Abstract
One of the circuits modified by early olfactory learning is in the olfactory bulb. Specifically, response patterns of mitral-tufted cells are modified by associative conditioning during the early postnatal period. In addition, previous work has demonstrated that mitral-tufted cell single units respond to both olfactory conditioned stimuli and rewarding stimulation of the medial forebrain bundle-lateral hypothalamus (MFB-LH). The present study suggests that norepinephrine β-receptor activation is required for early olfactory learning using MFB-LH stimulation as reward. Propranolol injected before odor-MFB-LH pairings blocks the acquisition of conditioned behavioral responses and their neural correlates to the conditioned odor. Furthermore, propranolol blocks a specific class of the mitral-tufted cell responses to MFB-LH reward stimulation. The relationship of this response to reward and early learning is discussed.
The olfactory bulb receives extensive centrifugal inputs conveying information regarding a variety of internal states such as arousal level, hunger, and sexual receptivity. Responses of the bulb to odors can be modulated by these centrifugal inputs (Cattarelli, 1982; Gray, Freeman, & Skinner, 1986; Pager, 1978; Potter & Chorover, 1976). For example, responses of mitral-tufted cells (the primary output neurons of the bulb) to specific odors are dependent on the current level of hunger or behavioral arousal (or both) of the animal (Gervais & Pager, 1979; Scott, 1977).
In addition, noradrenergic (NE) centrifugal input to the olfactory bulb may also modulate neural plasticity in that structure, allowing long-term changes in bulb function after associative conditioning or other memorial events (Cornwell-Jones & Bollers, 1983; Gray et al., 1986; Rosser & Keverne, 1985; Sullivan, McGaugh, & Leon, 1991; Sullivan, Wilson, & Leon, 1989). The role of NE in plasticity is not limited to the olfactory bulb because NE has been implicated in synaptic plasticity in other sensory systems (Kasamatsu & Pettigrew, 1979; Bear & Singer, 1986) as well as hippocampal long-term potentiation (Dahl & Sarvey, 1989; Neuman & Harley, 1983).
NE is present and functional in the neonatal rat olfactory bulb (McLean & Shipley, 1991; Wilson & Leon, 1988a). Indeed, the role of NE in olfactory bulb plasticity appears to manifest itself at least as soon as the first postnatal week. For example, association of an odor and reinforcer during the preweanling period produces a conditioned behavioral response to that odor that is correlated with specific changes in olfactory bulb neural response patterns. These neural changes include enhanced glomerular layer focal 2-deoxyglucose uptake and modified mitral-tufted cell single unit responses to the conditioned odor (Coopersmith & Leon, 1984; Sullivan & Leon, 1986; Wilson, Sullivan, & Leon, 1987). As early as the first postnatal week, blockade of NE activity during pairing of odor and reinforcement prevents both the behavioral and neural changes that normally occur with learning (Sullivan et al., 1989, 1991). Conversely, simultaneous pairing of an odor with an NE β-receptor agonist results in long-term modification of bulb physiology and olfactory behaviors in neonates (Sullivan et al., 1989, 1991). Furthermore, NE agonists combine additively with conventional rewards in early olfactory conditioning (Sullivan et al., 1991).
These results suggest that at least one of the loci of odor-reward convergence may occur in the olfactory bulb, which allows associative changes to occur. In support of this hypothesis, we have recently demonstrated that mitral-tufted cells respond to medial forebrain bundle-lateral hypothalamic (MFB-LH) stimulation in young rats (Wilson & Sullivan, 1990). Furthermore, pairing an odor with MFB-LH stimulation produces both a conditioned odor preference and modified mitral-tufted cell responses to subsequent presentations of that odor (Wilson & Sullivan, 1990). Because NE has been shown to be critically involved in olfactory conditioning, the present study examined the role of NE in associative conditioning using MFB-LH stimulation as reward and the role of NE in mitral-tufted cell responses to that reward. We demonstrated that propranolol, a NE antagonist, blocks early olfactory learning with MFB-LH stimulation as reward and that NE may mediate a specific component of mitral-tufted cell response to reward in young rats.
