Abstract
Newborn rat pups can learn to either approach or avoid odor cues through associative conditioning. The present results demonstrate that preference conditioning and avoidance conditioning both modify olfactory bulb responses (focal 2-deoxyglucose uptake and mitral-tufted cell single unit responses) to the conditioned odor. Despite opposing behavioral responses to the conditioned odor, however, olfactory bulb neural responses did not detectably differ between learned odor cues signaling approach and those signaling avoidance. Control pups exhibited neither the behavioral nor neural changes. Furthermore, both the behavioral and neural changes to these odor cues could be extinguished. These results suggest that the olfactory bulb in neonates may code learned odor importance, but specific information attached to that importance may require processing in other brain regions.
Olfactory associative conditioning in preweanling rat pups modifies behavioral (Brake, 1981; Johanson & Hall, 1982; Johanson & Teicher, 1980; Pedersen, Williams, &Blass, 1982; Sullivan, Brake, Hofer, & Williams, 1986; Sullivan & Hall, 1988; Sullivan, Hofer, & Brake, 1986) and olfactory bulb metabolic (Coopersmith & Leon, 1984; Sullivan & Leon, 1986) and neural responses (Wilson, Sullivan, & Leon, 1987) to subsequent presentations of that odor. This appetitive conditioning is believed to model the events underlying pup recognition of, and orientation toward, the dam. In fact, pup behavioral and neural responses to maternal odors are similar to those observed with artificial odors that have gained attractive value through classical conditioning (Sullivan, Wilson, Wong, Correa, & Leon, 1990).
Several lines of evidence suggest that this early olfactory conditioning may be a special form of learning, unique to the immature olfactory system. For example, early appetitive olfactory learning in rats is acquired very rapidly (Sullivan & Leon, 1987) during a sensitive period after birth (Woo & Leon, 1988) and lasts into adulthood (Coopersmith & Leon, 1986; Fillion & Blass, 1986). Thus, early olfactory learning in rat pups could be similar to imprinting to visual stimuli in birds (Horn, 1985) or imprinting to chemical stimuli in fish (Scholz, Horral, Cooper, & Hasler, 1976).
Further evidence in support of unique mechanisms of early olfactory learning arise from the nature of the neural changes associated with it. Olfactory bulb responses to appetitively conditioned odors are characterized by enhanced focal metabolic activity in odor-specific regions of the glomerular layer (2-deoxyglucose [2-DG] uptake) and modified response patterns of olfactory bulb output neurons (mitral-tufted cells) near these hyperactive glomeruli (Sullivan & Leon, 1986; Wilson & Leon, 1988). In addition, the size of the glomerular layer near the regions of hyperactivity is increased after early odor experience, suggesting either an experience-associated increase in neural size or number or decrease in normal postnatal death of these neurons (Woo, Sullivan, & Leon, 1987).
The present study was designed to further characterize early olfactory learning. Toward this aim, two specific issues were addressed. First, is the modified olfactory bulb response specific to learned preferred odors? Specifically, does an odor that an animal has learned to avoid also produce a modified olfactory bulb response? Rat pups can learn to avoid odors paired with shock (Kucharski & Spear, 1984; Rudy & Cheatle, 1977). The present experiment paired a peppermint odor with either footshock or positively reinforcing stroking and examined both behavioral and olfactory bulb neural responses to subsequent presentations of that odor. The second issue investigated was the apparent permanence of the learned neurobehavioral response. Odors experienced during the preweanling period can influence both behavioral and olfactory bulb neural responses to that odor even after the animal attains sexual maturity (Coopersmith & Leon, 1986; Fillion & Blass, 1986). If early olfactory learning is a special form of learning similar to imprinting, then it may be difficult to extinguish responses acquired through early associative conditioning. Therefore, the present experiment also examined extinction of responses to odors learned early in life.
Method
The subjects were 209 male and female preweanling rat pups from litters born of Wistar multiparous dams (offspring of Hilltop Lab Animals, Scottsdale, PA) in the animal care facilities at the University of Oklahoma (Norman, OK). Dams were housed in rectangular polypropylene cages (34 × 29 × 17 cm) lined with wood chips. The colony room was temperature controlled (23 °C) and maintained on a 12:12-hr light-dark cycle (lights on at 8:00 a.m.). Food and water were available at all times. Births were checked at 8:00 a.m. and 5:00 p.m. The day of birth was considered Postnatal Day (PN) 0. Litters were culled to 10 pups on PN 1.
