Abstract
We have recently shown that low intensity, intermediate frequency, electric fields inhibit by an anti-microtubule mechanism of action, cancerous cell growth in vitro. Using implanted electrodes, these fields were also shown to inhibit the growth of dermal tumors in mice. The present study extends these findings to additional cell lines [human breast carcinoma; MDA-MB-231, and human non-small-cell lung carcinoma (H1299)] and to animal tumor models (intradermal B16F1 melanoma and intracranial F-98 glioma) using external insulated electrodes. These findings led to the initiation of a pilot clinical trial of the effects of TTFields in 10 patients with recurrent glioblastoma (GBM). Median time to disease progression in these patients was 26.1 weeks and median overall survival was 62.2 weeks. These time to disease progression and OS values are more than double the reported medians of historical control patients. No device-related serious adverse events were seen after >70 months of cumulative treatment in all of the patients. The only device-related side effect seen was a mild to moderate contact dermatitis beneath the field delivering electrodes. We conclude that TTFields are a safe and effective new treatment modality which effectively slows down tumor growth in vitro, in vivo and, as demonstrated here, in human cancer patients.
Keywords: cancer, glioblastoma, tumor treating fields
Because living cells consist of ions, polar or charged molecules, membranes, and organelles, they are responsive to and often generate electric fields and currents. The electric activity of cells plays a key roll in many essential biological processes. The electric fields associated with all of the above phenomena are in the range of 0–10 V/cm, except within cell membranes (1) where they may reach 105 V/cm. Whereas electric fields induce ion flow, polar molecules only orient themselves along the lines of a uniform field (2). However, nonuniform electric fields exert forces on polar molecules forcing them to move toward higher field intensity, a well known process known as dielectrophoresis (3, 4). Electric fields and resulting currents, when sufficiently large, stimulate nerves, muscles, cardiac muscle, etc. Only much larger fields generate heat that may damage cells (5).
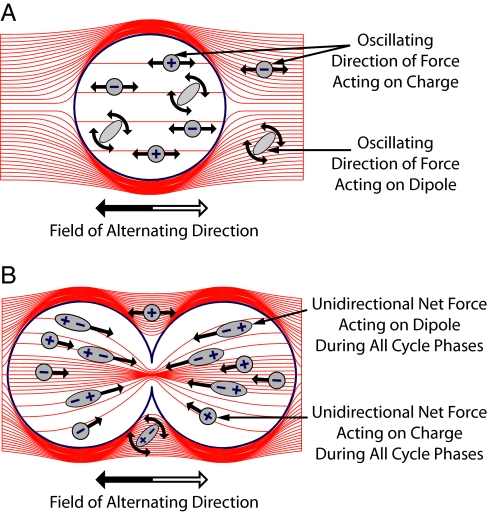
In an electric field of alternating direction (ac field) all charges and polar molecules are subjected to forces of alternating direction so that ionic flows and dipole rotation oscillate (Fig. 1). In view of the relatively slow kinetics of the bioelectrical responses, as the ac fields' frequency is elevated, their biological effect (except for heating) is reduced such that, >10 kHz, it becomes negligible. Therefore, it is generally believed that ac fields of 100 kHz or above have no meaningful biological effects (5), although a number of nonsignificant effects have been described (6–8).
Fig. 1.
ac field distribution in and around quiescent (A) and dividing (B) cells. Inside quiescent cells, the field is uniform, and the oscillating electric forces result only in “vibration” of ions and dipoles (the forces associated with each half cycle are denoted white and gray arrows). In contrast, the nonuniform field within dividing cells (B) induces forces pushing all dipoles toward the furrow. Note that at frequencies of 0.1–1.0 MHz, the cell membrane impedance is relatively high, so only a small fraction of the currents penetrate the cells as seen from the density of lines.
In contradiction to this belief, we have recently demonstrated (9) that 100 KHz to 1 MHz ac fields have significant specific effects on dividing cells. The basis of these effects during cytokinesis was shown to be the unidirectional forces induced by the inhomogeneous fields at the bridge separating the daughter cells (Fig. 1B) that interfere with spindle tubulin orientation and induce dielectrophoresis.
It is the aim of this work to further study the effects of ac fields on quiescent and proliferating cells in culture, animal cancer models, and cancerous tumors in humans. Following a basic work on cell cultures (9), we demonstrate here that such fields, termed tumor treating fields (TTFields), are effective when applied by insulated external electrodes to animal cancer models and patients with recurrent glioblastoma (GBM). In a pilot clinical trial conducted on this extremely malignant tumor of glial cell origin (10, 11), TTFields treatment was found to be both safe and effective in slowing tumor progression. These promising results raise the possibility that TTFields could become a new treatment modality for cancer.