Method
Subjects
The subjects were 49 male and female postnatal day 11 (PN11) Wistar rat pups born to females obtained from Hilltop Lab Animals (Scottdale, PA). The day of birth was considered PNO, and litters were culled to 8 pups on PN1. No more than 1 male, 1 female, or both were used from a litter for a treatment-test condition. Lights were on at 7:00 a.m. and offat 7:00 p.m. All procedures were in accordance with National Institutes of Health guidelines.
Electrode Implantation
On PN11, pups were anesthetized with a 1:1 mixture of ketamine and xylazine (25 mg/kg ip) and placed in a stereotaxic apparatus. A bipolar, stainless steel, Teflon-coated electrode (twisted 0.13-mm-diameter strands, 0.5 mm tip separation) was implanted in the MFB-LH (0.5 mm posterior to bregma, 1.5 mm lateral, and 7.5 mm ventral to skull surface) and attached to the skull with dental acrylic. The pups were kept warm during recovery from the anesthesia, which generally took 2-4 hr.
Drugs and Conditioning
At least 1 h after return to normal behavioral activity, the pups were injected subcutaneously with either saline or 20 mg/kg DL-propranolol hydrochloride (Sigma Chemical Co., St. Louis, MO). This dose of propranolol has been shown to be maximally effective at disrupting early olfactory learning (Sullivan et al., 1989, 1991). Thirty-five minutes later the pups were placed in glass training chambers (1-L beakers) for a 10-min habituation period, which was followed by olfactory conditioning. Pups were randomly assigned to receive either paired presentations of odor and MFB-LH stimulation or backward presentations of odor and MFB-LH stimulation. (Half the pups in each group were saline treated, and the other half were propranolol treated.) For paired presentations, the odor was delivered for 15 min, with the MFB-LH stimulation delivered every 30 s (total of 30 MFB-LH stimuli concurrent with the odor stimulus). Pups in the backward group received 30 MFB-LH stimuli (1/30 s) and then 15 min of odor (nonconcurrent MFB-LH-odor presentations). The odor was peppermint (Shilling) that was presented through a flow-dilution olfactometer at 2 L/min and at a 1:10 concentration of saturated vapor. The saturated vapor was obtained by circulating air over a pool of peppermint extract in a 25 ml filtering flask. For MFB-LH stimulation, the pups were connected to a constant-current stimulator via lightweight leads. Stimulation consisted of a 200-Hz, 300-ms-duration train of 0.1-ms-duration monophasic pulses at 100-1,300 μA. Stimulus intensity for training was set to reliably evoke behavioral activation. If a pup did not behaviorally respond to the stimulus, then intensity was set at 1,000 μA. Pups were tested for behavioral odor preference or mitral-tufted cell response patterns 18-24 h later.
Odor Preference Test
Testing was performed in the absence of any drugs. The odor preference test was a two-odor choice test consisting of an opaque Plexiglas arena (20 × 24 × 34 cm) with a stainless steel grate floor (7-mm-diameter holes). The floor was divided in half, perpendicular to the long side of the arena, by a neutral zone (2 cm). Under the floor, on each side of the neutral zone, was a tray filled with either clean pine wood chips or pine wood chips mixed with peppermint odor. The pup was placed on the central neutral zone, and the amount of time the pup spent over each odor was recorded. The test lasted 60 s and was repeated three times. Between tests, the floor was washed with water and dried. The direction the pup was placed in the test apparatus was counterbalanced between trials. All observations were made by observers who were unaware of the subjects’ treatment.
Neurophysiology
Details of the recording procedure have been presented previously (Wilson & Sullivan, 1990). All recordings were taken from urethane-anesthetized, normally respiring pups using an electrode positioned ipsilateral to the MFB-LH stimulating electrode. A bipolar simulating electrode was placed in the lateral olfactory tract (LOT), and mitral-tufted cell single unit responses were recorded in the olfactory bulb with a glass microelectrode filled with 2 M NaCl.
