Summary
The localization of oskar mRNA to the posterior of the Drosophila oocyte defines the site of assembly of the pole plasm, which contains the abdominal and germline determinants 1, 2, 3. oskar mRNA localization requires the polarization of the microtubule cytoskeleton, which depends on the recruitment of PAR-1 to the posterior cortex in response to a signal from the follicle cells, where it induces an enrichment of microtubule plus ends 4, 5, 6, 7. Here, we show that overexpressed oskar mRNA localizes to the middle of the oocyte, as well as the posterior. This ectopic localization depends on the premature translation of Oskar protein, which recruits PAR-1 and microtubule-plus-end markers to the oocyte center instead of the posterior pole, indicating that Oskar regulates the polarity of the cytoskeleton. Oskar also plays a role in the normal polarization of the oocyte; mutants that disrupt oskar mRNA localization or translation strongly reduce the posterior recruitment of microtubule plus ends. Thus, oskar mRNA localization is required to stabilize and amplify microtubule polarity, generating a positive feedback loop in which Oskar recruits PAR-1 to the posterior to increase the microtubule cytoskeleton's polarization, which in turn directs the localization of more oskar mRNA.
Keywords: CELLBIO, RNA, DEVBIO
Results and Discussion
Overexpression of oskar mRNA Causes Premature Translation of Oskar Protein and an Assembly of the Pole Plasm at an Ectopic Site
During stage 9 of oogenesis, oskar mRNA localizes to the posterior of the Drosophila oocyte, where it nucleates the pole plasm, which contains the determinants that specify the germ cells and abdomen at the posterior of the embryo 1, 2, 3. This localization depends on the RNA-binding proteins Staufen and HRP48 and on the exon-junction-complex components Mago nashi, Y14, eIF4AIII, and Barentsz 8, 9, 10, 11, 12, 13, 14. In addition, the posterior accumulation of oskar mRNA requires microtubules and the plus-end-directed microtubule motor protein, kinesin, suggesting that it is transported by kinesin toward microtubule plus ends at the posterior pole 4, 15.
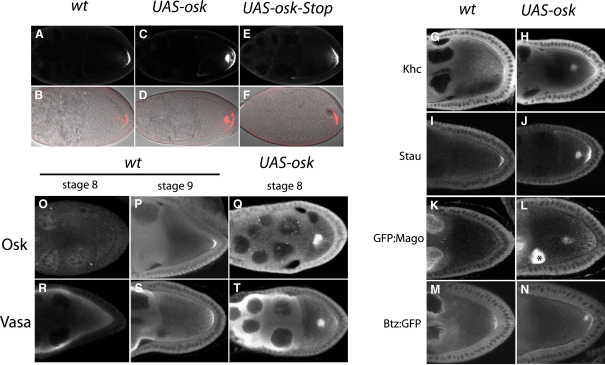
As part of a project to visualize the transport of oskar mRNA, we used the UAS-GAL4 expression system to overexpress oskar mRNA in the female germline [16]. Although increased amounts of oskar mRNA are localized to the oocyte posterior in these UAS-osk females, a significant proportion of the mRNA is found in an ectopic dot in the middle of the oocyte in all stage 9 egg chambers (62/62) and more than half of those at stage 10A (80/150) (Figures 1A–1D).
Figure 1.
Overexpression of oskar mRNA Causes Its Mislocalization and Premature Translation in the Middle of the Oocyte
(A–F) Fluorescence in situ hybridizations to oskar mRNA in wild-type (A and B), UAS-osk (C and D) and UAS-osk-Stop (E and F) stage 9 oocytes ([B], [D], and [F] were obtained by overlaying confocal pseudo differential-interference-contrast images with the in situ stainings [red channel] from the top panel [A, C, and E]). Overexpressed oskar mRNA is localized to both the posterior pole and an ectopic site in the middle of the UAS-osk oocytes. In flies overexpressing an untranslatable version of oskar mRNA (UAS-osk-Stop), the incidence of the ectopic localization is reduced, and most egg chambers show a wild-type pattern of localization (E and F).