Cells in Culture
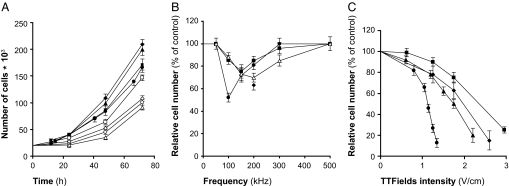
The effects of a 24-h exposure of four of the most common types of cancer [malignant melanoma, glioma (part of the data for malignant melanoma and glioma cells was taken from ref. 9), breast carcinoma, and non-small-cell lung carcinoma to TTFields] are illustrated in Fig. 2. It is seen that the number of unexposed (control) cells roughly doubles every 24 h, whereas the proliferation rate of the exposed cells is slowed down during exposure and gradually recovers after treatment is terminated (Fig. 2A). The frequency dependency of the effects is depicted in Fig. 2B. It is seen that the optimal frequency is 100 kHz for mouse melanoma (B16F1), 150 kHz for human breast carcinoma (MDA-MB-231), and 200 kHz for rat glioma (F-98). In addition, similar experiments were performed in two human glioma cell lines (U-118 and U-87). In both, the optimal TTFields frequency was identical to rat glioma cell lines (i.e., 200 kHz).
Fig. 2.
Time, frequency, and intensity dependence of the effect of TTFields on cancer cell proliferation. (A) The number of cells in untreated cultures (filled symbols) as compared with cultures treated with TTFields (open symbols) for 24 h (1.75 V/cm for MDA-MB-231, F-98, and H1299 cells and 1.1 V/cm for B16F1 cells). (B) The relative change in number of cells after 24 h of treatment of different frequencies (same TTFields intensity). (C) The effect of 24 h of exposure to TTFields of increasing intensities (at optimal frequencies). ● and ○, B16F1; ■ and □, MDA-MB-231; ▴ and ▵, F-98; ♦ and ♢, H1299.
The “dose–response curve,” i.e., the relationship between the TTFields effects and field intensity, is given in Fig. 2C. It is seen that effect on cell division and cell death (by apoptosis) is intensity dependent, the sensitivity being highest for mouse melanoma cells, decreasing for rat glioma and for human non-small-cell lung carcinoma and lowest for human breast carcinoma.
From the mechanism of action of TTFields, as illustrated in Fig. 1, it can be deduced that their efficacy must be a function of the angle between the field and axis of division; when the two are parallel its maximal and when one is perpendicular to the other, it must be minimal. Because in culture the axis of division is randomly oriented, only a fraction of the dividing cells are subjected to optimal treatment. To overcome this problem, multiple field directions were applied sequentially every 0.25–1 sec. Two perpendicular fields were found to be ≈20% more effective than the single-direction one for B16F1 and F-98 cells. This result is consistent with the previously reported effects on malignant melanoma cells (9).
Animal Tumor Models
Intracranial Glioblastoma.
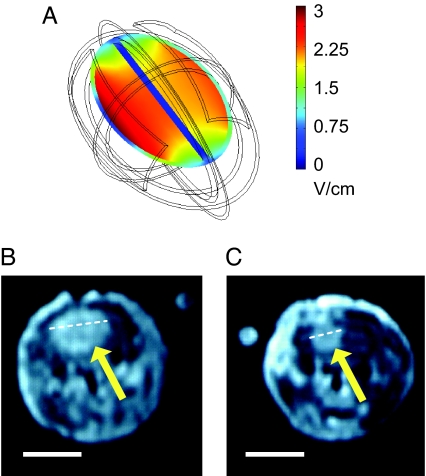
Our report (9) described the effects of TTFields applied by means of implanted electrodes to intradermal malignant melanoma in mice. This report compares 40 Fischer rats inoculated intracranially with glioma cells, treated by means of external electrodes with a temperature, and geometry matched electrode control group. The treatment duration was 6 days, using the optimal frequency of 200 kHz (see Fig. 2) at 2 V/cm. Fig. 3 depicts the computed field distribution in the rat brain (Fig. 3A), exemplary posttreatment MRI images of a control (Fig. 3B) and a treated tumor (Fig. 3C). The maximal diameter of the treated tumor is about half that of the control one.
Fig. 3.
