Abstract
Notch signalling affects most aspects of development, not least the determination of neural stem cell fate. Here, we describe the presence of the Notch-1 intracellular domain (N1ICD) in sub-nuclear bodies in SH-SY5Y neuroblastomas and in primary rat cortical neurons as well as several other mammalian cell lines. We also demonstrate that these N1ICD-positive sub-nuclear bodies are distinct from premyelocytic leukaemia (PML) and SC35 bodies. Furthermore, using Notch deletion constructs we determined that a region C-terminal of amino acid 2094 is involved in targeting the N1ICD into sub-nuclear bodies. These findings have ramifications for nuclear architecture and gene transcription.
Keywords: Notch, CBF-1, Transcription, Luciferase, Sub-nuclear bodies
Notch signalling regulates many aspects of invertebrate and vertebrate development ranging from somite formation [7] to epithelial–mesenchymal interactions [20] and the determination of neural stem cell fate [23]. Notch signalling is also active in adult tissues including the brain where it is involved in synaptic plasticity and memory [3,19]. There are four mammalian Notch receptors (Notch 1–4), which undergo a series of sequential proteolytic cleavages. The first cleavage (S1) occurs in the Golgi by a furin-like convertase generating two fragments that remain non-covalently attached to form the mature Notch receptor at the cell surface. Ligands of the Jagged and Delta families, expressed on neighbouring cells, bind to the Notch receptor leading to the second cleavage (S2) by tumor necrosis factor alpha converting enzyme (TACE), which results in the shedding of the extracellular domain. The second cleavage is followed by a constitutive cleavage (S3) within the transmembrane domain of Notch by γ-secretase. This cleavage releases the Notch intracellular domain (NICD), which migrates to the nucleus [20,14,15] where it interacts with the C-promoter binding factor-1 (CBF-1), the mammalian homologue of Suppressor of hairless (Drosophila) and Lag-1 (C. elegans), also known as RBP-Jκ [16]. In the absence of NICD, CBF-1 forms a complex with repressor proteins, resulting in the suppression of gene transcription. Interaction with NICD displaces the repressor proteins and allows the entry of transcriptional activators such as p300/CBP and Mastermind, which convert the CBF-1 repressor complex into an activator of gene transcription [8]. Notch/CSL target genes include the hairy and enhancer of split (HES) family and the HES related proteins (HERP/Hey) that encode basic helix-loop-helix transcription factors, which suppress differentiation by antagonising the expression of down-stream lineage-specifying genes. In this report, we demonstrate the presence of the N1ICD in sub-nuclear bodies in SH-SY5Y neuroblastomas and in primary rat cortical neurons.
To undertake this study the following cell lines, antibodies and plasmid constructs were used: SH-SY5Y neuroblastoma cells and chinese hamster ovary cells (CHO) were obtained from ECACC (UK) and human embryonic kidney 293a cells (HEK293a; an adherent clone of HEK293 cells) were purchased from Quantum Biotechnologies (Canada). Mouse monoclonal anti-splicing factor SC-35 antibody and rabbit polyclonal anti-FLAG tag antibody were from Sigma (UK). Mouse monoclonal anti-myc antibody was from Cell Signalling Technology (UK). Mouse monoclonal anti-promyelocytic leukaemia protein antibody, goat anti-Notch-1, goat anti-mouse IgG and donkey anti-goat IgG were from Santa Cruz Biotechnology (USA). Alexa Fluor 488 and 594 goat anti-mouse and donkey anti-goat IgG conjugates were from Molecular Probes (UK). C-terminally tagged myc-His human N1ICD, ΔTAD-N1ICD (lacking the transactivation domain TAD; the OPA domain and the proline (P), glutamate (E), serine (S), threonine (T) rich sequence—PEST) and ΔRA-N1ICD (lacking the RAM domain and the Ankyrin repeats) were gifts from Dr. Tom Kadesch (University of Pennsylvania, USA) [13]. Enhanced green fluorescent protein (EGFP) C-terminally tagged human N1ICD (N1ICD-EGFP) was from Dr. Allan Levey (Emory University School of Medicine, USA) [11]. The Notch-CBF-1 reporter, 4xwt-CBF-Luc, which contains four tandem repeats of the consensus CBF-1 DNA binding sequence, GTGGGAA and N-terminally tagged FLAG CBF-1 were generous gifts from Dr. Diane Hayward (Johns Hopkins University School of Medicine, USA) [5].