(G–N) Immunostainings for the Kinesin heavy chain (G and H), Stau (I and J), and localization of GFP-tagged variants of Mago nashi (K and L) and Barentsz (M and N) in wild-type (G, I, K, and M) and UAS-osk egg chambers (H, J, L, and N). All these components of the oskar mRNA localization complex are mislocalized to the center of the oocyte with overexpressed oskar mRNA. The asterisk in (L) indicates the strong accumulation of Mago:GFP in the oocyte nucleus.
(O–Q) Immunostainings for Oskar protein. In wild-type egg chambers, Oskar protein is not expressed at stage 8 (O) and is first translated at the posterior pole of the oocyte at stage 9 (P). In UAS-osk oocytes, Oskar protein is translated in the middle of the oocyte at stage 8 (Q).
(R–T) Immunostainings for Vasa protein. Oskar recruits Vasa protein to the posterior of the oocyte at stage 9 in wild-type egg chambers (R and S). In UAS-osk oocytes, the ectopic Oskar in the middle of the oocyte recruits Vasa at stage 8 (T).
The mislocalization of oskar mRNA to the center of the oocyte is reminiscent of the localization of oskar mRNA at stage 8 [17], suggesting that excess oskar mRNA saturates the transport pathway, causing a delay in its translocation to the posterior pole. We therefore examined the localization of other components of the oskar mRNA localization complex that are required for its transport to the posterior. Barentsz, Mago nashi, Staufen, and the Kinesin heavy chain all localize to the ectopic dot in the middle of the oskar-overexpressing oocytes, as well as to the posterior pole (Figures 1G–1N). Thus, none of these components appears to be limiting for transport to the posterior.
oskar mRNA is normally translationally repressed until it reaches the posterior pole, but overexpression of the oskar 3′ untranslated region (UTR) causes its premature translation [18]. We therefore examined whether this was also the case in the egg chambers overexpressing full-length oskar RNA. Oskar protein starts to accumulate at the posterior pole of wild-type egg chambers at stage 9 (Figures 1O and 1P). In UAS-osk egg chambers, robust Oskar expression is already visible in the ectopic dot at stage 8 (Figure 1Q). Thus, overexpressed oskar mRNA is not translationally repressed, even though it has not reached the posterior. Furthermore, the ectopic Oskar protein recruits Vasa, indicating that the premature translation of Oskar initiates the formation of the pole plasm in the middle of the oocyte (Figures 1R–1T).
To test whether Oskar protein is required for the mislocalization of oskar mRNA, we generated a second construct, UAS-oskar-Stop, in which we introduced a stop codon at position K178, which is equivalent to the mutation in the oskar protein-null allele oskar54 (Figures 1E and 1F). When oskar-STOP mRNA is overexpressed, it rarely localizes to the middle of the oocyte (13/79 at stage 9; 11/96 at stage 10A), although it is overexpressed at similar levels to the wild-type UAS-oskar mRNA (Figure S1E in the Supplemental Data online). Thus, the formation of the ectopic dot seems to be mainly caused by Oskar protein, rather than saturation of the localization pathway. The expression of the UAS-oskar-STOP construct induces the premature translation of endogenous oskar mRNA (see below), and this may account for the low penetrance of oskar mRNA mislocalization to the middle of these oocytes.
UAS-oskar-STOP Activates Endogenous oskar Translation
Whereas oskar mutants block abdomen formation, extra copies of oskar cause the opposite bicaudal phenotype, in which the head and thorax are replaced by a mirror-image copy of the abdomen [19]. Although UAS-osk females produce some bicaudal embryos, the vast majority show a more extreme phenotype, in which the gut and terminal structures are expanded at the expense of the cuticle (Figures S1A–S1D, Table S1). This is most probably because oskar mRNA is very highly expressed in UAS-osk ovaries (equivalent to at least ten copies of oskar; see Figure S1E), leading to Oskar and Nanos overexpression, which causes an expansion of the expression domains of the terminal-gap genes, tailless and huckebein (data not shown) [20].
Surprisingly, UAS-oskar-STOP gives rise to embryonic phenotypes similar to those of UAS-oskar, even though it cannot produce functional Oskar protein (Figure S1D and Table S1). A weaker bicaudal phenotype has been observed when the oskar-3′UTR is overexpressed, leading to the proposal that this RNA activates the translation of endogenous oskar mRNA by titrating out translational repressors [18]. To test whether this is also the case for UAS-oskar-STOP, we expressed the transgene in females that were transheterozygous for two oskar protein-null mutations (oskar54/oskar346). The resulting embryos showed a typical posterior-group phenotype that was indistinguishable from oskar54/oskar346 alone (Table S1). Thus, UAS-oskar-STOP produces a bicaudal phenotype by activating the translation of endogenous wild-type oskar mRNA.
