Abstract
Prolactin (PRL) is recognized as a metabolic regulator during lactation, but little information exists on its actions in male adipose tissue. We examined whether PRL affects the expression of its receptors (PRLR), lipolysis and adipokine secretion in male rats. Both long and short PRLR isoforms were induced 40 to 50-fold during differentiation of epididymal preadipocytes, with a 10 fold higher expression of the long isoform. PRL upregulated both isoforms before and after differentiation. PRL suppressed lipolysis in epididymal explants and mature adipocytes in a dose- and time-dependent manner, which was reversed by a Jak2 inhibitor. PRL also inhibited leptin, but not adiponectin, release. We conclude that PRL inhibits lipolysis and leptin release by acting directly on adipocytes via interaction with either of its receptors and activation of a Jak2-dependent signaling pathway(s). This is the first demonstration of substantial effects of PRL on male adipocytes.
Keywords: prolactin, metabolism, rat, adipose tissue, adipocytes, lipolysis, leptin, prolactin receptors
Introduction
Prolactin (PRL) is an essential hormone for mammary gland development and milk production. During lactation, PRL dramatically alters lipid metabolism by stimulating lipogenesis in the mammary gland while reducing lipid storage in peripheral adipose tissue [1]. Whereas growth hormone (GH) is known as a metabolic regulator, the potential actions of prolactin (PRL), its sister molecule, on adipose tissue under non-lactation conditions have received little attention. Although males are continuously exposed to circulating PRL, there is a misconception that their tissues are unresponsive to its actions.
Lipid accumulation in adipose tissue reflects a dynamic balance between formation of triglycerides (TG) from free fatty acids (FFA) and their breakdown by lipolysis. Insulin and catecholamines are the primary regulators of lipolysis via their opposite effects on the cAMP pathway. In addition, lipolysis is modulated by other circulating hormones and cytokines such as GH, TNFα and IL-6. Adipose tissue also functions as an endocrine organ through the release of adipokines which affect muscle and liver metabolism as well as brain control over food intake. Previous reports on the actions of PRL on lipid metabolism [2,3], leptin [4,5] or adiponectin [6] release were conducted with murine cell lines or adipose tissues from females. Dysregulation of either lipolysis or adipokine release contributes to the development of insulin resistance and vascular dysfunctions [7].
PRL acts by binding to its receptor (PRLR), a member of the PRL-GH-cytokine receptor superfamily, and activating Jak/Stat signaling pathway [8]. In rats, PRLR exists as two isoforms, long (PRLR-L) and short (PRLR-S), whose expression varies in a tissue-specific manner [9]. Whereas PRL upregulates the expression of its receptors in several cell types [10,11], there is no information on the expression of PRLR isoforms in rat adipose tissue or their regulation by PRL.
Given the paucity of information on the metabolic actions of PRL in males, our objectives were to: a) examine the expression and regulation of PRLR isoforms in male rat adipose tissue, b) compare the effects of PRL on lipolysis in adipose explants and mature adipocytes from male and female rats, and c) determine whether PRL affects the release of leptin and adiponectin. Our data indicate a direct action of PRL on male adipocytes by up regulating its receptor, inhibiting isoproterenol-stimulated lipolysis and suppressing leptin release.
Materials and Methods
Animals
Male and female Sprague Dawley rats (Harlan, Indianapolis, Indiana) were housed under a 12-h light cycle with food and water available ad libitum. Animals were fasted overnight before sacrifice by CO2 inhalation between 9-11 am. Animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Tissue and cell culture
Epididymal or periovarian adipose tissue was removed and minced. Explants (4-5 pieces/well, totaling about 50 mg) were incubated in M199 (Cellgro, Herndon VA) containing, 1% charcoal stripped serum (CSS; Hyclone, Logan UT) and recombinant ovine PRL (Dr. Arieh Gertler, Hebrew University, Israel). Explants were either used for determination of lipolysis or for harvesting preadipocytes and mature adipocytes.
Mature adipocytes and preadipocytes were isolated from epididymal adipose tissue using collagenase [12]. Mature adipocytes were collected by centrifugation and equal amounts of packed cells were embedded in Matrigel (BD Sciences, Franklin Lakes, NJ). The embedded cells were maintained in DMEM:F12 (Cellgro, Herdon, MS) with 1 nM insulin and 2% BSA.
