We identified and deleted in C albicans, homologs of components of these systems—the voltage-gated Cch1p channel [10] and the stretch-activated channel Mid1p [11]. Together, these two channels are thought to form a complex that defines the HACS. We also identified and deleted Fig1p [12]—a component of LACS [13].
CaCCH1 encodes a putative 2254 amino acid protein with 38.4% identity to its S. cerevisiae homolog. The 24 predicted transmembrane (TM) regions in CaCch1p are arranged in four repeated units (I to IV) of six TM domains, as they are in mammalian calcium channels where they tetramerize to form the core α1-subunit of L-type Ca2+ channels [14]. The TM regions include segments responsible for voltage-dependency, channel-specificity, and association with organic calcium-channel blockers [15]. The C. albicans Cch1p and the human voltage-gated calcium channel CaV1.2 are 62.9% similar and 37.7% identical over a 20 amino acid region in the four Ca2+ selective, pore-forming P domains. In the voltage-sensitive S4 domains, 13 of the 23 basic residues in CaV1.2 are identically positioned in CaCch1p. The CaMID1 gene sequence had 36.9% and 34.4% identity to ScMID1 and Schizosaccharomyces pombe yam8(+), respectively. The 559 amino acid protein it encodes contains four putative TM regions (H1-4), potential N-glycosylation sites, a helix-loop-helix domain and 10 conserved cysteines that, in S. cerevisiae, form the C1/C2 domains that are essential for activity and localization 11, 16. Unlike in S. cerevisiae, the C1/C2 regions in C. albicans are located between H3 and the C-terminal H4. CaFig1p shares 48.5% identity with ScFig1p, a putative homolog of mammalian PMP-22/EMP/MP20/Claudins, which are involved in the trafficking and assembly of membrane-associated proteins [17]. Consistent with EMP homology, CaFig1p has four predicted N-glycosylation sites in the first of its four TM domains. The role of ScFig1p in S. cerevisiae is not well-defined, but it localizes predominantly to the plasma membrane [13] and is required for low-affinity calcium transport and for the calcium-dependent fusion of mating projections [12].
Control strains were created by the generation of conditional mutants expressing a single remaining wild-type gene from the MRP1 maltose-regulatable promoter (CCH1 or MID1) or by reintegration of the gene at a high-expression locus (FIG1 or MID1) (see the Experimental Procedures in the Supplemental Data available with this article online).
HACS and LACS Are Expressed during Hyphal Vegetative Growth
In S. cerevisiae, HACS is activated by low Ca2+ conditions and is regulated by calcineurin, which controls calcium homeostasis and specific stress responses 8, 9, 10, 11, 18. ScFig1p (LACS) activity was only revealed under conditions when HACS was inhibited by rich media. In contrast to HACS, LACS is insensitive to calcineurin and its affinity for Ca2+ is 16-fold lower [8]. However, both systems are activated on exposure of cells to α-pheromone, which stimulates the formation of the polarized mating projection 8, 19. Localization of ScMid1p and ScFig1p to the mating projection is dependent on ScSPA2 and ScBNI1 19, 8. In C. albicans, CaSpa2p and CaBni1p are components of the hyphal polarisome and Spitzenkörper, respectively, and are required for polarized hyphal extension [20]. Mid1p and Fig1p may therefore be involved in polarized cell growth in both organisms. In C. albicans, mRNA encoding HACS and LACS component proteins was detected in both yeast and hyphal growth conditions and in C. albicans-infected rabbit kidney (data not shown). Also, both HACS and LACS mutants were affected in the thigmotropic response (see below). Therefore, both HACS and LACS are expressed in C. albicans during the normal in vitro and in vivo growth of this fungus.