TTFields inhibition of the growth of intracranial glioma. (A) FEM simulations (using a three-dimensional mesh) of the distribution of TTFields intensity within a simplified rat brain model. (B and C) Exemplary T1 weighted coronal MRI sections (after IV injection of Gd-DTPA) of the heads of a control and a TTFields treated (200 kHz, two-directional TTFields) rat, respectively. In both examples, the section shown is that with the largest diameter tumor. Head simulations are 3.1 × 1.9 cm ellipsoid; skin thickness, 0.4 mm (σ = 0.00045 S/m; ε = 1,120); skull thickness, 1.1 mm (σ = 0.015 S/m; ε = 16); thickness of the CSF surrounding the brain, 0.5 mm (σ = 2 S/m; ε = 109); and brain itself has the properties of a uniforms white matter (σ = 0.15 S/m; ε = 3,200). The electrodes placed over a 0.5-mm layer of hydrogel. Note the almost uniform field intensity in most brain volume. (Scale bars, 1 cm.)
The average inhibitory effect of unidirectional TTFields (in a temporal-temporal direction) was small and did not reach statistical significance (treated tumor volume 19.8% smaller than sham control tumors; n = 26; P = 0.19, Student's t test). However, increasing the number of TTFields directions caused statistically significant inhibition of tumor growth, reaching 42.6% and 53.4% for two (n = 42; P < 0.01, Student's t test) and three (n = 10; P < 0.01, Student's t test) directions positioned at 45–90° to each other, respectively.
Frequency Dependence of the Inhibitory Effect of TTFields.
The TTFields inhibitory efficacy vs. frequency was studied on mice inoculated with B16F1 melanoma. The mice (n = 26) were treated for 5 days by single-direction TTFields of different frequencies. The maximal growth inhibition was found at 100 kHz, with the treated tumor size 62.7 ± 8.9% that of control tumors. Although this frequency dependence in vivo did not reach statistical significance (single-factor ANOVA, P = 0.11), it shows the same frequency dependency as the dependence of cultured B16F1 cells reported in ref. 9, which supports the conclusion that this is the optimum frequency. In contrast, rats bearing intracerebral glioma were unaffected by 100 kHz TTFields, whereas 200 kHz TTFields caused significant inhibition of tumor growth.
Safety Profile of TTFields in Healthy Animals.
TTFields (100 kHz) at 6 V/cm were applied to the chest of three New Zealand rabbits. No changes were seen in the rate or regularity of cardiac rhythm throughout and following the exposure. To test the safety of chronic TTFields application TTFields were applied to either the head (n = 30, 1 V/cm for 4 weeks) or the chest (n = 10, 3 V/cm for 2 weeks) of New Zealand Rabbits. All animals were assessed weekly for weight, temperature, ECG, CBC, wide chemistry panel and coagulation. After a 1-month follow-up period, all animals were killed and had samples of major organs examined by a pathologist. No treatment-related toxicities were recorded in any of the animals.
GBM Patients
TTFields Treatment of Patients with Recurrent GBM Brain Tumor.
Ten patients with recurrent GBM were included in the trial [see Materials and Methods and supporting information (SI) Table 1].
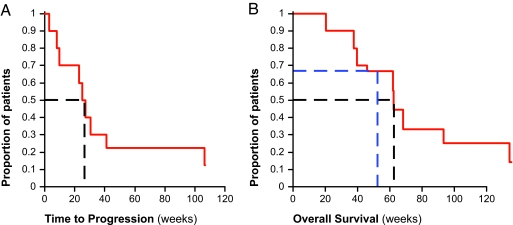
As seen in Fig. 4A, the median time to disease progression (TTP) of the patients is 26.1 weeks (range 3–124 weeks) and the progression-free survival at 6 months (PFS6) is 50% (23–77%; 95% confidence interval). Two of the patients were still progression free at study closure.
Fig. 4.
Efficacy of TTFields treatment in recurrent GBM. (A) TTP of treated patients (n = 10); median TTP is 26.1 weeks (dashed black line). (B) Kaplan–Meier OS curve for NovoTTF-100A treated patients (n = 10). The median OS in these patients is 62.2 weeks (black dashed line), and the 1-year survival rate is 67.5% (blue dashed line).
The median overall survival (OS) of TTFields treated patients is currently 62.2 weeks (range 20.3–124.0 weeks). These TTP and OS values are more than double the reported medians of historical control patients. Three of the patients are still alive at this time. The Kaplan–Meier survival curve (12) of the treatment results is shown in Fig. 4B.