Test odors were delivered to the external nares through glass tubing from a flow dilution olfactometer. Pups respired normally from a continuous flow (500 ml/min) of clean air to which a 1:10 dilution of saturated vapor could be added with a Harvard syringe pump controlled by a Zenith 286 computer. Mitral-tufted cell responses to repeated 4-s odor pulses were monitored. Stimuli were repeated three times, and responses were analysed with peristimulus time histograms (250-ms bin widths). At least 60 s elapsed between stimuli. Responses were categorized as either excitatory, suppressive, or no response as compared with a 5-s preodor baseline, as previously described (Wilson & Leon, 1988b; Wilson & Sullivan, 1990). Excitatory responses were characterized by an increase in firing rate during the 4-s stimulus above the variability of the 5-s baseline, whereas suppressive responses were characterized by a decrease in firing rate below baseline variability. The ratio (R) of the percent of excitatory responses to the total response rate (excitatory + suppressive) was determined (R = % excitatory/total % responding), and comparisons of this ratio were made between groups. All cells were recorded ipsilateral to the MFB-LH electrode.
Responses to MFB-LH Stimuli
An additional 10 naive pups (11-12 days old) were used to examine mitral-tufted cell single unit responses to MFB-LH stimulation alone (no odor presented). These animals were prepared as just described for single unit recording with a stimulating electrode in the MFB-LH and one in the LOT. MFB-LH stimulation was the same as that used in the conditioning procedure: 200 Hz, 300 ms, and 500-1,000 μA. Responses to MFB-LH stimulation were analyzed as for odor responses, by comparing poststimulus firing rate to a 5-s prestimulus baseline. Responses were either excitatory, suppressive, or null, as previously described (Wilson & Sullivan, 1990). After 2-5 cells were analyzed in a given animal, the pup was injected subcutaneously with 20 mg/kg DL-propranolol. Recording was resumed 45 min after the injection; the cells were localized, and the responses were characterized for an additional 60 min. Our previous research suggests that the effects of systemic propranolol injections on bulb physiology peaks approximately 60 min after injection and returns to baseline by 120 min at this age (Wilson & Leon, 1988a). Additional pups (n = 2) were injected with saline rather than propranolol. No difference was observed between responses during the preinjection period and postsaline period, thus these saline data are included in the preinjection baseline data for statistical analysis. Comparisons were made between mitral-tufted cell response patterns to MFB-LH stimulation pre- and postpropranolol.
Results
Stimulation of the MFB-LH produced behavioral activation in both saline- and propranolol-treated groups. This activation ranged from mouthing to probing to generalized activity, as previously reported (Moran, Schwartz, & Blass, 1983; Wilson & Sullivan, 1990). Stimulus intensity required to produce this activation varied across animals, from 200 to 1,300 μA. Using an analysis of variance, no significant difference was found in stimulus intensity used between the conditioning and drug conditions, F(l, 34) = 0.6 ns (mean stimulus intensity for all pups = 643 μA).
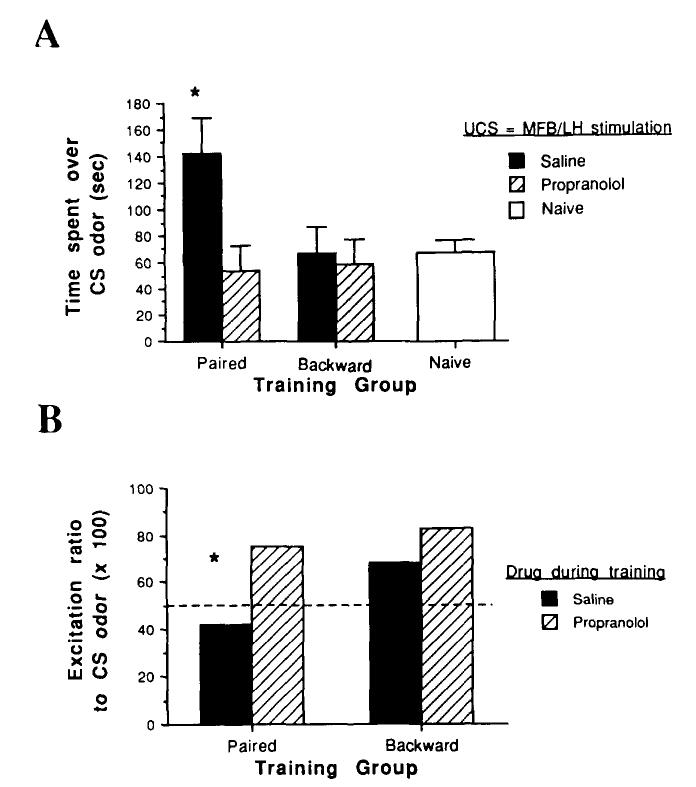
Temporal association of an odor and MFB-LH stimulation produced a relative odor preference in pups trained with saline (Figure 1A). Propranolol during training blocked acquisition of this learned response, Drug × Training interaction, F(2, 27) = 3.9, p < .05; post hoc Tukey tests revealed that the paired-saline group was significantly different from all other groups (p < .05). Pups in both saline-backward and propranolol-backward groups were not significantly different from naive pups.