Ectopic Pole Plasm Recruits Microtubule Plus Ends
At stage 7 of oogenesis, a signal from the posterior follicle cells induces a reorganization of the oocyte microtubule cytoskeleton, so that microtubules are then nucleated from the anterior and lateral cortex 21, 22, 23, 24, 25. The microtubule plus ends appear to localize to the middle of the oocyte during stages 7 and 8, as revealed by the localization of oskar mRNA, Staufen, and Kin:β-GAL (a marker for the plus ends of the microtubules) 4, 17, 26. This organization is only temporary, however, and plus-end markers start to accumulate at the posterior cortex at the beginning of stage 9, coincident with the onset of oskar mRNA localization to the posterior.
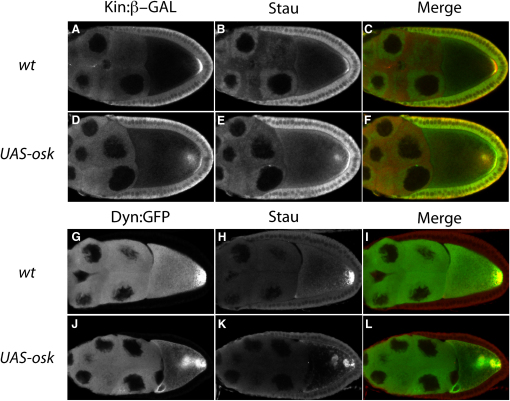
The mislocalization of oskar mRNA to the center of UAS-oskar oocytes resembles the polarity phenotype produced by par-1 hypomorphic mutant combinations, in which some of the microtubule plus ends localize to the middle of the oocyte, rather than the posterior pole 5, 6. We therefore examined the localization of the plus-end marker Kinesin-β-GAL in UAS-osk oocytes. Kinesin-β-GAL always localizes to an ectopic site in the center of these oocytes (18/18), as well as to the posterior, and colocalizes with Staufen, a marker for oskar mRNA (Figures 2A–2F).
Figure 2.
Markers for the Plus Ends of Microtubules Colocalize with Ectopic oskar mRNA
(A–C) A wild-type egg chamber; Kin:β-GAL (A) localizes to the posterior pole of the oocyte at stage 9 and colocalizes with Staufen (B).
(D–F) A UAS-osk egg chamber; Kin:β-GAL (D) and Staufen (E) are mislocalized to a dot in the center of the oocytes with overexpressed oskar mRNA, as well as to the posterior pole.
(G–L) Dynamitin:GFP (G) fusion protein localizes to the posterior pole in wild-type egg chambers and colocalize with pole-plasm markers, such as Stau (H). In UAS-osk flies, Dynamitin:GFP (J) and Stau (K) are mislocalized to the ectopic site in the middle of the oocyte.
The dynein/dynactin motor complex provides another marker for the polarity of the microtubules because it is localized by kinesin to the posterior of the oocyte independently of oskar mRNA 17, 27, 28. This can be visualized with GFP-Dynamitin, a subunit of the Dynactin complex (Figures 2G–2I) [29]. GFP-Dynamitin is mislocalized with Staufen to the ectopic dot in the middle of almost all oskar-overexpressing oocytes (16/18) (Figures 2J and 2K). Thus, two markers for microtubule plus ends colocalize with ectopic Oskar in the middle of the oocyte.
The ectopic Oskar does not appear to interfere with the organization of the microtubule minus ends, or with minus-end-directed processes. The overall morphology of the microtubules is not affected when visualized with tubulin antibodies (not shown) or with a GFP-tagged version of the microtubule-binding protein Tau (Figures S2C and S2D). In addition, the oocyte nucleus always migrates normally to the dorsal-anterior corner of the oocyte, and bcd mRNA shows a wild-type localization to the anterior cortex (Figures S2A and S2B). Surprisingly, some gurken mRNA is often mislocalized to the posterior of UAS-oskar oocytes. It is not translated there, however, because Gurken protein is only found at the correct location adjacent to the oocyte nucleus (Figures S2E–S2I). This phenotype requires high levels of Oskar protein because gurken mRNA is not mislocalized in UAS-oskar-STOP oocytes. These results suggest that prematurely translated Oskar protein and/or downstream components of the pole plasm somehow sequester gurken mRNA and take it with them to the posterior pole.