Cells were maintained in 5% CO2 in air in a humidified incubator at 37 °C. All culture media and supplements were from Invitrogen, UK, unless otherwise stated. SH-SY5Y neuroblastomas were grown in a 50/50 mix of F12/Eagle's minimal essential medium (EMEM) supplemented with 15% (v/v) fetal bovine serum (FBS; Autogen Bioclear, UK), 2 mM l-glutamine, non-essential amino acids (Sigma), 100 IU penicillin and 100 mg/ml streptomycin. HEK293a were cultured in low glucose Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) FBS, 2 mM l-glutamine, 100 IU penicillin and 100 mg/ml streptomycin. CHO cells were grown in F12 media supplemented as described above for HEK293a cells. Primary cortical neurons were prepared and cultured in Neurobasal media plus B27 supplement as previously described from E19 rat embryos [17].
Cells on coverslips were transfected with 500 ng of N1ICD-EGFP or 500 ng of myc-His N1ICD using FuGene 6 according to the manufacturer's instructions. Cells were fixed in ice-cold methanol 24 h after transfection then stained according to standard protocols. In brief, cells were incubated with goat anti-Notch-1 antibody (1:100), mouse monoclonal anti-promyelocytic leukaemia protein antibody (1:100) or mouse monoclonal anti-splicing factor SC-35 antibody (1:250) before being incubated with the appropriate fluorescent secondary antibody (1:200). Nuclei were stained with Hoescht 33342. Immunofluorescence was visualized and captured using a Zeiss LSM510 meta confocal microscope. Images were processed using LSM5 image examiner software (Zeiss). All experiments were performed in triplicate. Figures shown are representative images from a single experiment.
For luciferase reporter gene assays SH-SY5Y neuroblastomas were transfected with 400 ng of CBF-Luc (firefly luciferase based reporter DNA) and 500 ng of N1ICD-EGFP or 500 ng of myc-His N1ICD using FuGene 6 according to the manufacturer's instructions (Roche, UK). To control for transfection efficiency 50 ng of phTK-Renilla luciferase (Promega, UK) was also included in transfections. Empty vector DNA was included where necessary to maintain constant DNA concentrations. Twenty-four hours post-transfection the cells were lysed and firefly and Renilla luciferase activities were sequentially measured using Dual-Glo reagents (Promega) in a Wallac Trilux 1450 Luminometer (Perkin-Elmer, UK). Values were normalized by dividing firefly values by the Renilla value from the same well. Data for each set of four replica transfections was averaged, the control in each set normalized to 1 and data presented as fold increases over control. All experiments were performed in triplicate.
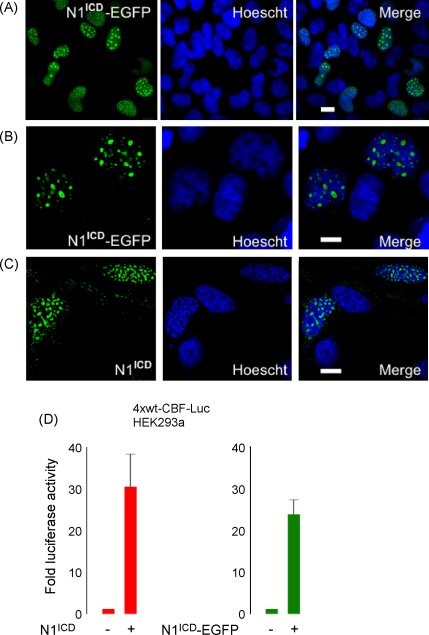
Endogenous S3 cleaved, nuclear, NICDs are expressed at very low levels and are difficult to detect in cell culture experiments [9], therefore we expressed exogenous N1ICD-EGFP in SH-SY5Y neuroblastomas to model N1ICD in vitro. In these cells N1ICD-EGFP exhibited a diffuse nuclear staining pattern with intense sub-nuclear bodies (Fig. 1A and B). In contrast, expression of exogenous p53 or β-catenin resulted in diffuse nuclear staining (data not shown), providing evidence that the N1ICD-positive sub-nuclear bodies are specific. The sub-nuclear bodies were not attributable to the EGFP protein since SH-SY5Y cells transfected with the EGFP expression vector containing no insert did not display a speckled nuclear staining pattern (data not shown). Furthermore, a myc-His tagged form of N1ICD was found in sub-nuclear bodies in SH-SY5Y neuroblastoma cells (Fig. 1C) as demonstrated using an anti-Notch-1 antibody, providing additional evidence that the observed speckled staining pattern is specific and not due to the presence of EGFP. Control cultures that were not exposed to primary antibody did not exhibit any positively stained cells (data not shown). To ensure the presence of nuclear bodies was not an artefact of the fixation method used (ice-cold methanol), SH-SY5Y cells were transfected with both EGFP and myc-His tagged Notch constructs and fixed with either 3.7% formaldehyde or 4% paraformaldehyde. With both methods very similar nuclear bodies were seen with both constructs (data not shown). Of note, both N1ICD-EGFP and myc-His tagged N1ICD were functionally active as demonstrated using CBF-Luc (Fig. 1D) and we have previously shown that exogenous N1ICD expression promotes the inhibition of retinoic acid-induced differentiation in SH-SY5Y neuroblastomas [4].