The Cacch1Δ and Camid1Δ Mutants Are Defective in Calcium Accumulation
After prolonged incubation on Ca2+-depleted solid minimal medium (>14 days), wild-type C. albicans colonies produced aberrant lobed margins that could be alleviated by the addition of 10 mM Ca2+ to the medium. Emerging colonies of the Cacch1Δ and Camid1Δ mutants produced lobed colonies at 2 days (see Supplemental Data). The aberrant morphologies of the colonies of Cafig1Δ, Cacna1Δ, and Cacnb1Δ mutants were partially alleviated on supplementation with exogenous Ca2+, supporting the view that these genes are involved in calcium signaling in C. albicans. The growth rates of the Cacch1Δ, Camid1Δ, and Cacch1-mid1Δ mutants were reduced by 19%, 23%, and 25%, respectively, when they were grown in the yeast form, compared to the control strain (p = <0.037), but the extension rates of hyphae were not affected by the mutations (data not shown). Consistent with the putative roles of CaMid1p and Cch1p as Ca2+ channels, Ca2+ accumulation in the Camid1Δ and Cacch1Δ mutants was significantly reduced after 2 hr culture in Ca2+-depleted medium supplemented with 45Ca2+ (p = <0.001; Supplemental Data). Induction of regulatable CaCCH1 or reintegration of CaMID1 abrogated this phenotype. The double Cacch1Δ-mid1Δ mutant had similar Ca2+ accumulation and yeast growth rates to the single mutants, consistent with the model that these proteins operate within the same pathway.
Deletion of CaFIG1 did not affect Ca2+ accumulation in low-Ca2+ minimal medium. This is consistent with reports that, in S. cerevisiae, Cch1-Mid1p are involved in Ca2+ homeostasis under low-Ca2+ conditions, where Fig1p activity is not detectable [13].
Calcium Ions and CaCch1p Mediate Cathodal Germ-Tube Emergence
Tropic growth responses to applied external electric fields (galvanotropism) have been observed in migratory and tip-growing cells [21]. Growing C. albicans hyphae orient toward the cathode in such fields [7]. To characterize hyphal orientation, we measured the angle at which germ tubes emerged from the mother cell (emergence angle) and the angle of the hyphal tip after 6 hr growth (final angle) relative to the cathode. To investigate the role of calcium ions and channels in galvanotropism, we measured the emergence and final angles of hyphae exposed to electrical fields in media of varying extracellular [Ca2+] or in the presence of pharmacological agents that block the activity of L-type voltage-gated cation channels. In C. albicans, site selection of germ tubes is not strictly controlled by the Bud proteins that regulate bud-site selection during axial and bipolar budding of S. cerevisiae [22]. We observed that, in electric fields, germ tubes were formed almost exclusively on the cathode-facing pole of cells, suggesting that imposed electrical fields can override cortical Bud evagination markers. The percentage cathodal germ-tube emergence was positively correlated with extracellular [Ca2+]. Cathodal orientation of wild-type cells was attenuated in Ca2+-depleted medium (Figures 1A and 1B) and was further significantly reduced in the presence of calcium-channel blockers (p = <0.05) (Figure 2A). Therefore, Ca2+ influx is important for cathodal evagination of the germ tube in C. albicans. Localized Ca2+ uptake correlates with sites of germination of other cell types, such as the zygotes of the brown alga Silvetia compressa [23]. Extracellular [Ca2+] did not affect the final angle of hyphae. Even in low Ca2+ medium, extending hyphae grew toward the cathode and reoriented their direction of growth when the field polarity was reversed (Figure 1C).
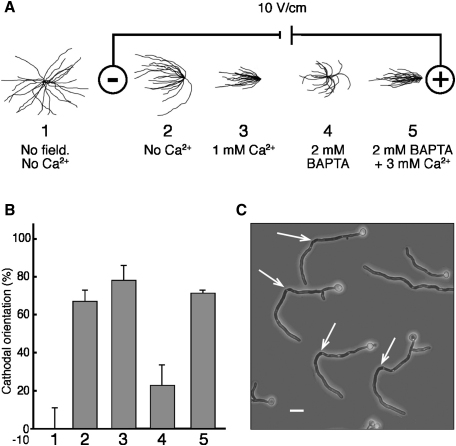
Figure 1.