Figure 1.
Neurobehavioral response to the conditioned stimulus (CS) odor. (No drugs were used during the test. A: Behavioral response in a two-odor choice test between a familiar odor [pine wood chips] and the CS odor. B: Mitral-tufted cell single unit response patterns to the CS odor. Excitation ratios above 50 correspond to more excitatory responses than suppressive responses to the odor, whereas ratios below 50 correspond to more suppressive responses than excitatory. Values are means ±SE. Asterisks in A and B signify significantly different from saline controls and propranolol groups, p < .05. UCS = unconditioned stimulus; MFB/LH = medial forebrain bundle-lateral hypothalamus.)
In addition to the learned behavioral response, mitral-tufted cell single unit responses to the conditioned odor were also modified (Figure 1B). A total of 97 cells from 12 pups were tested (3 pups/group, at least 24 cells/group). The naive group was not included in this analysis to reduce the total number of pups tested. Previous studies (Sullivan & Leon, 1986; Wilson et al., 1987) have shown that single unit responses in naive pups do not differ from other control groups. Mitral-tufted cells in the paired-saline group showed more suppressive and fewer excitatory responses to the conditioned odor (R = 0.42) than pups in the backward-saline group (R = 0.68). Propranolol during training blocked the acquisition of this modified mitral-tufted cell response (χ2 = 3.0, p < .05). There was no significant difference in overall response rates between the groups (paired-saline = 42%; paired-propranolol = 42%; backward-saline = 46%; backward-propranolol = 48%).
These results suggest that NE is required during training for acquisition of both learned behavioral responses and their neural correlates in young rats. To further examine the role that NE plays in odor-reward association in young rats, 10 additional naive PN11-12 rats were used to monitor mitral-tufted cell responses to MFB-LH stimulation before and after propranolol injections.
A total of 72 cells were tested, 45 before propranolol injection and 27 between 45 min and 105 min after injection (20 mg/kg sc). As previously reported (Wilson & Sullivan, 1990), cells recorded during the baseline period responded to MFB-LH stimulation by either increasing their firing rate (Type E; Figure 2), decreasing their firing rate (Type I), or did not demonstrate a significant response (null response). These different response types could be obtained with a single stimulation site and at a variety of stimuli intensities and did not appear to be dependent on recording location within the mitral cell body layer (Wilson & Sullivan, 1990).
Figure 2.