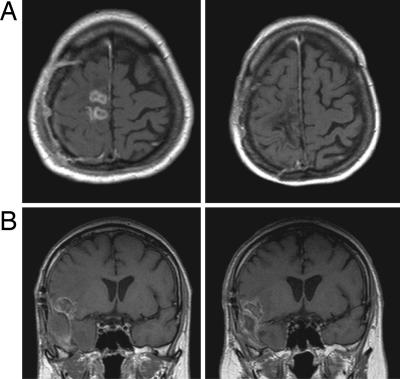
The TTFields treatment resulted in one complete response (Fig. 5A) which is still tumor free per MRI ten months after stopping treatment and one partial response (Fig. 5B) that is still responding 7 months after stopping treatment. Both are still progression free >2 years from treatment initiation. In addition one patient had minimal response and four had stable disease for over 4 months before progressing.
Fig. 5.
Exemplary T1-weighted, post contrast, MRI scans of recurrent GBM patients before (Left) and after (Right) TTFields treatment. (A) Complete response after 8 months of treatment. (B) Stable disease (10% reduction in contrast enhancing area) after 9 months of treatment.
Safety Profile of TTFields Applied to GBM Patients.
The 10 recurrent GBM Patients received treatment for a total of 280 weeks without a single treatment-related serious adverse event and no significant changes were seen in serum chemistry or blood count in any of the patients. The only changes seen consistently were elevated liver enzymes, attributed to anti-epileptic drug usage. Two patients had partial seizures that were unrelated to treatment. Nine of ten patients suffered from a mild to moderate contact dermatitis beneath the electrode gel. This treatment-related adverse event responded well to application of steroid creams and periodic electrode relocation.
Discussion
Alternating electric fields have been shown to have a wide range of effects on living tissues. At very low frequencies (<1 KHz), electric fields stimulate excitable tissues through membrane depolarization (13) and have been claimed to stimulate bone growth and accelerate fracture healing (14). However, as the frequency of the electric field increases the stimulatory effect diminishes, whereas above MHz a completely different biological effect, tissue heating, becomes dominant (15, 16).
Alternating electric fields of intermediate frequencies (10 kHz to 1 MHz) were considered not to have any meaningful non-thermal biological effects (5). An exception, are the TTFields described in ref. 9. This presumed lack of effect of such fields is consistent with the fact that when electric fields, that exert forces only on charges and dipoles reverse direction at a high frequency, their net effect tends to null out. Thus, the effects were minor and have neither been shown to be beneficial or detrimental to humans (5, 8, 17).
In this study we try to use TTFields as a new cancer treatment modality. We first extended the In-Vitro study of TTFields effect on glioma and melanoma cells (9) to several of the most prevalent cancers; breast carcinoma and non-small-cell lung carcinoma. It was found that the proliferation of these cells is arrested and the cells are destroyed (Fig. 2). The optimal frequencies differed between cancer cell types. To understand this finding we calculated the force on a 1 μm polarizable spherical particle in a dividing cell as function of cell radius, membrane thickness and cytoplasm conductivity. It was found that optimal TTFields frequency is inversely related to cell size (see SI Appendix A) in a way consistent the diameter variability of the different cell types studied.
In the previous study (9) animal treatment was done by using implanted electrodes. In the present study, we used the much more practical externally applied electrodes. Furthermore, as the available data suggests that treatment may need to be prolonged, the use of conducting electrodes may result in serious problems: local damage to the skin because of electrolysis and the generation of free radicals at the electrode-tissue interface, skin permeabilization by the transdermal currents (18, 19), and calcium accumulation within cells (20) that can result in cell death (21). Clearly, the first 2 adverse effects do not occur at the surface of insulated electrodes. Using fluorescence calcium imaging techniques, we could demonstrate that electric field induced calcium accumulation is eliminated by the use of insulated electrodes (see SI Appendix B). However, the large potential drop across the insulation high impedance poses a serious problem; to generate the fields of the required intensity potentials of >1,000 V must be used. As such high voltages may compromise patient safety, low impedance electrodes were developed. The impedance of insulation is lowered by using an insulating material, lead magnesium niobate–lead titanate (PMN-PT) (EDO, New York, NY), that has a dielectric constant of ε > 5,000. Under these conditions the electrodes have a capacitance of ≈10nF/cm2, i.e., an impedance of 100–200 Ω at the TTFields frequency range. Thus, only 50% of the applied voltage is lost on the insulation in the mice experiments. The corresponding potential drop on the 22.5 cm2 electrodes placed on the patient's head, in the trial presented here, is only ≈10% of the applied voltage.