Extracellular [Ca2+] Affects Cathodal Emergence of C. albicans Hyphae but Not Final Orientation in an Applied Electrical Field
(A) Tracings of individual hyphae grown in varying [Ca2+] were superimposed at a common point of origin for illustrating the distribution of hyphal orientation under the conditions used. Yeast cells adhered to poly-L-lysine-coated glass slides were grown in Ca2+-depleted, hypha-inducing medium for 6 hr and either not exposed to an electrical field (1) or exposed to an electrical field of 10 V/cm (2) supplemented with 1 mM CaSO4 (3), 2 mM BAPTA (a Ca2+ chelator) (4) or 2 mM BAPTA + 3 mM (excess) CaSO4 (5).
(B) Germ-tube-emergence angles relative to the cathode for cells in Figure 1A, where 100% cathodal orientation denotes perfect cathodal orientation, −100% denotes anodal orientation, and 0% is obtained for a randomly orientated population. Each error bar shows the SD of the mean values obtained from three independent experiments.
(C) The tropic growth of hyphal tips was not affected by extracellular [Ca2+]. The final angles of hyphal tips after 6 hr growth in an electrical field were cathodally oriented irrespective of germ-tube-emergence angle. Hyphae reoriented when the field polarity was reversed (arrows), even in low [Ca2+] medium. The scale bar represents 10 μM.
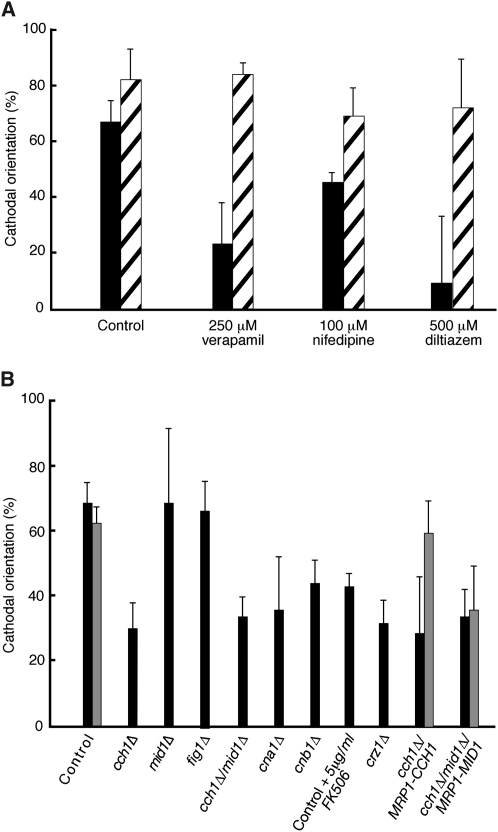
Figure 2.
Ca2+-Channel Blockers or Deletion of CaCch1p or Either Calcineurin Subunit Attenuates Cathodal Germ-Tube Emergence of C. albicans Hyphae
(A) Cells were grown in an electric field and the medium supplemented with 250 μM verapamil, 100 μM nifedipine, or 500 μM diltiazem. Emergence angles (black bars) and final angles (hatched bars) were measured in relation to the cathode for >100 hyphae per strain per experiment so that percentage cathodal orientation could be obtained [20].
(B) Mutant and control strains were exposed to an electric field of 10 V/cm for 6 hr in Ca2+-depleted medium containing glucose (black bars), maltose (conditional mutants, gray bars), or 5 μg/ml FK506, an inhibitor of calcineurin. Conditional MRP1p-regulated mutants were subcultured for 3 days in glucose containing medium prior to assaying.
Each error bar shows the SD of the mean values obtained from three independent experiments.