Oskar Is Required for the Normal Posterior Recruitment of Microtubule Plus Ends
The results above indicate that Oskar protein recruits microtubule plus ends when overexpressed, raising the possibility that Oskar normally plays a role in the recruitment of plus ends to the posterior in wild-type oocytes. To test this hypothesis, we re-examined the organization of the microtubule cytoskeleton in the absence of Oskar protein and in mutants that affect oskar mRNA localization and translation.
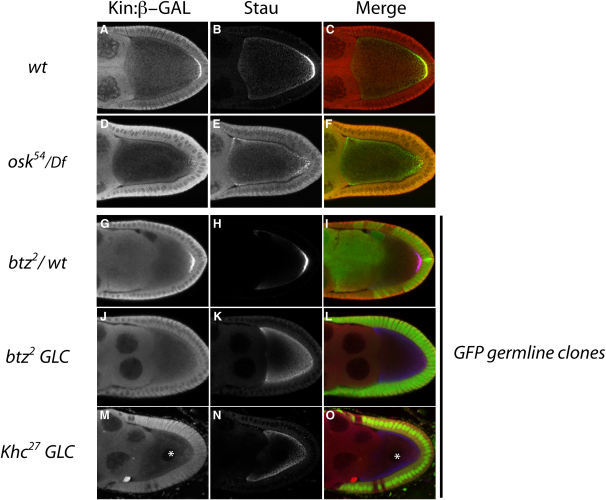
The plus-end marker Kin:β-GAL localizes to a tight posterior crescent in most wild-type stage 9 oocytes (88%, n = 58), with 12% displaying a weaker enrichment at the posterior pole (Figure 3A, Table S2). In an oskar protein null, however, only 16% of the oocytes show a robust posterior localization of Kin:β-GAL, whereas 72% show a weak posterior enrichment, and 12% show no localization to the posterior at all (Figure 3D). We also examined germline clones of btz2 and Khc27, which completely disrupt oskar mRNA localization 12, 15. In these cases, the distribution of Kin:β-GAL in the mutant egg chambers can be directly compared with its localization in the heterozygous egg chambers from the same ovaries, which are stained under identical conditions. In each case, the mutant egg chambers show a highly penetrant reduction or loss of the posterior localization of Kin:β-GAL compared to the nonmutant controls (Figures 3G–3O, Table S2). These results are in good agreement with those of Brendza et al, who noted that Kin:β-GAL is not detectably localized in a significant fraction of Khc mutant oocytes [15]. Thus, the posterior recruitment of plus ends is strongly impaired when Oskar protein is absent from the posterior pole.
Figure 3.
Oskar Is Required for the Efficient Recruitment of the Microtubule Plus Ends to the Posterior Pole
(A–C) Wild-type: the plus-end marker, Kin:β-GAL, localizes to the posterior pole of the oocyte at stage 9 ([A], red in [C]) and colocalizes with Staufen ([B], green in [C]).
(D–E) osk54/Df: The posterior localization of Kin:β-GAL ([D], red in [F]) and Staufen ([E], green in [F]) is strongly reduced in an oskar protein-null mutant.
(G–I) A nonrecombinant btz2/+ egg chamber from a germline clone experiment, showing a robust localization of Kin:β-GAL ([G], red in [I]) and Staufen ([H], blue in [I]) to the posterior pole. GFP staining is shown in green in the merged image (I) and marks the nonrecombinant wild-type cells. The signal in the nurse cells indicates that this is not a germline clone.
(J–L) A btz2 germline clone marked by the loss of GFP (green in [L]) from the same experiment as in (G)–(I). Kin:β-GAL (J) is barely detectable at the posterior of the oocyte, whereas Staufen (K) is not localized to the posterior pole, and accumulates at the anterior of the oocyte and diffusely around the oocyte cortex, consistent with the complete absence of oskar mRNA localization to the posterior pole in this mutant.