A major limitation of all current cancer treatments is their unfavorable therapeutic index. Two types of toxicities may be expected from an electric field based treatment. First, the fields could theoretically affect excitable tissues causing cardiac arrhythmias or seizures. However, such effects are not expected to occur, because for sinusoidal alternating fields of >10 kHz, excitation of nerves and muscles decreases dramatically, because of the parallel resistor–capacitor nature of the cell membrane (22). Indeed, in both acute and chronic application of TTFields to animals and patients, there was no trace of abnormal cardiac or neurological activity. Secondly, TTFields might be expected to damage rapidly dividing normal cells within the body, i.e., bone marrow and small intestine mucosa. However, no treatment-related toxicities were found in any of the treated patients or upon animal exposure to field intensities threefold higher than the effective anti-tumoral dose. With regards to hematopoesis the reason for this is that these cells, which reside mainly in the bone marrow, are protected from the TTFields by the high impedance of both the bone and bone marrow (23). This was demonstrated by calculating the TTFields distribution in an extremity, such as a leg, by using the finite element mesh (FEM) method. It was found that the field intensity is 100-fold lower within the bone marrow compared with the surrounding tissues. The lack of damage to intestinal mucosa probably reflects that the small intestine mucosal cells have a slower replication cycle than neoplastic cells (24) and that the intestine changes its orientation, relative to the applied field, often lowering the efficacy of the mitotic disruption.
The tumor inhibitory effect of TTFields has been attributed previously to two separate mechanisms (9): interference with the formation of the mitotic spindle microtubules and physical destruction of cells during cleavage, both of which are strongly dependant on the orientation of mitosis axis versus the field vectors. Because the relative orientation of the mitosis axis during cytokinesis is random, it would be expected that only a fraction of dividing cells would be affected by TTFields of any specific direction. To overcome this problem, we applied sequentially several field directions and have shown that increasing the number of directions from 1 to 3, resulted in a significant increase in the anti-proliferative efficacy of TTFields in vitro and in vivo.
Following encouraging evidence from experimental animals, a clinical trial of the effect of TTFields on patients with recurrent GBM was initiated. Because in vitro data indicate that TTFields are most effective when applied for >16 h continuously (data not shown), patients were treated daily for an average of 18 h per day until progression. The results reported here are the first evidence of the safety and efficacy of TTFields used to treat cancer in patients. Preliminary accounts of this data were published in abstract form.‡‡,§§,¶¶ Because this was a pilot trial there was no randomized control group and the results were evaluated by comparing to historical control data. Most historically controlled pilot studies in recurrent GBM are compared with a large metaanalysis performed by Wong et al. in 1999 (10) and to this data we added the four prospective trials (25–28), which included >50 GBM patients, performed since that date. The average historical PFS6 based on the above studies is 15.3 ± 3.8%, and the average historical TTP is 9.5 ± 1.6 weeks. OS averaged 29.3 ± 6 weeks (see SI Table 2). When compared with these outcomes, the efficacy data collected in the current pilot trial is extremely promising (TTP, 26.1 weeks; PFS6, 50%; and OS, 62.2 weeks). These results were not accompanied by hematological or gastrointestinal toxicities, epileptic seizures, cardiac arrhythmias, etc., despite >70 months of cumulative treatment. The only side effect detected was contact dermatitis beneath the electrodes. This reaction is most likely the result of a combination of factors, including chronic moisture, heat, and occlusion of the skin; chemical irritation by constituents of the hydrogel and medical tape (29); and possibly inhibition of cellular replication in the skin by the TTFields. Thus, in conclusion, this treatment modality was well tolerated and caused almost no toxicity at all.
In summary, we demonstrated initially that TTFields are effective in arresting the proliferation and inducing death in a wide range of tumor cells in culture as well as solid tumors in animals. On this basis a clinical trial was carried out treating human patients suffering from recurrent GBM, a malignant brain tumor. It was demonstrated that the TTFields inhibit the growth of this highly treatment-resistant tumor by using special insulated electrodes, with little or no side effects. Can we expect to have similar efficacy on other human tumors? The fact that in cultures and animal models TTFields were found to be effective on all cells and tumors tested is definitely encouraging. Furthermore, TTFields being a physical, rather than chemical, modality, their efficacy is likely to be highly insensitive to specific interactions with tumor and patient receptors and other characteristic elements. Thus, like irradiation, they have the potential to be effective over a wide range of tumors. However, from the above it is apparent that their practical specificity to cancerous cells is significantly higher than that of irradiation, the therapeutic efficacy of which is often severely limited by toxicity. Therefore, we believe that there is a high probability that TTFields may prove to be an effective and safe therapeutic modality to a large number of human cancers.
Materials and Methods
Cell Cultures.