Preadipocytes were seeded at 3 × 104 cells/cm2 and incubated in DMEM:F12 containing 3% CSS. Preadipocytes were induced to differentiate by adding 100 nM insulin, 10 μg/ml transferrin, 1 μM dexamethasone, 5 μM rosiglitazone, and 1nM T3. Either proliferating or differentiated adipocytes were serum-starved overnight before treatment with PRL. Staining with Oil Red O was used to confirm lipid accumulation [12]. On day 8, 70-80% of the cells contained lipid droplets.
Lipolysis
Explants or mature adipocytes were incubated with PRL for 2, 6 or 24 hrs. For basal lipolysis, samples were incubated for an additional 2 hrs in KRH (Krebs-Ringers-Hepes, 1.8 mM CaCl, 1 mM sodium pyruvate, 1% CSS) containing the same PRL concentrations. Conditioned media (CM) were collected and replaced with the same medium containing 100 nM isoproterenol, a β-adrenergic agonist, for 2 hrs. Glycerol release was measured by a colorimetric assay (Sigma). Glycerol concentration was normalized by tissue weight when appropriate and expressed as nmol glycerol/mg tissue/2 hrs. Isoproterenol-stimulated glycerol release was 3-4 fold above basal release in all experiments. In some experiments, explants were incubated with the Jak2 inhibitor, AG490 (LC Laboratories, Woburn, MA), one hr prior to and continuing throughout treatment with PRL.
Real-Time PCR
Total RNA was reverse transcribed as described [12]. Real-time PCR was performed on 200 ng of cDNA, using intron-spanning primers for the rat PRLR isoforms as follows: a) PRLR-L (forward, 5'-CTG GGC AGT GGC TTT GAA G-3'; reverse, 5'-CCA AGG CAC TCA GCA GCT CT-3'), b) PRLR-S (forward, 5'-CTG GGC AGT GGC TTT GAA G-3'; reverse, 5'-AAG GGC CAG GTA CAG ATC CA-3'), and c) β-actin as a control (forward, 5'-TGT CAC CAA CTG GGA CGA TAT GGA-3'; reverse, 5'-ATA CAG GGA CAA CAC AGC CTG GAT-3'). Products were amplified on a SmartCycler I (Cepheid, Sunnyvale, CA) using Immolase heat-activated Taq DNA polymerase (Bioline, Randolph MA) and SYBR Green I (Invitrogen). Cycle parameters were: 96C for 6 min followed by 40 cycles at 95C for 15 sec, 57C for 15 sec, 72C for 25 sec. Product purity was confirmed by melting curve analysis. Changes in gene expression were calculated from the cycle threshold (Ct), after correcting for β-actin, according to Pfaffl et al [13]. Data are expressed as fold induction over control as defined for each experiment.
Leptin and adiponectin determination
Leptin concentrations in CM were measured by an ELISA kit from R&D Systems (Minneapolis, MN). The lowest level of detection was 62.5 pg/ml. Adiponectin concentrations were measured using an ELISA kit from Biosource International (Camarillo, CA.). The lowest level of detection was 50 pg/ml.
Data analysis
Statistical analysis was done using one-way ANOVA followed by Fisher least significant difference post hoc analysis. P values <0.05 were considered significant. Experiments were repeated three times.
Results
Dramatic increase in PRLR expression following adipogenesis and further induction by PRL
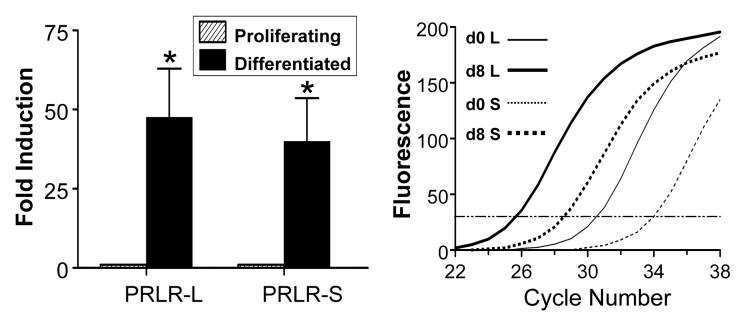
As determined by quantitative real-time PCR, expression of both PRLR isoforms was low but detectable in proliferating preadipocytes. During differentiation, the long and short isoforms were induced 48- and 40-fold, respectively (Fig. 1). The PRLR-L was ≈10-fold more abundant, as evident by the detection of its transcript 4 cycles earlier than that of the PRLR-S isoform.