Associative olfactory conditioning consisted of seventeen 10-min training sessions with an intertrial interval (ITI) of 24 hr. On PN 1, pups were assigned to one of the following training groups: (a) forward odor-stroke, pups were exposed to peppermint odor while being vigorously stroked with a sable hair brush (13 normal and 10 extinction for behavior; 7 normal and 7 extinction for 2-DG; 4 normal and 3 extinction for physiology); (b) random odor-stroke, pups were given nonoverlapping exposures to both odor and stroking using a pseudorandom schedule (13 normal and 10 extinction for behavior; 7 normal and 7 extinction for 2-DG; 5 normal and 3 extinction for physiology); (c) forward odor-shock, pups were exposed to the peppermint odor paired with a footshock coinciding with the last second of odor presentation (13 normal and 13 extinction for behavior; 7 normal and 7 extinction for 2-DG; 4 normal and 3 extinction for physiology); (d) random odor-shock, pups were given nonoverlapping exposures to both odor and shock using a pseudorandom schedule (13 normal and 13 extinction for behavior; 7 normal and 7 extinction for 2-DG; 4 normal and 3 extinction for physiology); or (e) naive (15 for behavior; 7 for 2-DG; 4 for physiology). The tactile stimulation produced by stroking was used to mimic maternal stimulation and has reinforcing properties as robust as those of milk to infant rats (Sullivan & Hall, 1988). The intensity of the stroking was sufficient to induce behavioral activation in the pups. The footshock was a 1-s, constant-current, 1.5-mA shock. This shock intensity has been shown to be capable of producing an odor aversion throughout development (Kucharski & Spear, 1984; Sullivan, 1990; Sullivan & Wilson, 1990). The odor for all shock and stroking groups was peppermint extract (Shilling, Baltimore, MD; the alcohol in this peppermint oil and alcohol mixture was allowed to evaporate before use) presented through a flow-dilution olfactometer (concentration 1:10 of saturated vapor; 1 L/min flow rate). On PN 18 and PN 19, half of the pups from each group were given six extinction trials: 10-min odor exposures, 2-hr ITIs, 3 times per day.
On PN 20, pups were given one of three tests: a behavioral odor preference test, measurement of l4C-2-DG uptake during conditioned stimulus-only exposure, or measurement of mitral-tufted cell single unit response patterns to the conditioned stimulus.
The behavior test consisted of a Plexiglas Y maze (start box: 8.5 cm width, 10 cm length, 8 cm height; choice arms: 8.5 × 24 × 8 cm) that required pups to choose between two odors:—the conditioned peppermint odor and a familiar pine odor. Both odors were delivered via flow-dilution olfactometers (1:10 concentration of saturated vapor; 1 L/min flow rate) into the ends of the choice arms. Pups were placed in the start box and allowed 60 s to choose an arm. The pups were removed from the maze for 30 s between trials and received five trials. Odors were exhausted from the maze between trials.
For the odor-2-DG test, pups were injected with 14C-2-DG (20 μCi/100 g) immediately before odor delivery and placed in a test chamber identical to that used during conditioning. Peppermint odor was delivered at 1 L/min (1:10 dilution) for 45 min (cycle of 3 min on, 1 min off). After odor exposure, pups were decapitated, and their brains were quickly removed and frozen in methylbutane at -45 °C. The frozen brain was equilibrated to -17 °C in a cryostat for 45 min, and the olfactory bulb was cut coronally in 20-μm sections. Brain sections were exposed on Kodak SB-5 film for 8 days at room temperature, and autoradiographs were analyzed with an image analysis system (Imaging Research, Inc., St. Catharines, Ontario, Canada) as previously described (Sullivan, Wilson, & Leon, 1989; Wilson & Sullivan, 1990).