Deletion of CaCCH1 resulted in a significant reduction in the cathodal orientation of germ-tube emergence (p = <0.001) (Figure 2B). In contrast, emergence angles in the Camid1Δ and Cafig1Δ mutants were unaffected. Cathodal orientation was restored in the conditional CaCCH1 strain when CaCCH1 expression was induced by growth on maltose but not when CaMID1 was induced in the cch1Δ/mid1Δ/MRP1-MID1 strain. We hypothesize that the voltage-gated CaCch1p Ca2+ channel is activated by membrane depolarization at the cathodal face of yeast cells and that this results in localized Ca2+ uptake and subsequent induction of localized polarized growth.
The effects of extracellular [Ca2+] deprivation and CaCCH1 deletion primarily affected the site of germ-tube emergence. In low [Ca2+], in the Cacch1Δ mutant and in the presence of L-type Ca2+-channel blockers, cathodal germ-tube emergence was approximately half that of the control strain, but after 6 hr exposure to an electric field, this effect was almost lost (Figure 2A). Even hyphae that had emerged from the anodal face of the Cacch1Δ mother cell eventually responded to the electric field. This suggested that differences exist between the mechanism that establishes cathodal growth and that which maintains it. Only the former appears to depend on calcium influx and Cch1p.
CaMid1p, CaCch1p, and CaFig1p Mediate C. albicans Thigmotropism
The ability of fungal hyphae to exhibit tropic growth responses in relation to changes in substratum topography is well-known in plant pathogens and has been demonstrated previously for C. albicans and certain dermatophytes 24, 25. Some plant pathogenic fungi also use topographical features to trigger formation of the appressorium infection structure (thigmodifferentiation) [26]. We tested whether treatments and mutations that attenuated galvanotropism also influenced thigmotropic orientation in C. albicans hyphae.
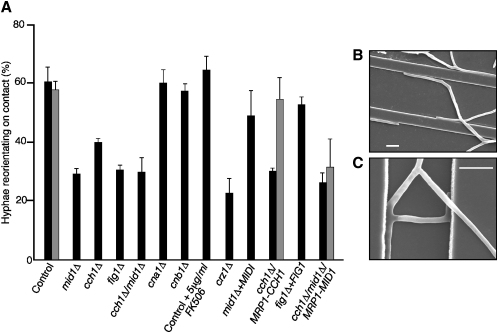
In wild-type cells, 60% of hyphae that contacted a 0.79 μm ridge responded by reorienting their growth axis (Figures 3A and 3B). Deletion of CaMID1 reduced the number of reorientation events by 50% (p = <0.0001) (Figures 3A and 3C). This is consistent with previous observations that thigmotropism was attenuated by inhibitors of stretch-activated Ca2+ channels [6] and supports a model whereby changes in the underlying topography induce stresses in the membrane that are sensed by Mid1p (Figure 4B). Reorientation of the Cacch1Δ strain was also significantly reduced (p = <0.001) compared to the control strain (Figure 3). Thigmotropism was attenuated in the regulatable Cacch1Δ/MRP1-CCH1 under repressing conditions and restored to normal under MRP1p-inducing conditions. Expression of CaMID1 in the Cacch1Δ/Camid1Δ/MRP1-MID1 conditional mutant did not restore the reorientation response, confirming that CaCch1p is required for thigmotropism. We propose that stretch activation of CaMid1p, and subsequent opening of the CaCch1p channel, results in localized Ca2+ influx that exerts an influence on the molecular machinery involved in polarized growth of the hyphal tip. This influence overrides or repositions, or both, the molecular markers that defined the original axis of growth. However, because hyphal reorientation was observed in almost 30% of ridge interactions in the Camid1Δ mutant, either CaCch1p is activated independently of CaMid1p or other sensing elements also contribute to the regulation of thigmotropism. Deletion of CaFIG1 also resulted in attenuation of the reorientation response. The function of Fig1p in C. albicans is not known, but in S. cerevisiae its deletion resulted in defective cell-cell fusion during mating [12], suggesting it could be involved in the delivery of components to the fusion site. Because its deletion in C. albicans reduces hyphal reorientation during contact-sensing, CaFig1p may again be involved in targeted delivery of secretory vesicles to the cell surface. No instances of tip bifurcation were observed when a hyphal tip contacted a ridge, even when the angle of approach was 90° (Figure 3C), suggesting that orientation-determining factors are a nondivisible entity or discrete protein complex.