Fig 1.
Induction of PRL receptor (PRLR) isoforms during adipogenesis in rat epididymal adipocytes. Both long (PRLR-L) and short (PRLR-S) isoforms are markedly induced during differentiation (left panel), as determined by real-time PCR. Data are expressed as fold induction relative to day 0 after corrections for β actin expression. The higher abundance of the long isoform (L) before (d0) and after (d8) differentiation is shown in the right panel. Each value is a mean±SEM of four separate experiments. * p<0.05 vs. proliferating cells.
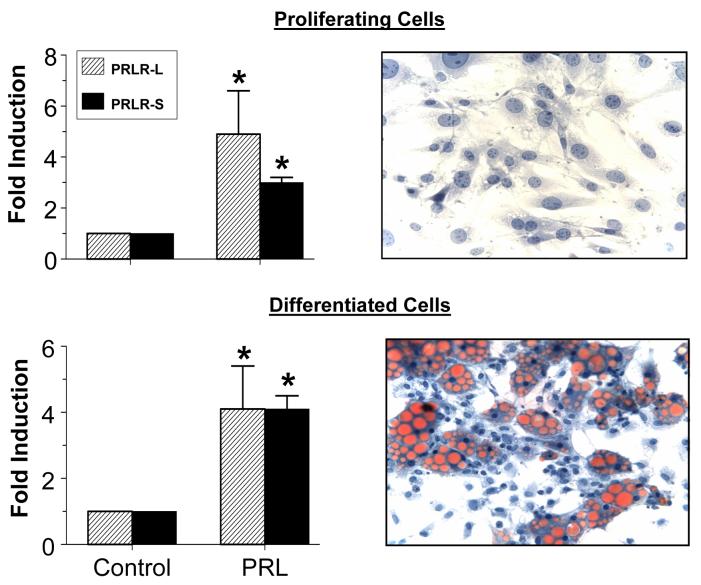
We next examined whether PRL alters the expression of its receptors before or after differentiation. Incubation of proliferating preadipocytes with PRL for 24 hrs caused 5 and 3.5 fold increases in expression of the PRLR-L and PRLR-S, respectively (Fig 2). In spite of the dramatic increase in their expression during differentiation, PRL further upregulated both isoforms 4 fold. Staining with Oil red O confirmed lipid accumulation following differentiation.
Fig 2.
PRL unpregulates the expression of both PRLR isoforms in proliferating (upper panel) and differentiated (lower panel) epididymal adipocytes. Lower panel: PRL causes a similar induction of the two isoforms in differentiated cells. Cells were incubated with 50 ng/ml PRL or vehicle (control) for 24 hrs. Staining with Oil-red O shows lipid accumulation after 8 days of differentiation. See Fig 1 for other details. Each value is a mean±SEM of four separate experiments. * p<0.05 vs. controls (0 PRL).
Inhibition of lipolysis by PRL
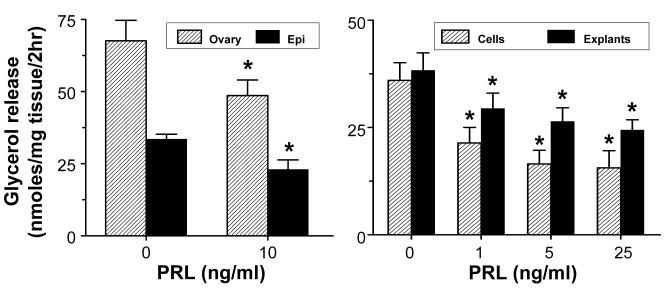
To compare the effects of PRL on lipolysis in each gender, we used a low dose of 10 ng/ml to match its circulating levels in male rats. Although isoproterenol-stimulated glycerol release was 2-fold higher in females than males, incubation with a low dose of PRL for 24 hrs similarly inhibited lipolysis in both sexes (Fig 3). All subsequent studies were conducted with epididymal adipose tissue.
Fig 3.