Pups used for single unit recording on PN 20 were anesthetized with urcthane (1.5 g/kg) and mounted in a stereotaxic apparatus. Their body temperature was maintained with a thermistatically controlled heating pad at 35 °C. Single unit responses were recorded in the olfactory bulb with a glass microclcctrode filled with 2 M NaCI. Single unit recordings were made from mitral-tufted cells along the lateral aspect of the olfactory bulb, 1.5-2.2 mm from the rostral pole of the bulb, in the area of glomerular layer focal 2-DG uptake to peppermint (Wilson & Leon, 1988). A bipolar stimulating electrode was placed in the lateral olfactory tract (LOT) to aid in identification of mitral-tufted cells. Mitral-tufted cells were identified by the ability to antidromically activate such neurons from the LOT. Animals respired normally. Odors were delivered to the external nares through glass lubing. Odor concentration was matched to that used during training (peppermint odor at a 1:10 dilution of saturated peppermint vapor; 0.5 L/min flow rate). Stimulus duration was 4 s with at least 60 s between stimuli. Stimulus concentration and timing were controlled by microcomputer that controlled a Harvard syringe pump (Model 22; Harvard Apparatus, South Natick, MA). The pump introduced saturated peppermint vapor into a stream of clean air. Odor concentration could be varied by varying pump rate. Responses were categorized as excitatory, suppressive, or no response according to specific criteria previously described (Wilson & Sullivan, 1990). The ratio of excitatory responses to total response rate was calculated for each animal, and mean values for each group were compared using analyses of variance (ANOVAs).
Results
As shown in Figure 1, pups that received the forward odor-stroke training developed a relative preference for the conditioned odor. Conversely, pups that received forward odor-shock training learned to avoid the conditioned odor, 2 (extinction) × 5 (training) ANOVA: Training × Extinction interaction, F(4, 101) = 8.90, p < .001. Post hoc Tukey tests revealed that the forward odor-shock and forward odor-stroke pups were significantly different from controls, p < 0.05. Furthermore, extinction trials before testing resulted in the elimination of both the conditioned preference and conditioned aversion. Post hoc tests revealed that forward odor-shock extinction and forward odor-stroke extinction pups were significantly different from nonextinction pups and not significantly different from controls, p < .05.
Figure 1.
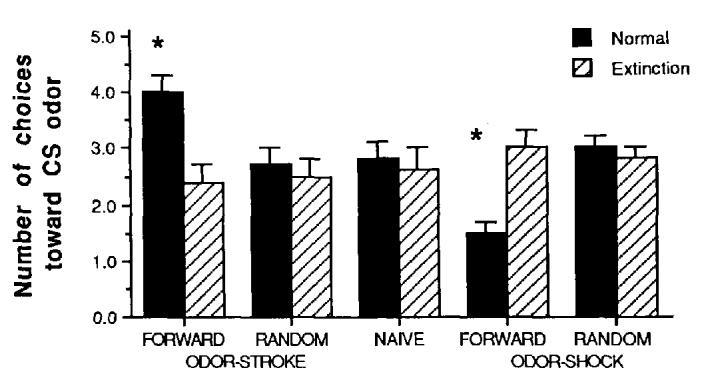
Mean (± SE) number of choices (five total possible) toward peppermint odor conditioned stimulus (CS) in the two odor preference test. (Pups were previously trained in the forward odor-stroke, random odor-stroke, naive, forward odor-shock, random odor-shock conditioning groups. Half of the pups in each training condition underwent extinction training before testing. Asterisks represent significantly different from control and extinction groups at p < .05.)
Despite the opposing conditioned behavioral responses in forward odor-stroke and forward odor-shock pups, both conditioning procedures produced similar modifications in olfactory bulb metabolic and neural activity (Figures 2 and 3). Forward odor-stroke and forward odor-shock pups demonstrated enhanced focal glomerular layer 2-DG uptake compared with random and naive controls. Relative focal 2-DG uptake in odor-specific regions of the glomerular layer was significantly enhanced in both forward odor-stroke and forward odor-shock pups, 2 × 5 ANOVA: Training × Extinction interaction, F(4, 60) = 6.9, p < .001. Post hoc Tukey tests revealed that focal 2-DG uptake in forward odor-shock and forward odor-stroke pups was significantly different from controls, p < .01. Relative 2-DG uptake in nonfocal glomerular layer areas did not significantly differ between groups. Extinction training eliminated the enhanced focal 2-DG uptake patterns in forward odor-stroke and forward odor-shock pups. Post hoc tests revealed that 2-DG uptake in forward odor-shock extinction and forward odor-stroke extinction pups were significantly different from nonextinction pups and not significantly different from controls, p < .01.