Figure 3.
The Thigmotropic Response Is Attenuated in Calcium-Signaling-Pathway Mutants
(A) Mutant strains were adhered to quartz slides with ridge height 0.79 μm and pitch 25 μm and grown in 20% (v/v) fetal-calf serum supplemented with 2 (w/v) glucose (black bars), maltose (conditional mutants, gray bars), or a maximal concentration of 10 μg/ml FK506. The number of hypha-ridge interactions resulting in hyphal reorientation was expressed as a percentage of the total number of interactions observed. Each error bar shows the SD of the mean values obtained from three independent experiments.
(B and C) In wild-type cells, 60% of interactions between growing tips and the 0.79 μm ridges in the substrate resulted in reorientation of the hyphal growth axis. In mutant strains, approximately 70% of interactions resulted in hyphae maintaining their direction of growth over the ridges. Scale bars represent 10 μm.
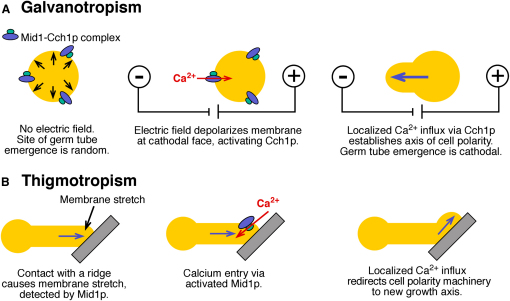
Figure 4.
Model for the Role of Ca2+ Influx in C. albicans Tropic Responses
Based on presented data, this model explains how exposure to an extracellular electric field (A) or contact with an obstacle (B) lead to activation of voltage-gated and stretch-activated calcium ion channels, respectively, and hence polarization of the site of germ-tube formation (A) or reorientation of the growth axis (B). The phosphatase, calcineurin, is required for cathodal germ-tube emergence but not for thigmotropism, suggesting that it is involved in interpretation of calcium gradients during the establishment of polarity but not after. The putative Ca2+-dependent transcription factor, CaCrz1p, is required for both tropic responses. Its activity may be responsible for the production of proteins involved in sensing or translating environmental signals.
Calcium-Signaling Factors Are Required for Both Tropic Responses
Calcium-dependent gene transcription in eukaryotes involves activation of a transcription factor by the phosphatase, calcineurin. Calcineurin regulates fungal morphogenesis [27], calcium flux, and homeostasis. In C. albicans, calcineurin acts on the transcription factor, Crz1p [28]. The deletion of CaCRZ1 affected both cathodal germ-tube emergence (Figure 2B) and thigmotropism (Figure 3), whereas the inhibition of calcineurin with FK506 or deletion of genes encoding either the catalytic CaCna1 or regulatory CaCnb1 calcineurin subunits only affected cathodal emergence (p = 0.006). Calcineurin modulates Cch1p activity [8] and can upregulate expression of CaCCH1 via Crz1p [28]. Our results suggest that calcineurin is required for the establishment of cathodal cell polarity in an electric field but not for the reorientation of already-polarized hyphal tips during contact-sensing. Thigmotropism was Crz1p dependent but was calcineurin independent. Five genes have been identified previously that are regulated in this manner [28]. Although CaCrz1p influences CaCCH1 expression, it does not appear to be essential for basal expression or activation of CaMid1p or CaCch1p because the morphology of Cacrz1Δ mutant colonies was the same as the wild-type strain, whereas the Camid1Δ and Cacch1Δ mutants formed aberrant colonies (see Supplemental Data). Other targets of CaCrz1p have been identified in C. albicans and are required for resistance to membrane damage and alkaline stress. It is not known whether CaCrz1p-mediated gene expression regulates events that are upstream or downstream of calcium-influx induced tropisms.