(M–O) A Khc27 germline clone stained as above. Kin:β-GAL (M) is only very weakly localized to the posterior, whereas Staufen (O) fails to localize there and is mislocalized to the oocyte anterior-lateral cortex, where oskar mRNA is detected in Khc27 mutants. Also note the misplacement of the oocyte nucleus, labeled with the asterisk; this misplacement reflects the requirement for Kinesin in nuclear anchoring 28, 29, 38.
Oskar May Reinforce Oocyte Polarity by Recruiting Par-1
The mechanism that recruits microtubule plus ends to the posterior pole at stage 9 is not well understood, but depends on the function of the conserved polarity kinase PAR-1 5, 6. In contrast to the wild-type, microtubules are nucleated all around the oocyte cortex of par-1 mutants, and the plus ends are focused at the center instead of the posterior pole. As a consequence, oskar mRNA is mislocalized to the middle of the oocyte, and the resulting embryos lack an abdomen and germline. PAR-1 plays a similar role in organizing the microtubule cytoskeleton in both Drosophila and mammalian epithelial cells, indicating that one of its major functions is in the establishment of polarized microtubule arrays 30, 31, 32.
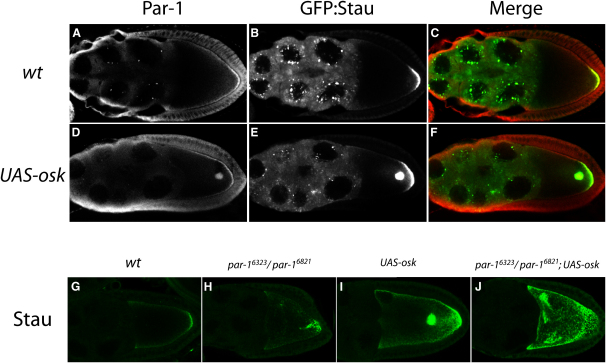
The PAR-1N1 short and long isoforms appear to play the major role in the polarization of the oocyte cytoskeleton because only these fully rescue the polarity defects of par-1 hypomorphs, and GFP-tagged PAR-1N1S and L are recruited to the posterior cortex of the oocyte at stage 7, making them the earliest markers for polarization of the anterior-posterior axis [7]. This localization is more cortical than that of the pole plasm and is not disrupted in staufen, mago nashi, and Kinesin heavy chain mutants, indicating that it is independent of oskar mRNA localization. Antibody stainings fail to detect the cortical PAR-1, perhaps because it is of too low abundance, but recognize a second population of PAR-1, which colocalizes to the posterior with the pole plasm 5, 6. This localization is disrupted in oskar and staufen mutants, but not vasa mutants, suggesting that PAR-1 is recruited by Oskar protein. These results suggest that oskar overexpression may interfere with polarity by recruiting PAR-1 to the center of the oocyte. In support of this view, antibody stainings revealed a strong localization of PAR-1 to the dot in the middle of the oocyte in UAS-osk-overexpressing flies (100%, n = 16; Figures 4A–4F). GFP-PAR-1N1S does not colocalize with ectopic Oskar in the center of the oocyte, however, consistent with the previous observation that this isoform is recruited to the posterior cortex, but not the pole plasm [25].
Figure 4.
PAR-1 May Mediate Plus-End Recruitment by Oskar Protein
(A–C) Wild-type: An immunostaining for PAR-1 ([A], red in [C]) shows its localization to the posterior with GFP-Staufen ([B], green in [C]).
(D–F) UAS-osk: Overexpression of oskar mRNA leads to the recruitment of PAR-1 ([D], red in [F]) and GFP-Staufen ([E], green in [F]) to the middle of the oocyte.
(G) An immunostaining for Stau protein shows its localization to the posterior pole of a wild-type oocyte.
(H) In the hypomorphic mutant combination, par-16323/ par-16821, some Staufen is occasionally mislocalized to the middle of the oocyte.
(I) In UAS-osk/GAL4, the overexpression of oskar mRNA also induces mislocalization of Staufen to the oocyte center.
(J) par-16323/ par-16821; UAS-osk: oskar mRNA overexpression strongly enhances the weak par-1 phenotype, leading to the complete loss of Staufen localization to the posterior.