Figure 2.
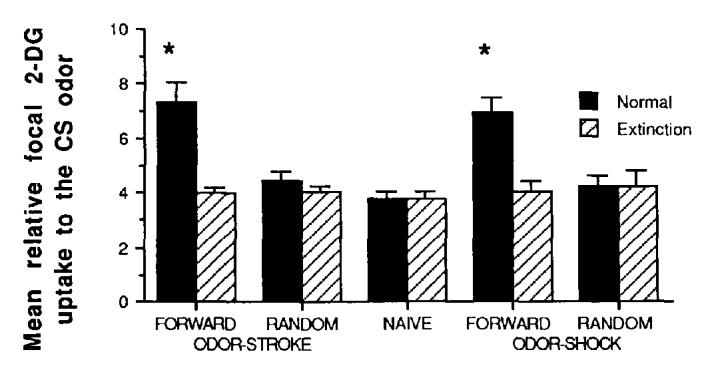
Mean (± SE) relative 2-dcoxyglucose (2-DG) uptake in the spatially odor-specific focal areas of the olfactory bulb during test exposure to the peppermint odor conditioned stimulus (CS). (Asterisks represent significantly different from control and extinction groups at p < .05.)
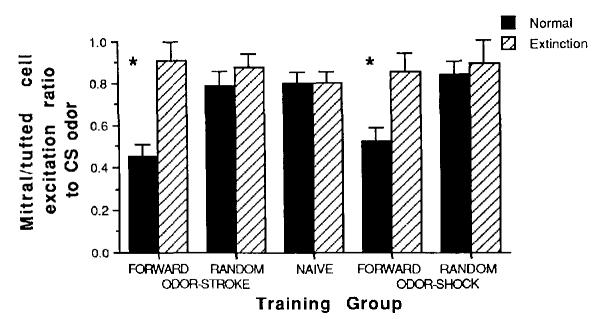
Figure 3.
Mean (± SE) mitral-tufted cell excitation ratios (percent excited/total percent responsive) to the conditioned stimulus (CS) odor. (An excitation ratio of less than 0.5 signified that, of the cells that responded to the odor, more were suppressed than excited. An excitation ratio of greater than 0.5 signified that, of the cells that responded to the odor, more were excited than suppressed. Asterisks represent significantly different from control and extinction groups at p < .05.)
A second measure of olfactory bulb response to the conditioned odor was mitral-tufted cell single unit recording. A total of 277 cells were examined in 33 pups (odor-stroke: forward normal, 41 cells; forward extinction, 20 cells; random normal, 42 cells; random extinction, 22 cells; odor-shock: forward normal, 41 cells; forward extinction, 22 cells; random normal, 40 cells; random extinction, 21 cells; naive controls: 28 cells). As shown in Figure 3, mitral-tufted cells recorded near the regions of focal 2-DG uptake demonstrated fewer excitatory and more suppressive responses (decreased excitation ratio) to the conditioned odor in forward odor-stroke and forward odor-shock pups compared with controls, 2 × 5 ANOVA: Training × Extinction interaction, F(4, 27) = 3.8, p < .02. Post hoc Tukey tests revealed that mitral-tufted cell excitation ratio in forward odor-shock and forward odor-stroke pups was significantly different from controls, p < .05. Total response rates (excitation and suppression) did not significantly differ between groups. As with the 2-DG uptake results, forward odor-stroke and forward odor-shock single unit response patterns were different from controls, although they were not different from each other. These modified responses were eliminated by extinction training (post hoc tests revealed that mitral-tufted cell excitation ratio in forward odor-shock extinction and forward odor-stroke extinction pups were significantly different from nonextinction pups and not significantly different from controls, p < .05.