Conclusion
The ability of external cues to influence the orientation of hyphal growth of the human pathogenic fungus, Candida albicans, may be relevant to their capacity to infiltrate between human cells during tissue invasion. We have found that reorientation of C. albicans hyphae in relation to electrical fields and topographical signals is Ca2+ dependent and is mediated by Ca2+ channels and a Ca2+-dependent transcription factor, CaCrz1p. Calcineurin, the primary regulator of CaCrz1p, was required for galvanotropism but not thigmotropism. We observed that the hyphae of mutants lacking the stretch-activated CaMid1p or voltage-activated CaCch1p proteins grew normally but were attenuated in orientation responses resulting from physical contact or imposed electric fields, respectively. We propose a model whereby localized Ca2+-channel activation, caused by localized changes in membrane potential or membrane stretch, results in calcium influx that directs polarized growth (Figure 4). Stretch-activated ion channel activity has been described in patch-clamp analysis of C. albicans membranes, but the ion selectivity of these channels is not known [6]. In plant cells, mechanosensory Ca2+ channels produce high cytosolic Ca2+ and initiation of localized cell-wall expansion at sites of shear stress [29]. Similarly, in mammalian synapses, localized channel activation produces intracellular Ca2+ microdomains where the spatial boundary of the domain correlates with the capacity for efficient vesicle exocytosis [30]. Localized Ca2+ influx may result in an asymmetry in the tip-high Ca2+ gradient in hyphae of C. albicans and other fungi. This alters the axis of growth by increasing the rate of vesicle fusion within a local Ca2+-high microdomain or by affecting the activity of calcium-binding proteins that are involved in polarized cell extension. Ca2+ influx may therefore override existing polarity determinants at the cortex of evaginating mother cells and growing hyphae. Thus, both thigmotropic and galvanotropic responses of C. albicans hyphae are dependent on a single Ca2+-regulated orientation mechanism.
Experimental Procedures
For strains used in this study, mutant-strain construction, growth conditions, and determination of Ca2+ accumulation, see the Supplemental Experimental Procedures.
Galvanotropism Assay
Yeast cells were adhered to poly-L-lysine-coated microscope slides and placed on the flat bed of a Biorad midi-sub cell electrophoresis tank [20] and cultured in modified Soll's medium at 37°C ± 1°C for 6 hr at 10 V/cm and a current of 33 ± 2 mA. This field strength could be applied without affecting germ-tube formation or hyphal extension rate, yet it is sufficient for inducing cytoplasmic [Ca2+] increases in other cell systems 3, 4. Hyphal orientation at the site of germ-tube emergence and at the germ-tube tip relative to the cathode were measured with Improvision Openlab 2.0 software. The percentage cathodal orientation (p) was calculated with p = Σ (−sin θ/n) × 100, for n measurements. A minimum of 100 cells was measured in each of three independent experiments for each treatment. Tracings of hyphal growth patterns were generated with Adobe Photoshop.
Thigmotropism Assay
Yeast cells were adhered to poly-L-lysine-coated quartz slides featuring ridges of 0.79 μm ± 40 nm and a pitch of 25 μm (Kelvin Nanotechnology, Glasgow, UK). This ridge height caused maximal hypha reorientation in a preliminary trial of five heights (data not shown). Slides were placed in 20 ml prewarmed 20% (v/v) newborn-calf serum and 2% (w/v) glucose at 37°C for 6 hr for inducing hyphae. The number of hyphae reorienting on contact with a ridge was expressed as a percentage of the total observed interactions. A minimum of 100 interactions was observed per strain in each experiment, and results were reported as the mean value from three independent experiments ± SD.