PRL suppresses lipolysis independent of gender and exerts a direct action on the adipocytes. Left panel: Epididymal (Epi) or periovarian (Ovary) adipose explants were incubated with 10 ng/ml of PRL for 24 hrs. Right panel: Dose-dependent inhibition of lipolysis by PRL in epididymal adipose explants (Explants) and mature adipocytes (Cells). Samples were incubated with PRL for 24 hrs. Lipolysis was determined by measuring isoproterenol-stimulated glycerol release. Data are expressed as nmol glycerol/mg tissue/2 hrs. Each value is a mean±SEM of 8 replicates. * p<0.05 vs. controls. Each experiment was repeated 2-3 times with similar results.
PRL suppressed lipolysis in both epididymal explants and mature adipocytes over a narrow range of physiological concentrations (Fig 3), suggesting that it exerts a direct action on adipocytes rather than through stromal-vascular elements. Notably, inclusion of a high dose of PRL (125 ng/ml), resulted in a ‘U shaped’ curve, indicating loss of responsiveness at high doses (data not shown).
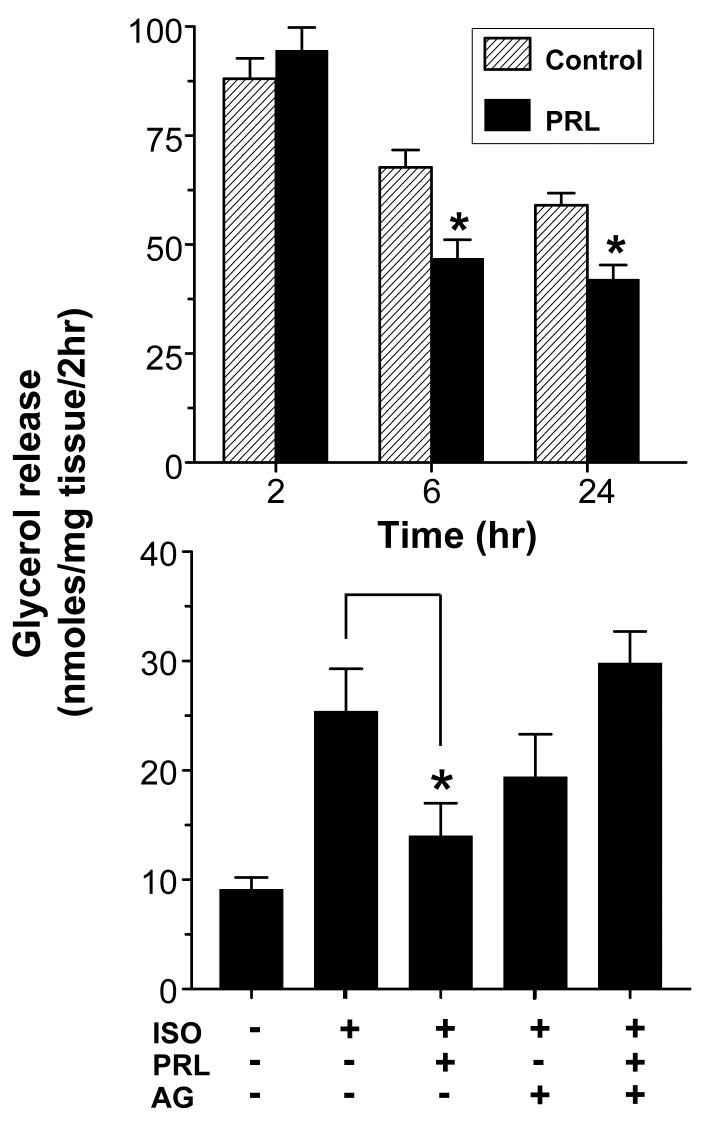
As shown in Fig 4, the anti-lipolytic action of 10 ng/ml PRL was evident after 6 and 24 hrs but not after 2 hrs, indicating lack of an acute effect. The suppression of lipolysis by PRL does not appear to involve transcriptional regulation of hormone sensitive lipase (HSL) or perilipin, since their expression, as determined by RT-PCR, was unchanged following a 24 hr treatment with PRL (data not shown).
Fig 4.