Fig. 1.
Exogenous N1ICD is present in sub-nuclear bodies in SH-SY5Y neuroblastomas. (A) Low magnification image of SH-SY5Y neuroblastomas transfected with N1ICD-EGFP (green) with nuclei counter stained with Hoescht (blue). (B) High magnification image of SH-SY5Y neuroblastomas transfected with N1ICD-EGFP (green) with nuclei counter stained with Hoescht (blue). (C) SH-SY5Y neuroblastomas were transfected with myc-His-tagged N1ICD, which was detected using an anti-Notch-1 antibody (green) and nuclei were counter stained with Hoescht (blue). Scale bars represent 10 μm. (D) N1ICD-EGFP and myc-His tagged N1ICD activate CBF-Luc. CBF-Luc was transfected into SH-SY5Y neuroblastomas along with either empty vector, N1ICD-EGFP or myc-His tagged N1ICD. Twenty-four hours post-transfection cells were lysed and luciferase activities determined. Data are presented as fold increases in luciferase activity over control.
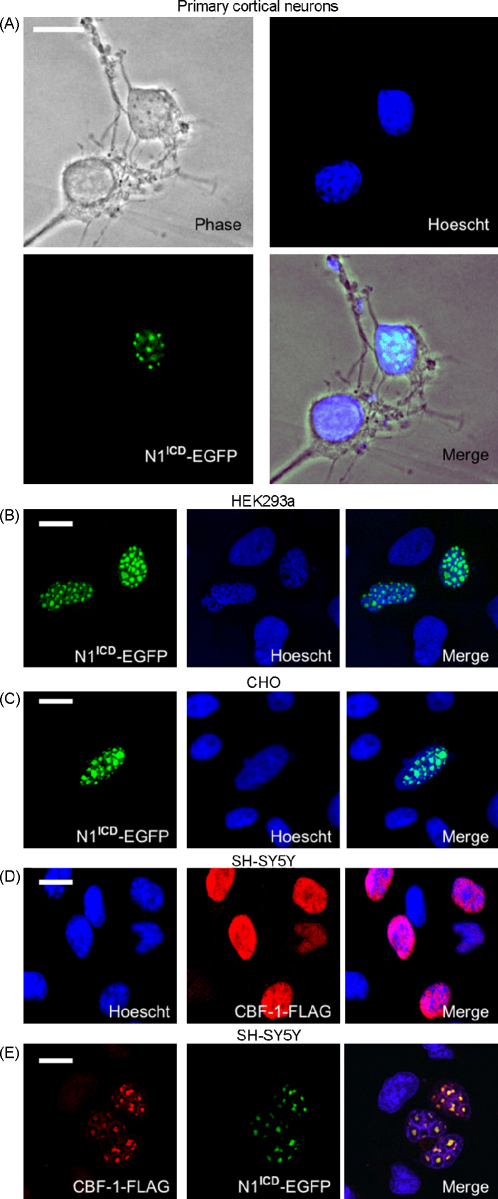
N1ICD-positive sub-nuclear bodies were not cell-type specific. We observed identical sub-nuclear structures in primary rat cortical neurones (Fig. 2A), HEK293a cells (Fig. 2B) and in CHO cells (Fig. 2C) with N1ICD-EGFP and myc-His tagged N1ICD (data not shown). As the punctate structures were detected with both constructs in a variety of cell lines and in primary neurons they are unlikely to be an artefact of tagging or a result of cellular transformation. Furthermore, we found that exogenous CBF-1 exhibited a diffuse nuclear staining pattern in SH-SY5Y neuroblastomas (Fig. 2D), but in the presence of N1ICD CBF-1 re-located into sub-nuclear bodies (Fig. 2E), indicative of functional gene transcription within these nuclear domains.