Cell cultures were grown in DMEM plus 10% FCS media in a CO2 incubator (5% CO2) at 37°C. Cell suspension (200 μl; total 20 × 103 cells) were placed as a drop in the centre of 35-mm Petri dishes, incubated for 24 h and then the cell number was estimated by using standard XTT method (Cell proliferation assay Kit, Biological Industries Ltd., Israel) and expressed as OD0. Temperature was measured by a thermocouple (Omega, Stamford, CT) placed at the center of the dish. Two pairs of electrodes, insulated by a high dielectric constant ceramic [lead magnesium niobate–lead titanate (PMN-PT)], positioned in the petri dish perpendicular to each other were connected to a sinusoid function generator and amplifier. Two-directional fields were generated sequentially (1) by switching the output of the amplifier between two pairs of electrodes every 0.25–1 sec. The electric field intensity in the culture medium was measured as described in ref. 1.
At the end of 24 h of treatment, the cell number was measured by using the XTT method and expressed as OD1. The rate of cell proliferation was expressed as the OD1/OD0 ratio.
Animal Models.
Tumor inoculation and in vivo size assessment.
Animal experiments were conducted after approval by the Technion–Israel Institute of Technology committee for the care of laboratory animals. Intracranial glioma (F-98) was inoculated stereotactically into the subcortical white matter in the right hemisphere of Fischer rats (Harlan laboratories, Israel) by using a modification of the method described in refs. 30 and 31. Briefly, a hole, 1 mm in diameter, was punched through the scalp, 2 mm to the right of the midline and 4 mm rostral to the line connecting the external ear canals. A 0.5 mm burr hole was drilled in the bone at same location and a 26G needle was inserted to a depth of 7 mm beneath the scalp surface. Five microliters of saline containing 2.5 × 105 F-98 cells was then injected by using a microsyringe operated by a micromanipulator. The needle was left in position for 60 sec and then retracted slowly at a rate of 2 mm/min. Rats were allowed to recuperate for 24 h before treatment initiation. Tumor volume was assessed based on serial (2-mm interval) T1 weighted axial MRI images (0.5 Tesla MRI; Gyrex orbital coil; Elscint, Haifa, Israel) obtained 10 min following injection of 0.7 ml of Gadolinium (Magnetol; Soreq Radiopharmaceuticals, Yavne, Israel) into the tail vein. Tumor volume was assessed by calculating the area in square milimeters of the contrast enhanced lesion in each section. In view of the small size of the head of the rat, only three electrodes could be positioned on it, generating one to three different field directions.
Computation of the distribution of electric fields generated by external insulated electrodes.
The distributions of the alternating electric field generated by external electrodes within the brains of rats were estimated by using FEM simulations. These field distributions are determined by the geometry and electrical properties of the electrodes and tissues. On average, the capacitance of each electrode is 8 nF. This translates into an impedance of 190 and 95 Ω at 100 and 200 kHz, respectively. Because the impedance of the rat head is on the order of 400 Ω, when applying 42 V, 200 kHz TTFields to rats, 14-V drop on the insulation of both electrodes and the remaining 28 V on the rat itself. The fields generated in the areas of interest are in the range of 1–2 V/cm. The calculated field distribution for the rat head is given in Fig. 3A.
Human GBM Trial.
GBM patient eligibility and characteristics.
Twelve patients, suffering from the brain tumor GBM were enrolled to the study. Patients eligible for enrollment had recurrence based on Macdonald criteria (32), were >18 years old, had histologically established GBM (World Health Organization grade IV), had a Karnofsky performance scale ≥ 70, and were at least 4 weeks from any brain surgery and at least 8 weeks from radiotherapy. Patients could be at any recurrence and may have received other salvage therapies before enrollment. All patients had received adjuvant Temozolomide for their primary tumor. No concomitant chemotherapy was allowed. Multifocal disease was allowed. Patients with significant comorbidities, infratentorial tumors, implanted pacemakers or documented clinically significant arrhythmias, were excluded from the trial. During review of the histology from postprogression debulking surgery, one patient was excluded from efficacy analysis because of failure to meet histological criteria for grade IV glioma. An additional patient dropped out of the trial immediately following the baseline visit because of withdrawal of consent. Individual patient characteristics are listed in SI Table 1.
The clinical trial.
A single arm, pilot trial of the safety and efficacy of TTField treatment was performed in 10 patients with recurrent GBM. Written informed consent was obtained from each subject. The trial was performed after approval by the Na Homolce Institutional Review Board and the Czech Ministry of Health. Efficacy analysis was performed for 10 recurrent GBM patients by comparing TTP, PFS6, and OS in recurrent GBM patients treated with the NovoTTF-100A device with the TTP, PFS6, and OS of recurrent GBM patients in a literature based historical control group (10, 25–28). No statistical hypothesis testing was planned because of the small sample size. Ninety-five percent confidence intervals of survival proportions were calculated from Kaplan–Meier survival curves, by using standard formulae (33).