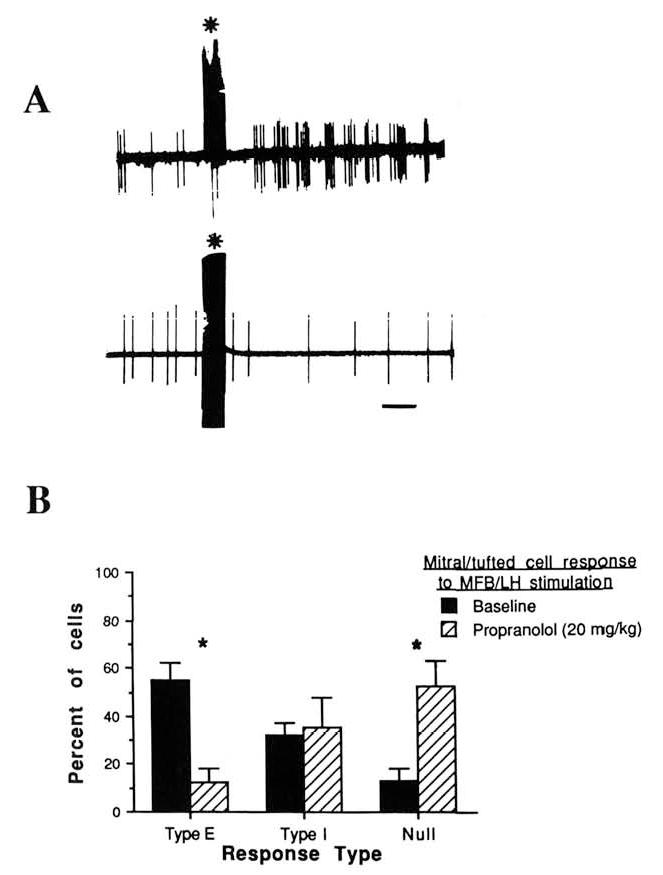
Mitral-tufted cell single unit responses to medial forebrain bundle-lateral hypothalamus (MFB/LH) stimulation. (A: Examples of Type E [top] and Type I [bottom] responses in control pups. Stimulus artifact produced by the MFB/LH stimulus is marked by an asterisk. Calibration bar is 0.5 s. B: Proportion of Type E, Type I, and null responses during baseline and after propranolol injection. Asterisks signify significantly different from saline, p < .05.)
Type E cells accounted for 54.6% of the cells sampled during the baseline period, and Type I cells accounted for 31.9% (Figure 2). Propranolol, however, greatly reduced Type E responses while leaving Type I responses unaffected, Drug × Response Type interaction, F(2, 45) = 13.6, p < .001; post hoc Tukey tests revealed a significant difference between saline and propranolol groups in Type E and null-response categories (p < .05). Altering MFB-LH stimulus intensity did not reinstate Type E responses. It should be noted that the stimulus intensities used here were sufficient to evoke behavioral activation in both saline- and propranolol-injected awake pups, as already described.
In addition to response patterns to MFB-LH stimulation, mitral-tufted cell spontaneous activity rates were significantly depressed by propranolol. Baseline firing rate before propranolol was 4.6 Hz (±0.6) and was reduced to 2.3 Hz (±0.5) after injection, t(49) = 2.7, p < .02. Antidromic response latencies to LOT stimulation were not significantly affected by propranolol (baseline = 8.1 ± 0.7 ms; postinjection = 7.6 ± 0.5 ms). The relatively low spontaneous activity rate and long antidromic latencies in the predrug conditions are characteristic of the bulb at this age (Wilson & Leon, 1986).
Discussion
The present results suggest that NE may be required for early olfactory learning with MFB-LH stimulation as reward. Blockade of NE β-receptors with propranolol during training prevents acquisition of behavioral and neural conditioned responses to the odor. These results extend our previous findings on the role of NE in early olfactory learning with tactile stimulation as reward (Sullivan et al., 1989, 1991) and correspond to the findings of others on NE and olfactory learning in mature animals (Gray et al., 1986; Rosser & Keverne, 1985).
Of importance, the present results suggest that the propranolol-induced deficit in early learning is correlated with a specific change in the olfactory bulb response to reward. Type E mitral-tufted cell responses to MFB-LH stimulation are greatly reduced by a dose of NE β-receptor antagonist that prevents learning. The occurrence of Type I responses is unaffected. A causal relationship between Type E responses and olfactory bulb modification cannot be determined from these data. However, the possibility remains that association of olfactory receptor neuron activation of mitral-tufted cells concurrent with NE-mediated mitral-tufted cell Type E responses to reward may be critical for synaptic modifications correlated with early learning. NE has also been implicated as an important mediator of reward information in the pigeon lateral geniculate nucleus during visual conditioning (Cohen, Gibbs, Siegelman, Gamlin, & Broyles, 1982).