Fig. 2.
Exogenous N1ICD is present in sub-nuclear bodies in primary neurons, HEK293a cells and in CHO cells. Primary cortical rat neurons (A), HEK293a cells (B) and CHO (C) cells were transfected with N1ICD-EGFP (green) and nuclei were counter stained with Hoescht (blue). SH-SY5Y neuroblastomas were transfected with FLAG tagged CBF-1 (red) independently (D) and in the presence of N1ICD-EGFP (green) (E). The FLAG tagged CBF-1 was detected using an anti-FLAG antibody and nuclei were counter stained with Hoescht (blue). Scale bars represent 10 μm.
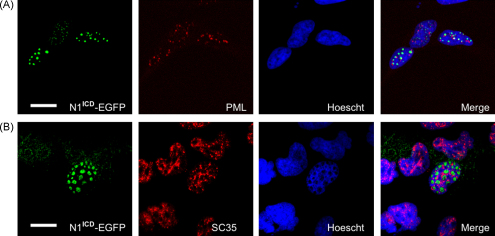
Several sub-nuclear structures, similar to those shown here, have been previously described including premyelocytic leukaemia (PML) bodies and SC35 bodies. These foci are believed to be areas of high transcriptional activity [2] and pre-mRNA splicing, respectively [10]. We therefore transfected SH-SY5Y cells with N1ICD-EGFP and used antibodies to detect endogenous PML and SC35 bodies. Both PML (Fig. 3A, mid left panel) and SC35 reactive bodies (Fig. 3B, mid left panel) were detected in SH-SY5Y cells. However, in both cases these formations were distinct from the N1ICD-EGFP positive foci (Fig. 3A and B, far right panels). The PML and SC35 bodies were more consistent in size and typically smaller and more numerous than the formations in which N1ICD was detected.
Fig. 3.
N1ICD does not co-localise with PML or SC35 bodies. SH-SY5Y neuroblastomas were transfected with N1ICD-EGFP (green). Twenty-four hours post-transfection cells were fixed and stained for endogenous PML protein (A, red, mid left panel) or SC-35 (B, red, mid left panel) and nuclei counter-stained with Hoescht (blue). Scale bar represent 10 μm.
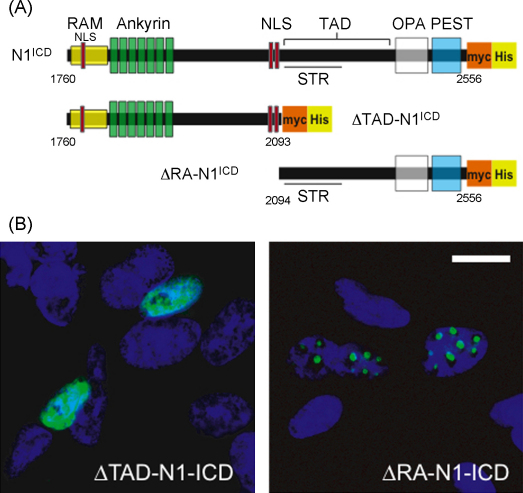
Next we examined which region of N1ICD was involved in targeting the protein to sub-nuclear bodies by using two myc-tagged N1ICD deletion constructs, ΔTAD-N1ICD and ΔRA-N1ICD. ΔTAD-N1ICD runs from a.a. 1760 to 2093 and lacks the transactivation domain, the OPA domain and the proline (P), glutamate (E), serine (S), threonine (T) rich sequence (PEST). ΔRA-N1ICD extends from a.a. 2094 to 2556 and lacks the RAM domain and the Ankyrin repeats (Fig. 4A). ΔTAD-N1ICD was detected diffusely throughout the nucleus in SH-SY5Y neuroblastomas (Fig. 4B, left panel), while ΔRA-N1ICD was located in sub-nuclear bodies very similar in appearance to those seen with N1ICD (Fig. 4B, right panel). This indicates that a region C-terminal of a.a. 2094, lying within the TAD, the OPA domain or the PEST domain or an unidentified motif within the intervening regions, is involved in targeting N1ICD into sub-nuclear bodies.
Fig. 4.