We also tested whether UAS-oskar enhances the phenotype of a weak par-1 mutant combination. Flies transheterozygous for the two hypomorphic par-1 alleles, par-16323 and par-16821, have mild polarity defects, in which oskar mRNA and Staufen protein are mislocalized to the center of the oocyte in 56% (n = 136) of egg chambers (Figures 4G–4J, Table S3). Despite this mislocalization, Stau is also visibly enriched at the posterior pole of the oocyte in the majority of cases (88%). In contrast, par-16323/par-16821 oocytes that also overexpress oskar mRNA show a much stronger phenotype: Stau protein is visibly enriched at the posterior pole of the oocyte in only 27% (n = 37) of these egg chambers and is mislocalized all around the oocyte cortex in most cases (Figure 4J; Table S3). In addition, these flies do not lay any eggs because of these severe defects in oogenesis. Thus, oskar overexpression strongly enhances the phenotype of a weak par-1 mutant combination, most probably because ectopic Oskar protein recruits some of the limited amount of PAR-1 away from the posterior to the oocyte center.
Although oskar mRNA localization has always been presumed to be downstream of the polarization of the oocyte microtubule cytoskeleton, here we show through both gain- and loss-of-function experiments that it also plays an active role in this process, through the recruitment of microtubule plus ends by Oskar protein. PAR-1 is a good candidate to be the effector of Oskar function in plus-end recruitment because it is required for the polarization of the microtubule cytoskeleton and is recruited by Oskar protein to the posterior of the oocyte. Furthermore, the polarity phenotype of par-1 hypomorphs is strongly enhanced by oskar mRNA overexpression, suggesting that PAR-1 and Oskar function in the same pathway to regulate the microtubules.
On the basis of our results, we would like to suggest a revised model for how the polarity of the oocyte is established (Figure S3). The polarization of the oocyte is initiated by the posterior-follicle-cell signal, which induces the localization of the PAR-1N1 isoforms to the posterior cortex. This localized PAR-1 then recruits or stabilizes some microtubule plus ends at the posterior cortex, leading to the kinesin-dependent transport of a small amount of oskar mRNA from the center of the oocyte to the posterior pole. Once oskar mRNA reaches the posterior, its translational repression is relieved, and the resulting Oskar protein recruits another population of PAR-1 to the posterior. This PAR-1 can then recruit further microtubule plus ends, which in turn direct the posterior localization of more oskar mRNA. Thus, Oskar protein initiates a positive feedback loop that amplifies the polarization of the microtubule cytoskeleton, leading to an increase in the localization of its own mRNA. The microtubule polarity is not reinforced in oskar protein-null mutants or in mutants that disrupt the localization of translation of oskar mRNA, and this results in only a partial polarization of the microtubule cytoskeleton. On the other hand, oskar mRNA overexpression and premature translation in the middle of the oocyte initiates the positive feedback loop in the wrong place. As a consequence, PAR-1 recruits some of the microtubule plus ends to the center of the oocyte and away from the posterior pole. PAR-1 has also been shown to stabilize Oskar protein through direct phosphorylation [33]. This therefore generates a second positive feedback loop that further reinforces the posterior localization of PAR-1, Oskar protein, and microtubule plus ends.
Amplification through positive feedback loops appears to be an emerging theme in cell polarity. For example, the polarization of migrating neutrophils depends on the local enrichment of phosphatidylinositol 3,4,5 triphosphate (PtdInsP3) at the leading edge of the cell, and this is amplified by a Rho-dependent positive feedback loop, in which PtdInsP3 recruits PtdIns-3-OH kinase to the leading edge, which in turn generates more PtdInsP3 [34]. A similar mechanism operates during bud-site selection in S. cerevisiae. The bud site is defined by the localization of activated Cdc42-GTP, and this localization induces the assembly of actin cables that extend into the cytoplasm. This signal is then reinforced by the transport of more Cdc42-GTP along the actin cables to the bud site, where it can induce the assembly of further actin cables [35]. This mechanism is enhanced by a second feedback loop, in which Cdc42-GTP recruits the adaptor protein, Bem1, which binds Cdc24, which activates Cdc42 36, 37. Our results add a third example of the use of multiple feedback loops to reinforce an initially weak cell polarity, and they provide the first case where this amplification involves the microtubule cytoskeleton rather than actin.