Measurement and simulation of TTFields intensity within the human brain.
To plan the TTFields intensity necessary to treat patients with intracranial tumors, we performed FEM simulations of the intensity distribution of TTFields within a three-dimensional model of the human head. Field intensity was slightly higher in the cortex than in the center of the brain (by ≈30%), but effective (1–2 V/cm) TTFields could be generated at the center of the brain by applying ≈50 V to surface electrodes placed on the scalp. To validate these findings, TTFields intensity was measured within the brain of a volunteer undergoing surgery because of obstructive hydrocephalus because of a huge meningioma of the pineal region. The study was performed according to an experimental protocol approved by the Rambam Medical Center ethics committee. The measured TTFields intensity was accurate within 10% of the FEM simulated values.
TTFields treatment of GBM patients.
TTFields were applied to recurrent GBM patients by using the NovoTTF-100A device (NovoCure Ltd., Haifa, Israel). This portable battery-operated device generates TTFields in GBM patients by means of insulated electrodes placed on their shaved scalps. The area of each insulated electrode array used was 22.5 cm2. Fields of 1–2 V/cm were generated by controlling the current density through the electrodes <31 mA/cm2 RMS, approximately one-third of the level that is generally recognized to present a risk of skin injury (100 mA/cm2) (34). In addition, the maximal power density beneath the electrodes was kept beneath 0.22 W/cm2, i.e., below the level associated with thermal skin injury (35). Electrode temperature was monitored and the power was lowered automatically when the temperature of any electrode exceeded 41°C. This value is well below the threshold of 44°C, i.e., the lowest prolonged temperature that can cause thermal injury (34).
TTFields having the optimal frequency of 200 kHz for rat and human gliomas (see Fig. 2) and an intensity of 1–2 V/cm (peak) were used in the trial. TTFields were switched sequentially every 1 sec between two perpendicular directions; lateral and anterior–posterior, through two sets of insulated electrode pairs. Patients received treatment continuously until disease progression or for a maximum of 18 months. Treatment was applied daily for an average of 18 h per day.
Patient evaluation.
Objective tumor assessment was performed by Gd-enhanced MRI according to a strictly defined protocol. MRI scanning was performed at trial entry within one week of NovoTTF-100A treatment initiation and after every treatment course (28–30 days). All scans were reviewed by a board certified radiologist (J.V.). The assessment of tumor response was based on criteria defined by Macdonald et al. (32). Study visits were performed once per week during the first month of treatment and monthly thereafter. The following examinations were carried out at each visit: Neurological evaluation, EKG, complete blood count with differential, chemistry panel, and coagulation studies. Adverse events occurring during treatment or up to 60 days after termination of therapy were scored according to the common toxicity criteria scale (version 3). Disease progression was not captured as a serious adverse event.
Supplementary Material
Acknowledgments
This work was supported by NovoCure Ltd.
Abbreviations
- FEM
finite element mesh
- GBM
glioblastoma
- OS
overall survival
- PFS6
progression-free survival at 6 months
- TTFields
tumor treating fields
- TTP
time to disease progression.
Footnotes
Conflict of interest statement: Y.P. has a minority holding in NovoCure Ltd. and is a member of the company board of directors; E.D.K., A.I., D.M., S.S.-S., Z.G., R.S., and Y.W. are employed in full or part by NovoCure Ltd.; and M.S. is a clinical trial consultant to NovoCure Ltd.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702916104/DC1.
Kirson, E. D., Dbalý, V., Rochlitz, C., Tovaryš, F., Salzberg, M., Palti, Y., AACR Meeting Abstracts, April 5, 2006, Washington, DC, Abstract 5259.
Dbalý, V., Kirson, E. D., Palti, Y., Gutin, P.H., Congress of Neurological Surgeons, October 13, 2005, Boston, MA (abstr.).
Gutin, P., Kirson, E., Palti, Y., Dbalý, V., International Brain Tumor Research and Therapy Meeting, April 26, 2006, Napa Valley, CA (abstr.).