Discussion
The results of the present experiment suggest that learned olfactory behaviors in infant rats are associated with modified olfactory bulb metabolic and neural responses to the conditioned odor. Of importance, the modified olfactory bulb response to learned odor cues signaling avoidance do not detectably differ from bulb responses to learned odor cues signaling approach in pups. Pups that learned to either avoid or approach peppermint odor exhibited enhanced focal 2-DG uptake in odor-specific glomeruli and decreased excitation ratios in mitral-tufted cell single unit response patterns to that odor. It should be noted that these results are in contrast to an earlier study of odor avoidance learning in young rats (Coopersmith, Lee, & Leon, 1986). Coopersmith, Lee, and Leon used a single pairing of odor with LiCl at PN 18 and found no change in olfactory bulb 2-DG uptake in response to the conditioned odor compared with odor-naive pups. The difference in age at training, the duration of training, or the use of LiCl-induced toxicosis as the aversive stimulus may account for the difference in results. We are currently examining the effects of odor-shock training at different ages and different shock intensities to isolate critical factors.
Of importance, both the behavioral and neural responses to conditioned odors could be extinguished. This suggests that early olfactory learning is not a special form of learning or imprinting but rather conforms to criteria of normal associative conditioning. The reversal of conditioned neural changes by extinction found in the present study is similar to results obtained in mature cats and rabbits (Applegate, Frysinger, Kapp, & Gallagher, 1982; Diamond & Weinberger, 1989; Woody & Brozek, 1969). It should be noted, however, that given the techniques used here, it is not clear whether the similarity between the extinguished responses and responses in control animals are due to similar mechanisms; that is, we do not know whether extinction training eliminated the modifications produced by conditioning or whether extinction produced additional changes that resulted in responses being similar to those of control animals.
The present results suggest that although olfactory bulb metabolic and neural responses to odors are modified by conditioning, the modified response does not distinguish between learned odors signaling approach and those signaling avoidance. This result was found despite dramatic differences in learned behavioral responses to that odor. Although we are unaware of a direct comparison, these results appear to compare favorably with those obtained in mature rabbits. In two separate studies using adult rabbits, olfactory bulb electroencephalographic burst amplitude was reduced to odors previously conditioned to either aversive shock (Bressler, 1988) or water reward (Viana Di Frisco & Freeman, 1985).
The mechanisms of change in olfactory bulb response are, as yet, unknown. A variety of previous studies have demonstrated that the enhanced 2-DG uptake to learned odors in pups is not due to modified respiration (Coopersmith & Leon, 1984; Sullivan, Wilson, Kim, & Leon, 1988). Furthermore, the changes in both focal 2-DG uptake and single unit activity have been shown to be specific to the conditioned odor; neurobehavioral responses to nonconditioned stimuli are not modified by associative conditioning in pups (Coopersmith, Henderson, & Leon, 1986; Wilson ctal., 1987). However, it has not been definitively demonstrated whether the change in bulb response is due to synaptic changes intrinsic to the bulb or is due to centrifugal modulation of bulb activity. Although the present article does not directly address this issue, there are several lines of evidence suggesting that neural changes intrinsic to the bulb do occur after early olfactory experience. First, odor-stroke pairings in pups induce an increase in glomerular size near odor-specific 2-DG foci (Woo et al., 1987). Second, blockade of olfactory bulb/β-noradrenergic receptors with intrabulbar infusions during training blocks acquisition of learned odor preferences (Lin, Wilson, & Sullivan, 1990). Third, although noradrenergic centrifugal projections to the bulb appear to be required for acquisition of learned olfactory behaviors in pups (Sullivan et al., 1989), norepincphrine is not necessary for expression of the learned response (Lin et al., 1990). However, given the inability of the bulb to distinguish between learned cues signaling opposite behavioral responses, as shown here, it is clear that other brain regions must be modified or at least participate in the learned response.
Based on the present results, we hypothesize that the acquisition of even a simple association during olfactory conditioning in rat pups requires the subject to store at least two bits of information. One bit signals that a stimulus has acquired significance (“I know that odor”), and the second bit signals what the significance is (“approach-avoid”). The results of the present experiment suggest that the first bit may be encoded by activity in the olfactory bulb—the bulb responds differentially to learned odors. Other brain regions (Kucharski & Hall, 1987, 1988), however, must be involved in the encoding of the hypothesized second bit, given that bulb activity cannot distinguish learned preferred odors from learned aversivc odors. One possible locus for information regarding this remaining information is the amygdala. The amygdala receives direct olfactory input (Price, 1987), and amygdala neurons respond to olfactory stimuli (Cain & Bindra, 1972). Furthermore, the amygdala has long been associated with learning and memory, and damage to this area can impair associative learning (Sarter & Markowitsch, 1985), even in infant rats (Sananes & Campbell, 1989). The olfactory cortex, anterior olfactory nucleus, frontal cortex, hippocampus, and a variety of other areas have also been implicated in olfactory memories.