It has previously been shown the olfactory bulb NE depletion does not affect olfactory thresholds (Doty, Ferguson-Segall, Lucki, & Kreider, 1988). Furthermore, NE antagonists have limited effects on the magnitude of MFB-LH stimulation-induced reward (Stellar & Rice, 1989). In fact, in the present study, no difference in stimulus thresholds for MFB-LH-induced behavioral activation was observed between saline and propranolol pups. Thus, in the paired condition, both saline and propranolol pups were similarly aroused. Furthermore, we believe these results do not represent a “state-dependent” or context effect, but rather the failure of propranolol pups to express an odor preference when tested without propranolol is due to a disruption of acquisition. We have recently demonstrated that pups acquire and express normal olfactory memory after being trained in the absence of propranolol but tested in the presence of propranolol (Sullivan & Wilson, in press). Thus, despite being in different propranolol-induced states, pups can express an acquired odor preference. Together, these findings suggest that propranolol directly or indirectly affects neural plasticity underlying early learning rather than reducing responsiveness to olfactory stimuli or modifying the hedonic value of the MFB-LH stimulation. The role of other neurotransmitters-neuromodulators in early learning is currently being investigated.
Mitral-Tufted Cell Responses to Reward
The majority of centrifugal inputs to the olfactory bulb terminate on inhibitory interneurons, granule cells. Granule cells, in turn, form reciprocal dendrodendritic synapses with mitral-tufted cells. NE modulates granule cell-mediated inhibition in both the mature and immature bulb, apparently by inhibiting or suppressing granule cells (Jahr & Nicoll, 1982; Wilson & Leon, 1988a). Therefore, activation of the NE input to the bulb would suppress granule cell activity, thereby releasing mitral-tufted cells from inhibition. The Type E responses to MFB-LH rewarding stimulation, thus, may represent a disinhibition of mitral-tufted cells by direct or indirect activation of NE inputs to the bulb (Figure 3). Type I responses, on the other hand, may represent excitatory input to granule cells, perhaps via acetylcholine, serotonin, or other centrifugal inputs (Macrides & Davis, 1983).
Figure 3.
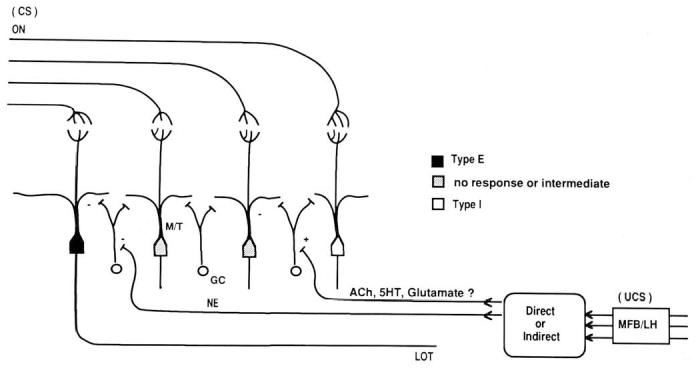
Hypothesized circuitry underlying modification of bulb responses to learned odors in neonates using olfactory associative conditioning with brain stimulation. (It should be emphasized that expression of the learned behavioral response may involve plasticity in additional brain structures. CS = conditioned stimulus; ON = olfactory nerve; M/T = mitral-tufted cell; GC = granule cell; LOT = lateral olfactory tract; UCS = unconditioned stimulus; NE = norepinephrine; ACh = acetylcholine; 5HT = serotonin; MFB/LH = medial forebrain bundle-lateral hypothalamus.)
If this is correct, then blockade of NE β-receptors should release granule cells from inhibition, thereby suppressing mitral-tufted cell activity. This is evident from the decrease in mitral-tufted cell spontaneous activity shown here and from an increase in LOT-induced paired-pulse inhibition (Wilson & Leon, 1988a). Furthermore, as shown in the present study, NE antagonists should prevent the disinhibitory response of mitral-tufted cells to MFB-LH stimulation (termed here Type E responses).
NE and Early Learning
The information required for expressing a learned odor preference is almost certainly stored in a variety of brain circuits in the newborn (Kucharski & Hall, 1987; Sullivan & Wilson, 1991). Our investigation has focused on elucidating the mechanisms underlying changes in one of those circuits— the olfactory bulb. We have demonstrated that early olfactory learning, using a variety of reinforcers, modifies an odor-specific group of mitral-tufted cells in an unique way. That is, after associative conditioning, mitral-tufted cells show an increase in suppressive responses and a decrease in excitatory responses to the conditioned odor (Wilson & Leon, 1988b; Wilson et al., 1987). The change is odor specific, lasts at least 24-48 hr and is NE-dependent for its acquisition (Sullivan et al., 1989, 1991) but not expression (Sullivan & Wilson, in press).