PRL suppresses lipolysis via a chronic mechanism involving activation of a Jak-dependent signaling pathway(s). Upper panel: Time-dependent suppression of lipolysis in epididymal adipose explants by PRL. Explants were incubated with 10 ng/ml PRL or vehicle (Control) for 2, 6 and 24 hrs. Lower panel: Reversal of the anti-lipolytic effect of PRL by a Janus kinase inhibitor. Epididymal adipose explants were pre-incubated with 25 μM AG490 (AG) 1 hr before treatment with 10 ng/ml of PRL for 24 hrs. Lipolysis was determined by measuring basal and isoproterenol-stimulated (ISO) glycerol release. Each value is a mean±SEM of 8 replicates. * p< 0.05 vs. controls. Each experiment was repeated 2-3 times with similar results.
To confirm involvement of a Jak2-dependent pathway, epididymal explants were pre-incubated with the Jak2 inhibitor AG-490 before treatment with 10 ng/ml PRL. Isoproterenol increased glycerol release 3-fold over basal levels. PRL alone caused a 40% inhibition of lipolysis while AG490 alone had no significant effects (Fig 4). Co-incubation of PRL with AG490 abrogated the inhibitory effect of PRL.
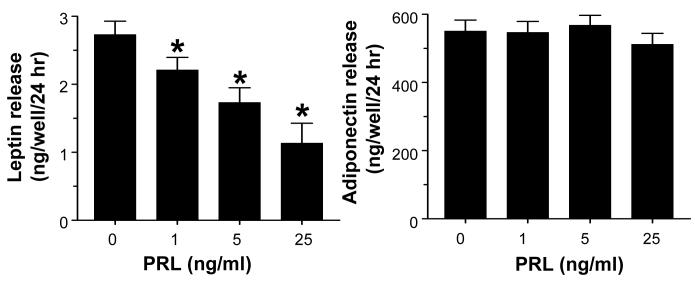
Suppression of leptin, but not adiponectin, release by PRL
Fig 5 shows a significant, dose-dependent inhibition of leptin release by PRL but no effect on adiponectin. This was supported by real-time PCR data showing suppression of leptin, but not adiponectin expression, by PRL (data not shown).
Fig 5.
PRL inhibits leptin (left panel), but not adiponectin (right panel) release from mature epididymal adipocytes. Mature fat cells embedded in Matrigel were incubated with PRL for 24 hrs. Leptin and adiponectin concentrations in conditioned media were determined by respective ELISA kits. Each value is a mean±SEM of 8 replicates. * p < 0.05 vs. controls. Each experiment was repeated twice with similar results.
Discussion
We are reporting that PRL up regulates its receptors, inhibits lipolysis and suppresses leptin release in epididymal adipose explants and adipocytes from male rats. These actions likely involve interactions of PRL with either the long or short PRLR isoforms and activation of a Jak2-dependent signaling pathway(s). These data indicate that PRL affects adipocyte functions in males and its impact on metabolic homeostasis is broader than previously appreciated.
The PRLR isoforms are expressed at various ratios in rat tissues, with the long isoform predominant in the mammary gland and hypothalamus, while the short isoform is more abundant in the liver and luteinized ovary [9]. However, there is no information on isoform-specific expression of PRLR or their regulation in adipose tissue. In this study, both receptor isoforms were dramatically induced in epididymal preadipocytes during adipogenesis. A marked increase in total PRLR expression during adipocyte differentiation was also observed by others [14,15,16]. Regardless of the expression level of the PRLR, PRL further increased the expression of both isoforms (Fig 2). We suspect that upregulation of the PRLR in adipose tissue by PRL involves CCAAT enhancer-binding proteins (C/EBPs), since PRL increases the expression of C/EBP-β in NIH-3T3 cells [17], and the PRLR promoter contains a consensus C/EBP-β binding element [18]. A functional association between PRL, C/EBP-β and PRLR expression in adipose tissue should be further explored.
The long and short PRLR isoforms have an identical extracellular ligand binding domain but varying lengths of the intracellular domain [19]. The intracellular domain of the long isoform contains all the putative elements for activation of Jak/Stat signaling, whereas the truncated cytoplasmic domain of the short isoform lacks many of these [8]. It has been argued that the long isoform mediates most of PRL actions while the short isoform acts as a dominant negative [20]. However, the short form mediates PRL actions in the rat corpus luteum [10], and its overexpression compensates for a partial loss of the long form in PRLR+/− knockout mice [21]. This, together with the activation of signaling pathways other than Jak/Stat by PRL [8], suggest that the short isoform has distinct functions.