Regardless of which areas beyond the olfactory bulb are involved in the further processing of olfactory memories, our results suggest that simple associative learning in infants may be a distributed process. These results are particularly interesting in light of recent studies of human cognitive memory investigating the time course of word-pair recognition (Gronlund & Ratcliff, 1989). These studies suggest that information regarding an association may be stored (retrieved) independently of the items associated. The results reported in the present article may provide a neurobiological example of this memory characteristic in newborn rats.
Footnotes
This research was supported by National Institutes of Health-National Institute of Deafness and Other Communications Disorders Grant DC00489 to Regina M. Sullivan and National Science Foundation Grants BNS8606786 and BNS88191891 to Donald A. Wilson.
References
- Applcgate CD, Frysinger RC, Kapp BS, Gallagher M. Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Research. 1982;238:457–462. doi: 10.1016/0006-8993(82)90123-8. [DOI] [PubMed] [Google Scholar]
- Brake SC. Suckling infant rats learn a preference for a novel olfactory stimulus paired with milk delivery. Science. 1981;211:506–508. doi: 10.1126/science.7192882. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Changes in electrical activity of rabbit olfactory bulb and cortex to conditioned odor stimulation. Behavioral Neuroscience. 1988;102:740–747. doi: 10.1037//0735-7044.102.5.740. [DOI] [PubMed] [Google Scholar]
- Cain DP, Bindra D. Responses of amygdala single units to odors in the rat. Experimental Neurology. 1972;35:98–110. doi: 10.1016/0014-4886(72)90062-3. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Henderson SR, Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Developmental Brain Research. 1986;27:191–197. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Lee S, Leon M. Olfactory bulb responses after odor aversion learning by young rats. Developmental Brain Research. 1986;24:271–277. doi: 10.1016/0165-3806(86)90195-1. [DOI] [PubMed] [Google Scholar]
- Coopersmith RM, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Coopersmith RM, Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Research. 1986;371:400–403. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Role of context in the expression of learning induced plasticity of single neurons in auditory cortex. Behavioral Neuroscience. 1989;103:471–494. doi: 10.1037//0735-7044.103.3.471. [DOI] [PubMed] [Google Scholar]
- Fillion TJ, Blass EM. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science. 1986;231:729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- Gronlund SD, Ratcliff R. Time course of item and associative information: Implications for global memory models. Journal of Experimental Psychology: Learning, Memory and Cognition. 1989;15:278–287. doi: 10.1037//0278-7393.15.5.846. [DOI] [PubMed] [Google Scholar]
- Horn G. Memory, imprinting and the brain. Oxford University Press; Oxford, England: 1985. [Google Scholar]
- Johanson IB, Hall WG. Appetitive conditioning in neonatal rats: Conditioned orientation to a novel odor. Developmental Psychobio/ogy. 1982;15:379–397. doi: 10.1002/dev.420150410. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Teicher M. Classical conditioning of an odor preference in 3-day-old rats. Behavioral and Neural Biology. 1980;29:132–136. doi: 10.1016/s0163-1047(80)92596-0. [DOI] [PubMed] [Google Scholar]
- Kucharski D, Hall WG. New routes to old memories. Science. 1987;238:786–788. doi: 10.1126/science.3672125. [DOI] [PubMed] [Google Scholar]
- Kucharski D, Hall WG. Developmental change in the access to olfactory memories. Behavioral Neuroscience. 1988;102:340–348. doi: 10.1037//0735-7044.102.3.340. [DOI] [PubMed] [Google Scholar]
- Kucharski D, Spear NE. Conditioning of aversion to an odor paired with peripheral shock in the developing rat. Developmental Psychobiology. 1984;17:465–480. doi: 10.1002/dev.420170505. [DOI] [PubMed] [Google Scholar]