Mitral-tufted cells receive direct excitatory input from olfactory receptor axons (Wellis, Scott, & Harrison, 1989; Hamilton & Kauer, 1988) and respond to reward stimuli (Wilson & Sullivan, 1990; present study), presumably via centrifugal inputs to the bulb. Given these response characteristics, it is hypothesized that excitatory olfactory nerve input concurrent with NE-mediated disinhibition produced by reward allows long-term changes in synaptic strength that are subsequently expressed as an increase in inhibitory responses to olfactory nerve input alone. NE receptor antagonists during training, however, should enhance inhibition and decrease input from MFB-LH afferents, which may prevent associative changes from occurring in the olfactory bulb.
Interestingly, the requirement for multiple coactive inputs and the NE dependence of olfactory learning are reminiscent of hippocampal long-term potentiation (LTP), as has been previously noted (Brennan, Kaba, & Keverne, 1990; Wilson & Sullivan, 1990). Although LTP does not develop in the hippocampus or neocortex until several days after PN11-12 in the rat (Wilson, 1984), we are currently examining LTP and LTP-like events in the neonatal olfactory bulb.
Footnotes
This research was supported by National Science Foundation Grant BNS8819189 to Donald A. Wilson and by National Institute of Development and Childhood Disease Grant DC00480 to Regina M. Sullivan.
References
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Brennan P, Kaba H, Keverne EB. Olfactory recognition memory: A simple memory system. Science. 1990;250:1223–1226. doi: 10.1126/science.2147078. [DOI] [PubMed] [Google Scholar]
- Cattarelli M. The role of the medial olfactory pathways in olfaction: Behavioral and electrophysiological data. Behavioural Brain Research. 1982;6:339–364. doi: 10.1016/0166-4328(82)90017-1. [DOI] [PubMed] [Google Scholar]
- Cohen DH, Gibbs CM, Siegelman J, Gamlin P, Broyles J. Is locus coeruleus involved in plasticity of lateral geniculate neurons during learning. Society for Neuroscience Abstracts. 1982;8:666. [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Cornwell-Jones CA, Boilers HR. Neonatal 6-hydroxydopamine alters conspecific odor investigation by male rats. Brain Research. 1983;268:291–294. doi: 10.1016/0006-8993(83)90495-x. [DOI] [PubMed] [Google Scholar]
- Dahl D, Sarvey JM. Norepinephrine induces pathwayspecific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proceedings of the National Academy of Science, USA. 1989;86:4776–4780. doi: 10.1073/pnas.86.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Ferguson-Segall M, Lucki I, Kreider M. Effects of intrabulbar injections of 6-hydroxydopamine on ethyl acetate odor detection in castrate and no-castrate male rats. Brain Research. 1988;444:95–103. doi: 10.1016/0006-8993(88)90917-1. [DOI] [PubMed] [Google Scholar]
- Gervais R, Pager J. Combined modulating effects of the general arousal and the specific hunger arousal on the olfactory bulb responses in the rat. Electroencepholography and Clinical Neurophysiology. 1979;46:87–94. doi: 10.1016/0013-4694(79)90053-1. [DOI] [PubMed] [Google Scholar]
- Gray CM, Freeman WJ, Skinner JE. Chemical dependencies of learning in the rabbit olfactory bulb: Acquisition of the transient spatial pattern change depends on norepinephrine. Behavioral Neuroscience. 1986;100:585–596. doi: 10.1037//0735-7044.100.4.585. [DOI] [PubMed] [Google Scholar]
- Hamilton KA, Kauer JS. Responses of mitral/tufted cells to orthodromic and antidromic electrical stimulation in the olfactory bulb of the tiger salamander. Journal of Neurophysiology. 1989;59:1736–1755. doi: 10.1152/jn.1988.59.6.1736. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Noradrenergic modulation of dendro-dendritic inhibition in the olfactory bulb. Nature. 1982;297:227–229. doi: 10.1038/297227a0. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Pettigrew JD. Preservation of binocularity after monocular deprivation in the striate cortex of kittens treated with 6-hydroxydopamine. Journal of Comparative Neurology. 1979;185:139–162. doi: 10.1002/cne.901850109. [DOI] [PubMed] [Google Scholar]