A region C-terminal of the Ankyrin repeats directs the N1ICD sub-nuclear bodies. (A) Schematic diagram showing the N1ICD and the deletion constructs, ΔRA-N1ICD and ΔTAD-N1ICD. Top, the intact S3 cleaved N1ICD in which the major recognised domains are depicted; the RAM domain; the 7 Ankyrin repeats; three nuclear localization signals (NLS, red rectangles); the transactivation domain (TAD), within which lies the Serine/Threonine Rich region (STR); the OPA sequence; and the proline (P), glutamate (E), serine (S), threonine (T) rich sequence (PEST). Below are depicted ΔTAD-N1ICD, which terminates at a.a. residue 2093 and ΔRA-N1ICD, which starts at a.a 2094 and ends at 2556. (B) SH-SY5Y neuroblastomas were transfected with the myc-His tagged deletion constructs, ΔTAD-N1ICD (left panel) or ΔRA-N1ICD (right panel). Twenty-four hours post-transfection myc-tagged proteins were detected using an anti-myc antibody (green, both panels) and nuclei were counter-stained with Hoescht (blue). Myc-tagged ΔTAD-N1ICD appeared diffuse throughout the nucleus (left panel), while myc-tagged ΔRA-N1ICD appeared in sub-nuclear bodies (right panel). Scale bars represent 10 μm.
In summary, we demonstrate that exogenous N1ICD is expressed in sub-nuclear bodies in SH-SY5Y neuroblastomas, primary rat cortical neurons as well as other mammalian cell lines and these sub-nuclear structures were distinct from SC35 or PML bodies. N1ICD foci were detectable as early as 5 h after transfection (cells were routinely fixed 24 h post-transfection in all other experiments described in this report), which suggests that the nuclear inclusions are not merely a consequence of protein aggregation with time (data not shown). The presence of the N1ICD in sub-nuclear bodies has previously been demonstrated when associated with the Epstein-Barr virus derived protein, RPMS [24] and the Mastermind family of Notch/CBF-1 co-activators [18,21,22]. Here, we report that the N1ICD appeared in foci independently of other exogenously expressed proteins. A number of endogenous Notch associated proteins, including CBF-1, have recently been observed in similar nuclear foci in primary hippocampal neurons [12], although the presence of NICDs was not examined. Interestingly, we found that exogenously expressed CBF-1 exhibited a diffuse nuclear staining pattern, however in the presence of exogenous N1ICD CBF-1 was found in sub-nuclear bodies. A number of previous reports [1,6] concerning Notch cytochemistry contain figures in which N1ICD appears to be in punctate formations, which with greater resolution could have been interpreted as distinct sub-nuclear bodies. It is feasible then that the N1ICD-positive bodies form part of a physiologically relevant, multimeric, protein complex involved in gene transcription. Moreover, the presence of such sub-nuclear bodies provides evidence that the nucleus contains defined domains and is not merely a homogenous pool of nucleic acids and proteins.
Acknowledgement
We are grateful to the Wellcome Trust for funding.
References
- 1.Berezovska O., Jack C., McLean P., Aster J.C., Hicks C., Xia W., Wolfe M.S., Kimberly W.T., Weinmaster G., Selkoe D.J., Hyman B.T. Aspartate mutations in presenilin and gamma-secretase inhibitors both impair Notch1 proteolysis and nuclear translocation with relative preservation of Notch1 signaling. J. Neurochem. 2000;75:583–593. doi: 10.1046/j.1471-4159.2000.0750583.x. [DOI] [PubMed] [Google Scholar]
- 2.Ching R.W., Dellaire G., Eskiw C.H., Bazett-Jones D.P. PML bodies: a meeting place for genomic loci? J. Cell Sci. 2005;118:847–854. doi: 10.1242/jcs.01700. [DOI] [PubMed] [Google Scholar]