Acknowledgments
This work was supported by a Darwin Trust scholarship to V.Z. and by the Wellcome Trust. We also would like to acknowledge everyone in the lab for the help given, especially Trent Munro for his comments on the manuscript.
Published online: February 1, 2007
Footnotes
Supplemental Data include Experimental Procedures, three figures, and three tables and are available with this article online at: http://www.current-biology.com/cgi/content/full/17/4/353/DC1/.
Supplemental Data
References
- 1.Ephrussi A., Dickinson L.K., Lehmann R. oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- 2.Kim-Ha J., Smith J.L., Macdonald P.M. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- 3.Ephrussi A., Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- 4.Clark I., Giniger E., Ruohola-Baker H., Jan L., Jan Y. Transient posterior localisation of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr. Biol. 1994;4:289–300. doi: 10.1016/s0960-9822(00)00068-3. [DOI] [PubMed] [Google Scholar]
- 5.Shulman J.M., Benton R., St. Johnston D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localisation to the posterior pole. Cell. 2000;101:1–20. doi: 10.1016/s0092-8674(00)80848-x. [DOI] [PubMed] [Google Scholar]
- 6.Tomancak P., Piano F., Riechmann V., Gunsalus K.C., Kemphues K.J., Ephrussi A. A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat. Cell Biol. 2000;2:458–460. doi: 10.1038/35017101. [DOI] [PubMed] [Google Scholar]
- 7.Doerflinger H., Benton R., Torres I.L., Zwart M.F., St Johnston D. Drosophila anterior-posterior polarity requires actin-dependent PAR-1 recruitment to the oocyte posterior. Curr. Biol. 2006;16:1090–1095. doi: 10.1016/j.cub.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 8.St Johnston D., Beuchle D., Nüsslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- 9.Newmark P.A., Boswell R.E. The mago nashi locus encodes an essential product required for germ plasm assembly in Drosophila. Development. 1994;120:1303–1313. doi: 10.1242/dev.120.5.1303. [DOI] [PubMed] [Google Scholar]
- 10.Hachet O., Ephrussi A. Drosophila Y14 shuttles to the posterior of the oocyte and is required for oskar mRNA transport. Curr. Biol. 2001;11:1666–1674. doi: 10.1016/s0960-9822(01)00508-5. [DOI] [PubMed] [Google Scholar]
- 11.Mohr S.E., Dillon S.T., Boswell R.E. The RNA-binding protein Tsunagi interacts with Mago Nashi to establish polarity and localize oskar mRNA during Drosophila oogenesis. Genes Dev. 2001;15:2886–2899. doi: 10.1101/gad.927001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Eeden F.J.M., Palacios I.M., Petronczki M., Weston M.J.D., St Johnston D. Barentsz is essential for the posterior localization of oskar mRNA and colocalizes with it to the posterior. J. Cell Biol. 2001;154:511–524. doi: 10.1083/jcb.200105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palacios I.M., Gatfield D., St Johnston D., Izaurralde E. An eIF4AIII-containing complex required for mRNA localization and nonsense-mediated mRNA decay. Nature. 2004;427:753–757. doi: 10.1038/nature02351. [DOI] [PubMed] [Google Scholar]
- 14.Huynh J.R., Munro T.P., Smith-Litiere K., Lepesant J.A., St Johnston D. The Drosophila hnRNPA/B homolog, Hrp48, is specifically required for a distinct step in osk mRNA localization. Dev. Cell. 2004;6:625–635. doi: 10.1016/s1534-5807(04)00130-3. [DOI] [PubMed] [Google Scholar]
- 15.Brendza R.P., Serbus L.R., Duffy J.B., Saxton W.M. A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science. 2000;289:2120–2122. doi: 10.1126/science.289.5487.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 17.Palacios I.M., St Johnston D. Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming, and posterior localization in the Drosophila oocyte. Development. 2002;129:5473–5485. doi: 10.1242/dev.00119. [DOI] [PubMed] [Google Scholar]
- 18.Filardo P., Ephrussi A. Bruno regulates gurken during Drosophila oogenesis. Mech. Dev. 2003;120:289–297. doi: 10.1016/s0925-4773(02)00454-9. [DOI] [PubMed] [Google Scholar]