We observed consistent anti-lipolytic effects of PRL at low physiological doses while previous reports have been conflicting. For instance, placental lactogens, which bind to the PRLR, stimulated lipolysis in human adipose tissue [22], whereas PRL itself had no effect on lipolysis in rabbits [23]. Pharmacological doses of PRL caused stimulation of lipolysis in mouse adipose tissue [24]. Such discrepancies may be due to different incubation conditions or species variability. Indeed, we reported that PRL at low concentrations had anti-lipolytic actions on both human and rat adipose explants, but did not affect lipolysis in mouse adipose explants [25].
Given the lack of information on the actions of PRL on lipolysis in males, it was important to characterize the physiology of this response. First, the comparable anti-lipolytic actions of PRL in males and females established that it inhibits lipolysis in both sexes. Second, a direct effect of PRL on adipocytes was confirmed by its similar actions on adipose explants and isolated mature adipocytes. Third, doses of PRL well within its circulating levels in male rats are anti-lipolytic. Notably, at either 10 or 25 ng/ml, PRL inhibited lipolysis by as much as 30-40%. A suppression of this magnitude could have a significant impact on energy homeostasis in vivo.
We raise a note of caution on the use of superphysiological doses of PRL and/or media additives that contain PRL. When PRL concentrations exceed 100 ng/ml, we have observed a loss of dose-response relationships in several PRL target tissues, including breast cancer cells and adipocytes. This can lead to erroneous interpretation if only high doses of PRL are used. Very high doses of PRL can cause receptor downregulation, hinder receptor dimerization, activate the dominant negative short receptor, or induce suppressors of cytokine signaling proteins [26]. The effects of exogenous PRL can also be masked by incubation with fetal bovine serum which contains high levels of lactogenic hormones [27].
Lipolytic agents such as catecholamines rapidly increase cAMP levels and stimulate TG hydrolysis by phosphorylating HSL and perilipin, whereas insulin antagonizes lipolysis by acutely decreasing cAMP levels [28]. Since the effects of PRL were not seen within 2 hrs of incubation, its ability to suppress lipolysis likely involves transcriptional regulation of key proteins rather than their phosphorylation/dephosphorylation. Although PRL did not alter HSL and perilipin expression, it may affect other targets such as adipose triglyceride lipase [29] or β-adrenergic receptors. Reversal of the anti-lipolytic action of PRL by the Jak2 inhibitor indicates involvement of a Jak-mediated pathway(s). Potential intracellular mediators include Stat proteins, MAP kinase and PI3 kinase, all of which are affected by PRL. Still, identification of signaling pathway(s) involved in inhibition is more challenging than those associated with stimulation.
PRL suppressed leptin release from mature rat adipocytes but did not affect adiponectin. Previous studies in mice and humans generated disparate data. For example, serum leptin levels are lower [5] or unchanged [30] in PRLR-deficient mice, and are elevated in PRL-overexpressing mice [4]. An inhibitory effect of PRL, however, is suggested by the higher serum leptin in male PRL-knockout mice [25], and the inhibition of insulin-stimulated leptin release in mouse adipocytes [4]. Data on PRL and adiponectin are also inconsistent. Whereas serum adiponectin levels are unchanged in PRLR- [6] or PRL-knockout mice [25], PRL at very high doses inhibits adiponectin production in cultured human adipose tissue and in mice in vivo [6,31]. Lower circulating adiponectin levels were reported in lactating women, with inhibition of adiponectin production in cultured human adipocytes by PRL [32]. These discrepancies are likely due to differences in experimental design, doses of PRL, gender or species.
Our results, focusing on an overlooked aspect of PRL biology, should inspire renewed interests in the metabolic functions of PRL. The rat is an excellent experimental model, given the extensive data base on the regulation of PRL secretion and its functions in this species. In addition, adipose explants represent a suitable in vitro model since tissue cytoarchitecture is maintained and experiments can be conducted soon after tissue removal with minimal processing. Future studies should explore potential interactions between PRL, insulin and catecholamines as well as the role of the different PRLR isoforms and their signaling in regulating both lipolysis and adipokine release.