- 3.Costa R.M., Honjo T., Silva A.J. Learning and memory deficits in Notch mutant mice. Curr. Biol. 2003;13:1348–1354. doi: 10.1016/s0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- 4.Hooper C., Tavassoli M., Chapple J.P., Uwanogho D., Goodyear R., Melino G., Lovestone S., Killick R. TAp73 isoforms antagonize Notch signalling in SH-SY5Y neuroblastomas and in primary neurones. J. Neurochem. 2006;99:989–999. doi: 10.1111/j.1471-4159.2006.04142.x. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh J.J., Henkel T., Salmon P., Robey E., Peterson M.G., Hayward S.D. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol. Cell Biol. 1996;16:952–959. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikura N., Clever J.L., Bouzamondo-Bernstein E., Samayoa E., Prusiner S.B., Huang E.J., DeArmond S.J. Notch-1 activation and dendritic atrophy in prion disease. PNAS. 2005;102:886–891. doi: 10.1073/pnas.0408612101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y.J., Smithers L., Lewis J. Vertebrate segmentation: the clock is linked to Notch signalling. Curr. Biol. 1998;8:R868–R871. doi: 10.1016/s0960-9822(07)00547-7. [DOI] [PubMed] [Google Scholar]
- 8.Kadesch T. Notch signaling: a dance of proteins changing partners. Exp. Cell Res. 2000;260:1–8. doi: 10.1006/excr.2000.4921. [DOI] [PubMed] [Google Scholar]
- 9.Kopan R. Notch: a membrane-bound transcription factor. J. Cell Sci. 2002;115:1095–1097. doi: 10.1242/jcs.115.6.1095. [DOI] [PubMed] [Google Scholar]
- 10.Lallena M.J., Correas I. Transcription-dependent redistribution of nuclear protein 4.1 to SC35-enriched nuclear domains. J. Cell Sci. 1997;110:239–247. doi: 10.1242/jcs.110.2.239. [DOI] [PubMed] [Google Scholar]
- 11.Levy O.A., Lah J.J., Levey A.I. Notch signaling inhibits PC12 cell neurite outgrowth via RBP-J-dependent and -independent mechanisms. Dev. Neurosci. 2002;24:79–88. doi: 10.1159/000064948. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie G.J., Stevenson P., Ward G., Papadia S., Bading H., Chawla S., Privalsky M., Hardingham G.E. Nuclear Ca2+ and CaM kinase IV specify hormonal- and Notch-responsiveness. J. Neurochem. 2005;93:171–185. doi: 10.1111/j.1471-4159.2005.03010.x. [DOI] [PubMed] [Google Scholar]
- 13.Ross D.A., Kadesch T. The Notch intracellular domain can function as a coactivator for LEF-1. Mol. Cell Biol. 2001;21:7537–7544. doi: 10.1128/MCB.21.22.7537-7544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeter E.H., Kisslinger J.A., Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 15.Struhl G., Adachi A. Nuclear access and action of Notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K., Taniguchi Y., Minoguchi S., Sakai T., Tun T., Furukawa T., Honjo T. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-J kappa/Su(H) Curr. Biol. 1995;5:1416–1423. doi: 10.1016/s0960-9822(95)00279-x. [DOI] [PubMed] [Google Scholar]
- 17.Theuns J., Remacle J., Killick R., Corsmit E., Vennekens K., Huylebroeck D., Cruts M., Broeckhoven C.V. Alzheimer-associated C allele of the promoter polymorphism −22C > T causes a critical neuron-specific decrease of presenilin 1 expression. Hum. Mol. Genet. 2003;12:869–877. doi: 10.1093/hmg/ddg098. [DOI] [PubMed] [Google Scholar]
- 18.Tonon G., Modi S., Wu L., Kubo A., Coxon A.B., Komiya T., O’Neil K., Stover K., El-Naggar A., Griffin J.D., Kirsch I.R., Kaye F.J. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat. Genet. 2003;33:208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Chan S.L., Miele L., Yao P.J., Mackes J., Ingram D.K., Mattson M.P., Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinmaster G., Roberts V.J., Lemke G. A homolog of Drosophila Notch expressed during mammalian development. Development. 1991;113:199–205. doi: 10.1242/dev.113.1.199. [DOI] [PubMed] [Google Scholar]
- 21.Wu L., Aster J.C., Blacklow S.C., Lake R., rtavanis-Tsakonas S., Griffin J.D. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat. Genet. 2000;26:484–489. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- 22.Wu L., Sun T., Kobayashi K., Gao P., Griffin J.D. Identification of a family of mastermind-like transcriptional coactivators for mammalian Notch receptors. Mol. Cell Biol. 2002;22:7688–7700. doi: 10.1128/MCB.22.21.7688-7700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon K., Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Chen H., Weinmaster G., Hayward S.D. Epstein-Barr virus BamHI-A rightward transcript-encoded RPMS protein interacts with the CBF1-associated corepressor CIR to negatively regulate the activity of EBNA2 and NotchIC. J. Virol. 2001;75:2946–2956. doi: 10.1128/JVI.75.6.2946-2956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]