Acknowledgments
We thank Dominique Sanglard (Swiss Research National Foundation 3200B0-100747/1) for strains and comments on the manuscript and Joe Heitman for kind gifts of mutants. We thank the Medical Research Council (grant no. MBOO4 RGA 0688), the Biotechnology and Biological Sciences Research Council, the Natural Environment Research Council, Wellcome Trust, and EC SIGNALPATH consortium for financial support and Brendan Casey at Kelvin Nanotechnology for guidance on the customized design of etched quartz topographies.
Published online: February 1, 2007
Footnotes
Supplemental Data include Experimental Procedures, two figures, and one table and can be found with this article at http://www.current-biology.com/cgi/content/full/17/4/347/DC1/.
Supplemental Data
References
- 1.Pierson E.S., Miller D.D., Callaham D.A., Shipley A.M., Rivers B.A., Cresti M., Hepler P.K. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell. 1994;6:1815–1828. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta R., Robinson K.R. Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 2004;135:1398–1406. doi: 10.1104/pp.104.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedlack R.S., Jr., Wei M., Loew L.M. Localized membrane depolarizations and localized calcium influx during electric field-guided neurite growth. Neuron. 1992;9:393–402. doi: 10.1016/0896-6273(92)90178-g. [DOI] [PubMed] [Google Scholar]
- 4.Davenport R.W., Kater S.B. Local increases in intracellular calcium elicit local filopodial responses in Helisoma neuronal growth cones. Neuron. 1992;9:405–416. doi: 10.1016/0896-6273(92)90179-h. [DOI] [PubMed] [Google Scholar]
- 5.Silverman-Gavrila L.B., Lew R.R. An IP3-activated Ca2+ channel regulates fungal tip growth. J. Cell Sci. 2002;115:5013–5025. doi: 10.1242/jcs.00180. [DOI] [PubMed] [Google Scholar]
- 6.Watts H.J., Very A.A., Perera T.H., Davies J.M., Gow N.A. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiol. 1998;144:689–695. doi: 10.1099/00221287-144-3-689. [DOI] [PubMed] [Google Scholar]
- 7.Crombie T., Gow N.A.R., Gooday G. Influence of applied electrical fields on yeast and hyphal growth of Candida albicans. J. Gen. Microbiol. 1990;136:311–317. doi: 10.1099/00221287-136-2-311. [DOI] [PubMed] [Google Scholar]
- 8.Muller E.M., Locke E.G., Cunningham K.W. Differential regulation of two Ca2+ influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics. 2001;159:1527–1538. doi: 10.1093/genetics/159.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locke E.G., Bonilla M., Liang L., Takita Y., Cunningham K.W. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 2000;20:6686–6694. doi: 10.1128/mcb.20.18.6686-6694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paidhungat M., Garrett S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 1997;17:6339–6347. doi: 10.1128/mcb.17.11.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iida H., Nakamura H., Ono T., Okumura M., Anraku Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 1994;14:8259–8271. doi: 10.1128/mcb.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdman S.E., Lin L., Malczynski M., Snyder M. Pheromone-regulated genes required for yeast mating differentiation. J. Cell Biol. 1998;140:461–483. doi: 10.1083/jcb.140.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller E.M., Mackin N.A., Erdman S.E., Cunningham K.W. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:38461–38469. doi: 10.1074/jbc.M304089200. [DOI] [PubMed] [Google Scholar]
- 14.Catterall A.W. Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 15.Hockerman G.H., Peterson B.Z., Johnson B.D., Catterall W.A. Molecular determinants of drug binding and action on L-type calcium channels. Annu. Rev. Pharmacol. 1997;37:361–396. doi: 10.1146/annurev.pharmtox.37.1.361. [DOI] [PubMed] [Google Scholar]