Acknowledgments
Supported by NIH grants ES012212, CA096613, DOD BC05725 and Susan G. Komen Foundation Grant BCRT87406 (to NBJ), and NIH training grant T32 HD07463 (to TDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flint DJ, Binart N, Kopchick J, Kelly P. Effects of growth hormone and prolactin on adipose tissue development and function. Pituitary. 2003;6:97–102. doi: 10.1023/b:pitu.0000004800.57449.67. [DOI] [PubMed] [Google Scholar]
- 2.Spooner PM, Chernick SS, Garrison MM, Scow RO. Development of lipoprotein lipase activity and accumulation of triacylglycerol in differentiating 3T3-L1 adipocytes. Effects of prostaglandin F2alpha, 1-methyl-3-isobutylxanthine, prolactin, and insulin. J.Biol.Chem. 1979;254:1305–1311. [PubMed] [Google Scholar]
- 3.Ling C, Svensson L, Oden B, Weijdegard B, Eden B, Eden S, Billig H. Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. J.Clin.Endocrinol.Metab. 2003;88:1804–1808. doi: 10.1210/jc.2002-021137. [DOI] [PubMed] [Google Scholar]
- 4.Ling C, Billig H. PRL receptor-mediated effects in female mouse adipocytes: PRL induces suppressors of cytokine signaling expression and suppresses insulin-induced leptin production in adipocytes in vitro. Endocrinology. 2001;142:4880–4890. doi: 10.1210/endo.142.11.8514. [DOI] [PubMed] [Google Scholar]
- 5.Freemark M, Fleenor D, Driscoll P, Binart N, Kelly P. Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinology. 2001;142:532–537. doi: 10.1210/endo.142.2.7979. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson L, Binart N, Bohlooly Y, Bramnert M, Egecioglu E, Kindblom J, Kelly PA, Kopchick JJ, Ormandy CJ, Ling C, Billig H. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem.Biophys.Res.Commun. 2005;331:1120–1126. doi: 10.1016/j.bbrc.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best.Pract.Res.Clin.Endocrinol.Metab. 2005;19:471–482. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr.Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 9.Nagano M, Kelly PA. Tissue distribution and regulation of rat prolactin receptor gene expression. J.Biol.Chem. 1994;269:13337–13345. [PubMed] [Google Scholar]
- 10.Duan WR, Parmer TG, Albarracin CT, Zhong L, Gibori G. PRAP, a prolactin receptor associated protein: its gene expression and regulation in the corpus luteum. Endocrinology. 1997;138:3216–3221. doi: 10.1210/endo.138.8.5336. [DOI] [PubMed] [Google Scholar]
- 11.Liby K, Neltner B, Mohamet L, Burd C, Ben-Jonathan N. Prolactin overexpression by MDA-MB-535 human breast cancer cells accelerates tumor growth. Breast Cancer Res.Treat. 2003;79:241–252. doi: 10.1023/a:1023956223037. [DOI] [PubMed] [Google Scholar]
- 12.Hugo ER, Brandebourg TD, Comstock CES, Gersin KS, Sussman JJ, Ben Jonathan N. LS14: A novel human adipocyte cell line that produces prolactin. Endocrinology. 2006;147:306–313. doi: 10.1210/en.2005-0989. [DOI] [PubMed] [Google Scholar]
- 13.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAveney KM, Gimble JM, Yu-Lee L. Prolactin receptor expression during adipocyte differentiation of bone marrow stroma. Endocrinology. 1996;137:5723–5726. doi: 10.1210/endo.137.12.8940406. [DOI] [PubMed] [Google Scholar]
- 15.Fleenor D, Arumugam R, Freemark M. Growth hormone and prolactin receptors in adipogenesis: STAT-5 activation, suppressors of cytokine signaling, and regulation of insulin-like growth factor I. Horm.Res. 2006;66:101–110. doi: 10.1159/000093667. [DOI] [PubMed] [Google Scholar]
- 16.Brandebourg TD, Hugo ER, Ben-Jonathan N. Adipocyte prolactin: regulation of release and putative functions. Diabetes Obes. Metab. doi: 10.1111/j.1463-1326.2006.00671.x. in press. [DOI] [PubMed] [Google Scholar]