- 19.Smith J.L., Wilson J.E., Macdonald P.M. Overexpression of oskar directs ectopic activation of nanos and presumptive pole cell formation in Drosophila embryos. Cell. 1992;70:849–859. doi: 10.1016/0092-8674(92)90318-7. [DOI] [PubMed] [Google Scholar]
- 20.Cinnamon E., Gur-Wahnon D., Helman A., St Johnston D., Jimenez G., Paroush Z. Capicua integrates input from two maternal systems in Drosophila terminal patterning. EMBO J. 2004;23:4571–4582. doi: 10.1038/sj.emboj.7600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theurkauf W.E., Smiley S., Wong M.L., Alberts B.M. Reorganization of the cytoskeleton during Drosophila oogenesis: Implications for axis specification and intercellular transport. Development. 1992;115:923–936. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- 22.González-Reyes A., Elliott H., St Johnston D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature. 1995;375:654–658. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- 23.Roth S., Neuman-Silberberg F.S., Barcelo G., Schüpbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 24.Clark I., Jan L.Y., Jan Y.N. Reciprocal localization of Nod and kinesin fusion proteins indicates microtubule polarity in the Drosophila oocyte, epithelium, neuron and muscle. Development. 1997;124:461–470. doi: 10.1242/dev.124.2.461. [DOI] [PubMed] [Google Scholar]
- 25.Cha B., Koppetsch B.S., Theurkauf W.E. In Vivo Analysis of Drosophila bicoid mRNA Localization Reveals a Novel Microtubule-Dependent Axis Specification Pathway. Cell. 2001;106:35–46. doi: 10.1016/s0092-8674(01)00419-6. [DOI] [PubMed] [Google Scholar]
- 26.Cha B.J., Serbus L.R., Koppetsch B.S., Theurkauf W.E. Kinesin I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat. Cell Biol. 2002;4:592–598. doi: 10.1038/ncb832. [DOI] [PubMed] [Google Scholar]
- 27.Li M.-g., McGrail M., Serr M., Hays T.H. Drosophila cytoplasmic dynein, a microtubule motor that is asymmetrically localized in the oocyte. J. Cell Biol. 1994;126:1475–1494. doi: 10.1083/jcb.126.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brendza R., Serbus L., Saxton W., Duffy J. Posterior localization of Dynein and dorsal-ventral axis formation depend on Kinesin in Drosophila oocytes. Curr. Biol. 2002;12:1541–1545. doi: 10.1016/s0960-9822(02)01108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Januschke J., Gervais L., Dass S., Kaltschmidt J.A., Lopez-Schier H., St Johnston D., Brand A.H., Roth S., Guichet A. Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Curr. Biol. 2002;12:1971–1981. doi: 10.1016/s0960-9822(02)01302-7. [DOI] [PubMed] [Google Scholar]
- 30.Drewes G., Ebneth A., Preuss U., Mandelkow E.M., Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule- associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 31.Doerflinger H., Benton R., Shulman J.M., St Johnston D. The role of PAR-1 in regulating the polarised microtubule cytoskeleton in the Drosophila follicular epithelium. Development. 2003;130:3965–3975. doi: 10.1242/dev.00616. [DOI] [PubMed] [Google Scholar]
- 32.Cohen D., Brennwald P.J., Rodriguez-Boulan E., Musch A. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol. 2004;164:717–727. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riechmann V., Gutierrez G.J., Filardo P., Nebreda A.R., Ephrussi A. Par-1 regulates stability of the posterior determinant Oskar by phosphorylation. Nat. Cell Biol. 2002;4:337–342. doi: 10.1038/ncb782. [DOI] [PubMed] [Google Scholar]
- 34.Weiner O.D., Neilsen P.O., Prestwich G.D., Kirschner M.W., Cantley L.C., Bourne H.R. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wedlich-Soldner R., Altschuler S., Wu L., Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- 36.Irazoqui J.E., Gladfelter A.S., Lew D.J. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 2003;5:1062–1070. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- 37.Wedlich-Soldner R., Wai S.C., Schmidt T., Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan J.E., Warrior R. The cytoplasmic Dynein and Kinesin motors have interdependent roles in patterning the Drosophila oocyte. Curr. Biol. 2002;12:1982–1991. doi: 10.1016/s0960-9822(02)01303-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.