- Kucharski D, Hall WG. New routes to old memories. Science. 1987;238:786–788. doi: 10.1126/science.3672125. [DOI] [PubMed] [Google Scholar]
- Macrides F, Davis BJ. The olfactory bulb. In: Emson PC, editor. Chemical neuroanatomy. Raven Press; New York: 1973. pp. 391–426. [Google Scholar]
- McLean JH, Shipley MT. Postnatal development of the noradrenergic projection from locus coeruleus to the olfactory bulb in the rat. Journal of Comparative Neurology. 1991;304:467–477. doi: 10.1002/cne.903040310. [DOI] [PubMed] [Google Scholar]
- Moran TH, Schwartz GJ, Blass EM. Organized behavioral responses to lateral hypothalamic electrical stimulation in infant rats. Journal of Neuroscience. 1983;3:10–19. doi: 10.1523/JNEUROSCI.03-01-00010.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman RS, Harley CW. Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Research. 1983;273:162–165. doi: 10.1016/0006-8993(83)91106-x. [DOI] [PubMed] [Google Scholar]
- Pager J. Ascending olfactory information and centrifugal influxes contributing to a nutritional modulation of the rat mitral cell responses. Brain Research. 1978;140:251–269. doi: 10.1016/0006-8993(78)90459-6. [DOI] [PubMed] [Google Scholar]
- Potter H, Chorover SL. Response plasticity in hamster olfactory bulb: Peripheral and central processes. Brain Research. 1976;116:417–429. doi: 10.1016/0006-8993(76)90490-x. [DOI] [PubMed] [Google Scholar]
- Rosser AE, Keverne EB. The importance of central noradrenergic neurons in the formation of an olfactory memory in the prevention of pregnancy block. Neuroscience. 1985;15:1141–1147. doi: 10.1016/0306-4522(85)90258-1. [DOI] [PubMed] [Google Scholar]
- Scott JW. A measure of extracellular unit responses to repeated stimulation applied to observations of the time course of olfactory responses. Brain Research. 1977;132:247–258. doi: 10.1016/0006-8993(77)90419-x. [DOI] [PubMed] [Google Scholar]
- Stellar JR, Rice MB. Pharmacological basis of intracranial self-stimulation reward. In: Liebman JM, Cooper SJ, editors. The neuropharmacologicalbasis of reward. Oxford University Press; New York: 1989. pp. 14–65. [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Developmental Brain Research. 1986;27:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, McGaugh J, Leon M. Norepinephrine induced plasticity and one-trial olfactory learning in neonatal rats. Developmental Brain Research. 1991;60:219–228. doi: 10.1016/0165-3806(91)90050-s. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Neural correlates of conditioned odor avoidance in infant rats. Behavioral Neuroscience. 1991;105:85–90. doi: 10.1037//0735-7044.105.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The role of norepinephrine in the expression of learned olfactory neurobehavioral responses in infant rats. Psychobiology. doi: 10.3758/bf03332084. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. Journal of Neuroscience. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellis DP, Scott JW, Harrison TA. Discrimination among odorants by single neurons of the rat olfactory bulb. Journal of Neurophysiology. 1989;61:1161–1177. doi: 10.1152/jn.1989.61.6.1161. [DOI] [PubMed] [Google Scholar]
- Wilson DA. A comparison of the postnatal development of post-activation potentiation in the neocortex and dentate gyrus of the rat. Developmental Brain Research. 1984;16:61–68. doi: 10.1016/0165-3806(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Early appearance of inhibition in the neonatal rat olfactory bulb. Developmental Brain Research. 1986;26:289–292. doi: 10.1016/0165-3806(86)90295-6. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Developmental Brain Research. 1988;42(a):69–75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. Journal of Neurophysiology. 1988;59(b):1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Olfactory associative conditioning in infant rats with brain stimulation as reward: I. Neurobehavioral consequences. Developmental Brain Research. 1990;53:215–221. doi: 10.1016/0165-3806(90)90009-n. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output response patterns to learned attractive odors. Journal of Neuroscience. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]