- 17.Nanbu-Wakao R, Fujitani Y, Masuho Y, Muramatu M, Wakao H. Prolactin enhances CCAAT enhancer-binding protein-beta (C/EBP beta) and peroxisome proliferator-activated receptor gamma (PPAR gamma) messenger RNA expression and stimulates adipogenic conversion of NIH-3T3 cells. Mol.Endocrinol. 2000;14:307–316. doi: 10.1210/mend.14.2.0420. [DOI] [PubMed] [Google Scholar]
- 18.Hu ZZ, Zhuang L, Meng J, Dufau ML. Transcriptional regulation of the generic promoter III of the rat prolactin receptor gene by C/EBPbeta and Sp1. J.Biol.Chem. 1998;273:26225–26235. doi: 10.1074/jbc.273.40.26225. [DOI] [PubMed] [Google Scholar]
- 19.Shirota M, Banville D, Ali S, Jolicoeur C, Boutin JM, Edery M, Djiane J, Kelly PA. Expression of two forms of prolactin receptor in rat ovary and liver. Mol.Endocrinol. 1990;4:1136–1143. doi: 10.1210/mend-4-8-1136. [DOI] [PubMed] [Google Scholar]
- 20.Berlanga JJ, Garcia-Ruiz JP, Perrot-Applanat M, Kelly PA, Edery M. The short form of the prolactin (PRL) receptor silences PRL induction of the beta-casein gene promoter. Mol.Endocrinol. 1997;11:1449–1457. doi: 10.1210/mend.11.10.9994. [DOI] [PubMed] [Google Scholar]
- 21.Binart N, Imbert-Bollore P, Baran N, Viglietta C, Kelly PA. A short form of the prolactin (PRL) receptor is able to rescue mammopoiesis in heterozygous PRL receptor mice. Mol.Endocrinol. 2003;17:1066–1074. doi: 10.1210/me.2002-0181. [DOI] [PubMed] [Google Scholar]
- 22.Williams C, Coltart TM. Adipose tissue metabolism in pregnancy: the lipolytic effect of human placental lactogen. Br.J.Obstet.Gynaecol. 1978;85:43–46. doi: 10.1111/j.1471-0528.1978.tb15824.x. [DOI] [PubMed] [Google Scholar]
- 23.FortunLamothe L, Langin D, Lafontan M. Influence of prolactin on in vivo and in vitro lipolysis in rabbits. Comp. Biochem. Physiol. C-Pharmacol. Toxicol. Endocrinol. 1996;115:141–147. doi: 10.1016/s0742-8413(96)00069-2. [DOI] [PubMed] [Google Scholar]
- 24.Fielder PJ, Talamantes F. The Lipolytic Effects of Mouse Placental Lactogen-Ii, Mouse Prolactin, and Mouse Growth-Hormone on Adipose-Tissue from Virgin and Pregnant Mice. Endocrinology. 1987;121:493–497. doi: 10.1210/endo-121-2-493. [DOI] [PubMed] [Google Scholar]
- 25.LaPensee CR, Horseman ND, Tso P, Brandebourg TD, Hugo ER, Ben Jonathan N. The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology. 2006;147:4638–4645. doi: 10.1210/en.2006-0487. [DOI] [PubMed] [Google Scholar]
- 26.Paukku K, Silvennoinen O. STATs as critical mediators of signal transduction and transcription: lessons learned from STAT5. Cytokine Growth Factor Rev. 2004;15:435–455. doi: 10.1016/j.cytogfr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Biswas R, Vonderhaar BK. Role of serum in the prolactin responsiveness of MCF-7 human breast cancer cells in long-term tissue culture. Cancer Res. 1987;47:3509–3514. [PubMed] [Google Scholar]
- 28.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem.Soc. Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 29.Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem.J. 2004;379:11–22. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleenor D, Oden J, Kelly PA, Mohan S, Alliouachene S, Pende M, Wentz S, Kerr J, Freemark M. Roles of the lactogens and somatogens in perinatal and postnatal metabolism and growth: studies of a novel mouse model combining lactogen resistance and growth hormone deficiency. Endocrinology. 2005;146:103–112. doi: 10.1210/en.2004-0744. [DOI] [PubMed] [Google Scholar]
- 31.Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 32.Asai-Sato M, Okamoto M, Endo M, Yoshida H, Murase M, Ikeda M, Sakakibara H, Takahashi T, Hirahara F. Hypoadiponectinemia in lean lactating women: Prolactin inhibits adiponectin secretion from human adipocytes. Endocr.J. 2006;53:555–562. doi: 10.1507/endocrj.k06-026. [DOI] [PubMed] [Google Scholar]