References
- 1.Cole KS. Membranes, Ions and Impulses: A Chapter of Clasical Biophysics. Berkeley: Univ of Calif Press; 1968. [Google Scholar]
- 2.Keller FJJ, Gettys WE, Skove MJ. Physics. New York: McGraw–Hill; 1993. [Google Scholar]
- 3.Clague DS, Wheeler EK. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64 doi: 10.1103/PhysRevE.64.026605. 026605. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez CF, Remcho VT. J Chromatogr A. 2005;1079:59–68. doi: 10.1016/j.chroma.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 5.Polk C, Postow E. Biological Effects of Electromagnetic Fields Handbooks, Manuals, Etc. Boca Raton, FL: CRC; 1995. p. 618. [Google Scholar]
- 6.Goater AD, Pethig R. Parasitology. 1998;117:S177–S189. doi: 10.1017/s0031182099004114. [DOI] [PubMed] [Google Scholar]
- 7.Sowers AE. J Cell Biol. 1984;99:1989–1996. doi: 10.1083/jcb.99.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takashima S, Schwan HP. Biophys J. 1985;47:513–518. doi: 10.1016/S0006-3495(85)83945-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y. Cancer Res. 2004;64:3288–3295. doi: 10.1158/0008-5472.can-04-0083. [DOI] [PubMed] [Google Scholar]
- 10.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 11.DeVita VT, Rosenberg SA, Hellman S. Cancer, Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 12.Kaplan EL, Meier P. J Am Stat Assoc. 1958:457–481. [Google Scholar]
- 13.Polk C. In: The Biomedical Engineering Handbook. Bronzino JD, editor. Hartford, CT: CRC; 1995. pp. 1404–1416. [Google Scholar]
- 14.Basset CA. Clin Plast Surg. 1985;12:259–277. [PubMed] [Google Scholar]
- 15.Elson E. In: The Biomedical Engineering Handbook. Bronzino JD, editor. Hartford, CT: CRC; 1995. pp. 1417–1423. [Google Scholar]
- 16.Chou CK. In: The Biomedical Engineering Handbook. Bronzino JD, editor. Hartford, CT: CRC; 1995. pp. 1424–1430. [Google Scholar]
- 17.Maier H. Biophys J. 1997;73:1617–1626. doi: 10.1016/S0006-3495(97)78193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster JG, Clark JW. Medical Instrumentation: Application and Design. New York: Wiley; 1998. [Google Scholar]
- 19.Burnette RR, Ongpipattanakul B. J Pharm Sci. 1988;77:132–137. doi: 10.1002/jps.2600770208. [DOI] [PubMed] [Google Scholar]
- 20.Cho MR, Thatte HS, Silvia MT, Golan DE. FASEB J. 1999;13:677–683. doi: 10.1096/fasebj.13.6.677. [DOI] [PubMed] [Google Scholar]
- 21.Orrenius S, McCabe MJ, Jr, Nicotera P. Toxicol Lett. 1992:64–65. doi: 10.1016/0378-4274(92)90208-2. Spec no:357–364. [DOI] [PubMed] [Google Scholar]
- 22.Palti Y. Bull Res Counc Isr Sect E Exp Med. 1962;10:54–56. [PubMed] [Google Scholar]
- 23.Bronzino JD. The Biomedical Engineering Handbook. Boca Raton, FL: CRC, IEEE Press; 1995. [Google Scholar]
- 24.Ross MH, Kaye GI, Pawlina W. Histology: a Text and Atlas. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 25.Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, et al. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, et al. Ann Oncol. 2001;12:259–266. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- 27.Chang SM, Theodosopoulos P, Lamborn K, Malec M, Rabbitt J, Page M, Prados MD. Cancer. 2004;100:605–611. doi: 10.1002/cncr.11949. [DOI] [PubMed] [Google Scholar]
- 28.Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon RE, et al. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 29.Ancona A, Arevalo A, Macotela E. Dermatol Clin. 1990;8:95–105. [PubMed] [Google Scholar]
- 30.Langen KJ, Clauss RP, Holschbach M, Muhlensiepen H, Kiwit JC, Zilles K, Coenen HH, Muller-Gartner HW. J Nucl Med. 1998;39:1596–1599. [PubMed] [Google Scholar]
- 31.Saini M, Bellinzona M, Meyer F, Cali G, Samii M. J Neurooncol. 1999;42:59–67. doi: 10.1023/a:1006128825766. [DOI] [PubMed] [Google Scholar]
- 32.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 33.Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1999. [Google Scholar]
- 34.Moritz AR, Henriques FCJ. Am J Pathol. 1947;23:695–720. [PMC free article] [PubMed] [Google Scholar]
- 35.Becker CM, Malhotra IV, Hedley-Whyte J. Anesthesiology. 1973;38:106–122. doi: 10.1097/00000542-197302000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.