- Lin W, Wilson DA, Sullivan RM. Olfactory bulb norepinephrine may be required for early olfactory learning. Society for Neuroscience Abstracts. 1990;16:1236. [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. Journal of Experimental Psychology: Animal Behavior. 1982;8:329–341. [PubMed] [Google Scholar]
- Price JL. The central olfactory and accessory olfactory systems. In: Finger TE, Silver WL, editors. Neurobiology of taste and smell. Wiley; New York: 1987. pp. 179–203. [Google Scholar]
- Rudy JW, Cheatle MD. Odor-aversion learning in neonatal rats. Science. 1977;198:845–846. doi: 10.1126/science.918668. [DOI] [PubMed] [Google Scholar]
- Sananes CB, Campbell BA. Role of the central nucleus of the amygdala in olfactory heart rate conditioning. Behavioral Neuroscience. 1989;103:519–525. [PubMed] [Google Scholar]
- Sarter M, Markowitsch HJ. Involvement of the amygdala in learning and memory: A critical review, with emphasis on anatomical relationship. Behavioral Neuroscience. 1985;99:342–380. doi: 10.1037//0735-7044.99.2.342. [DOI] [PubMed] [Google Scholar]
- Scholz AT, Horral RM, Cooper JC, Hasler AD. Imprinting to chemical cues: The basis for home stream selection in salmon. Science. 1976;192:1247–1249. doi: 10.1126/science.1273590. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Associative learning in infant rats and neonatal human infants. Teratology. 1990;41:613. [Google Scholar]
- Sullivan RM, Brake SC, Hofer MA, Williams CL. Huddling and independent feeding of neonatal rats can be facilitated by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19:625–635. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hall WG. Reinforcers in infancy: Classical conditioning using stroking or intra-oral infusions of milk as a UCS. Developmental Psychobiology. 1988;21:215–223. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Developmental Brain Research. 1986;27:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. One-trial olfactory learning enhances olfactory bulb responses to an appetitive conditioned odor in 7-day-old rats. Developmental Brain Research. 1987;35:301–311. doi: 10.1016/0165-3806(87)90056-3. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Plasticity in the reinforcement system of infant rats. Society for Neurosciences Abstracts. 1990;16:917. [Google Scholar]
- Sullivan RM, Wilson DA, Kim MH, Leon M. Behavioral and neural correlates of postnatal olfactory conditioning: I. Effects of respiration on conditioned neural responses. Physiology and Behavior. 1988;44:85–90. doi: 10.1016/0031-9384(88)90349-6. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Associative processes in early olfactory preference acquisition: Neural and behavioral consequences. Psychobiology. 1989;17:29–33. doi: 10.3758/bf03337814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Developmental Brain Research. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Viani Di Frisco G, Freeman WJ. Odor-related bulbar EEG spatial pattern analysis during appetitive conditioning in rabbits. Behavioral Neurosdence. 1985;99:964–978. doi: 10.1037//0735-7044.99.5.964. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. Journal of Neurophysiology. 1988;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan R:M. Olfactory conditioning in infant rats with brain stimulation as reward: 1. Neurobehavioral consequences. Developmental Brain Research. 1990;53:215–221. doi: 10.1016/0165-3806(90)90009-n. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output responses patterns to learned attractive odors. Journal of Neurosdence. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CC, Coopersmith RM, Leon M. Morphological and metabolie changes in the olfactory bulb accompany the enhanced neural response to familiar odors in rat pups. Journal of Comparative Neurology. 1987;263:113–125. doi: 10.1002/cne.902630110. [DOI] [PubMed] [Google Scholar]
- Woo CC, Leon M. Sensitive period for neural and behavioral response development to learned odors. Developmental Brain Research. 1987;36:309–313. doi: 10.1016/0165-3806(87)90038-1. [DOI] [PubMed] [Google Scholar]
- Woody CD, Brosek G. Changes in evoked responses from facial nucleus of cat with conditioning and extinction of an eyeblink. Journal of Neurophysiology. 1969;32:717–726. doi: 10.1152/jn.1969.32.5.717. [DOI] [PubMed] [Google Scholar]