- 16.Ozeki-Miyawaki C., Moriya Y., Tatsumi H., Iida H., Sokabe M. Identification of functional domains of Mid1, a stretch-activated channel component, necessary for localization to the plasma membrane and Ca2+ permeation. Exp. Cell Res. 2005;311:84–95. doi: 10.1016/j.yexcr.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Zheng N.N., Dudgeon D.D., Paliwal S., Levchenko A., Grote E., Cunningham K.W. Multiple signaling pathways regulate yeast cell death during the response to mating pheromones. Mol. Biol. Cell. 2006;17:3409–3422. doi: 10.1091/mbc.E06-03-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonilla M., Nastase K.K., Cunningham K.W. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noma S., Iida K., Iida H. Polarized morphogenesis regulator Spa2 is required for the function of putative stretch-activated Ca2+-permeable channel component Mid1 in Saccharomyces cerevisiae. Eukaryot. Cell. 2005;4:1353–1363. doi: 10.1128/EC.4.8.1353-1363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crampin H., Finley K., Gerami-Nejad M., Court H., Gale C., Berman J., Sudbery P. Candida albicans hyphae have a Spitzenkörper that is distinct from the polarisome found in yeast and pseudohyphae. J. Cell Sci. 2005;118:2935–2947. doi: 10.1242/jcs.02414. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M., McCaig C.D., Agius-Fernandez A., Forrester J.V., Araki-Sasaki A. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr. Eye Res. 1997;16:973–984. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]
- 22.Herrero A.B., Lopez M.C., Fernandez-Lago L., Dominguez A. Candida albicans and Yarrowia lipolytica as alternative models for analysing budding patterns and germ tube formation in dimorphic fungi. Microbiol. 1999;145:2727–2737. doi: 10.1099/00221287-145-10-2727. [DOI] [PubMed] [Google Scholar]
- 23.Pu R., Robinson K.R. The involvement of Ca2+ gradients, Ca2+ fluxes, and CaM kinase II in polarization and germination of Silvetia compressa zygotes. Planta. 2003;217:407–416. doi: 10.1007/s00425-003-1012-9. [DOI] [PubMed] [Google Scholar]
- 24.Read N.D., Kellock L.J., Collins T.J., Gundlach A.M. Role of topography sensing for infection-structure differentiation in cereal rust fungi. Planta. 1997;202:163–170. [Google Scholar]
- 25.Sherwood J., Gow N.A.R., Gooday G., Gregory D.W., Marshall D. Contact sensing in Candida albicans: A possible aid to epithelial penetration. J. Med. Vet. Mycol. 1992;30:461–469. doi: 10.1080/02681219280000621. [DOI] [PubMed] [Google Scholar]
- 26.Hoch H.C., Staples R.C., Whitehead B., Comeau J., Wolf E.D. Signalling for growth orientation and cell differentiation by surface topography in Uromyces. Science. 1987;235:1659–1662. doi: 10.1126/science.235.4796.1659. [DOI] [PubMed] [Google Scholar]
- 27.Sanglard D., Ischer F., Marchetti O., Entenza J., Bille J. Calcineurin A of Candida albicans: Involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 2003;48:959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- 28.Karababa M., Valentino E., Pardini G., Coste A.T., Bille J., Sanglard D. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 2006;59:1429–1451. doi: 10.1111/j.1365-2958.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 29.Pickard B.G. Wall to membrane linkers, stretch activated channels, and the detection of tension, voltage, temperature, auxin, and pH. ASGSB Bull. 1992;6:31. [PubMed] [Google Scholar]
- 30.Beaumont V., Llobet A., Lagnado L. Expansion of calcium microdomains regulates fast exocytosis at a ribbon synapse. Proc. Natl. Acad. Sci. USA. 2005;102:10700–10705. doi: 10.1073/pnas.0501961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.