Abstract
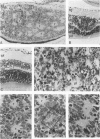
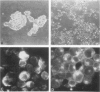
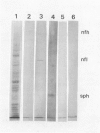
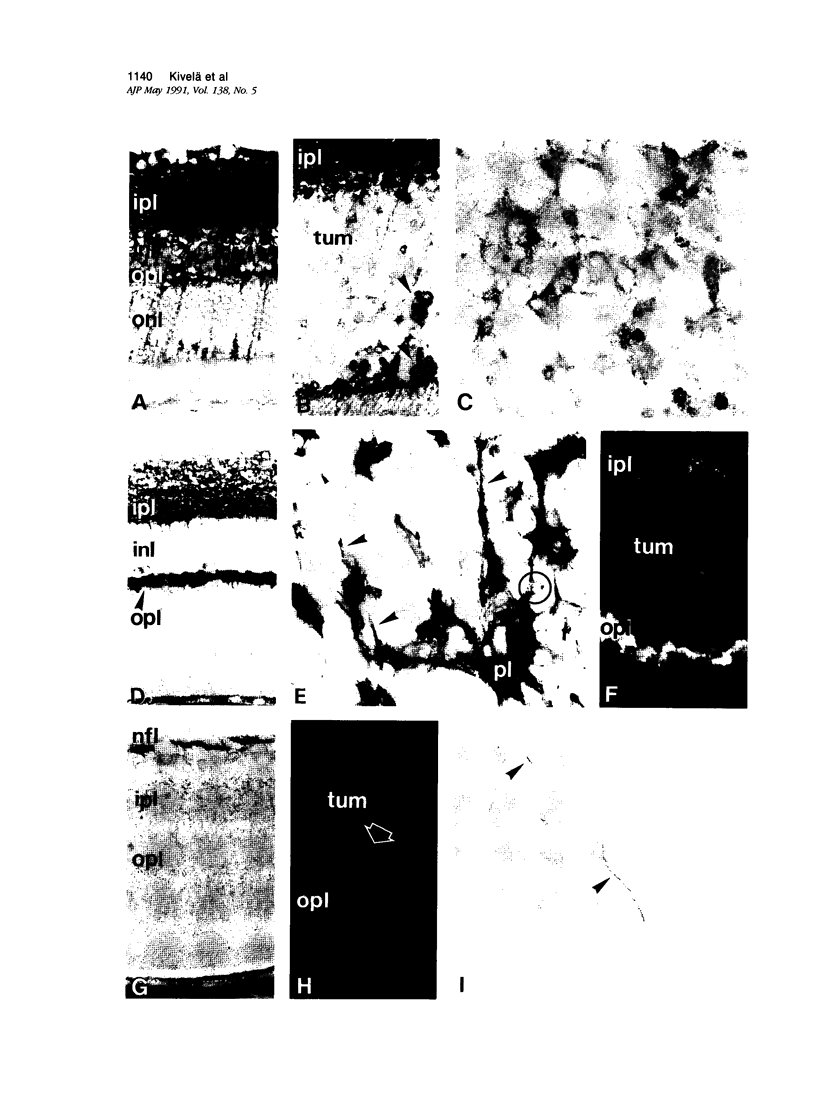
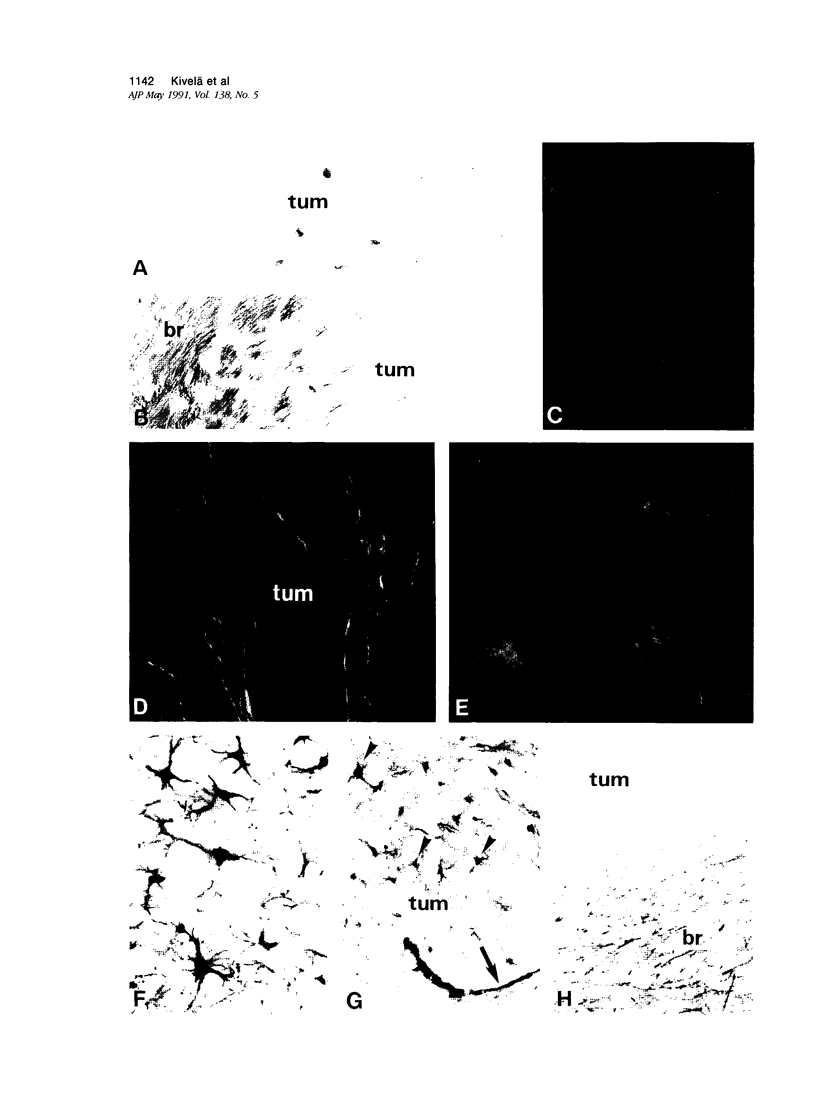
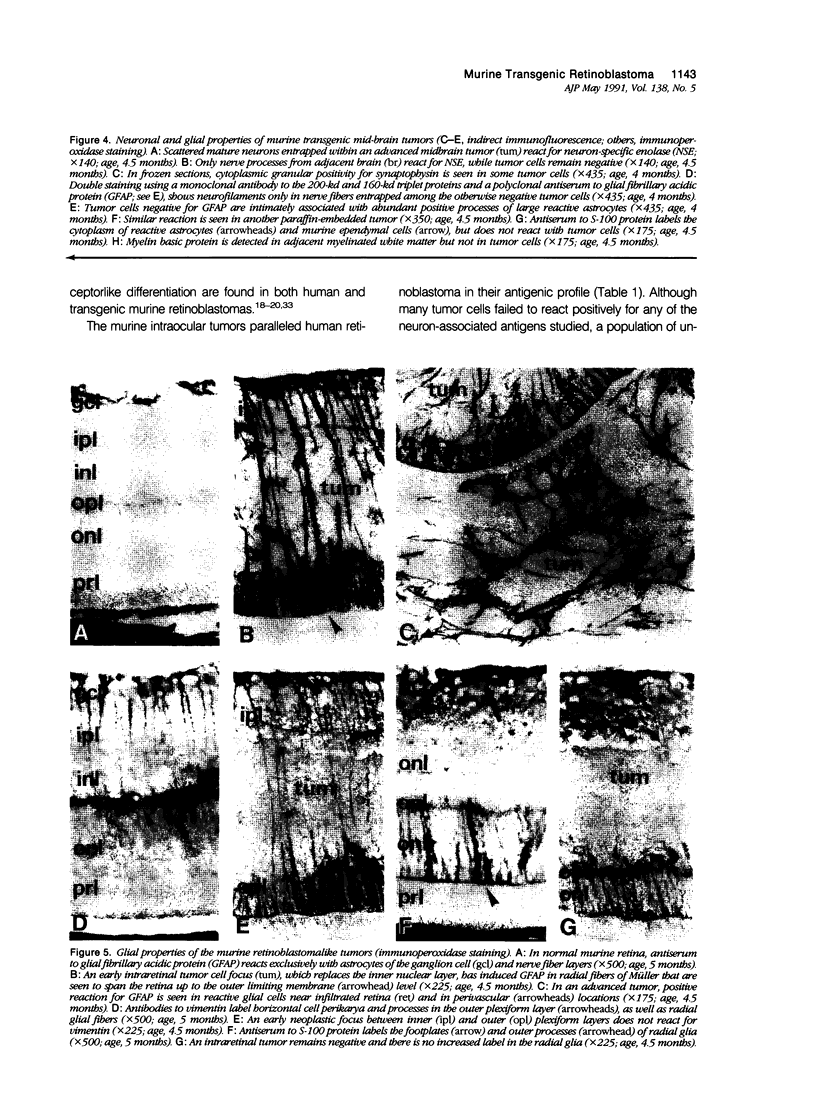
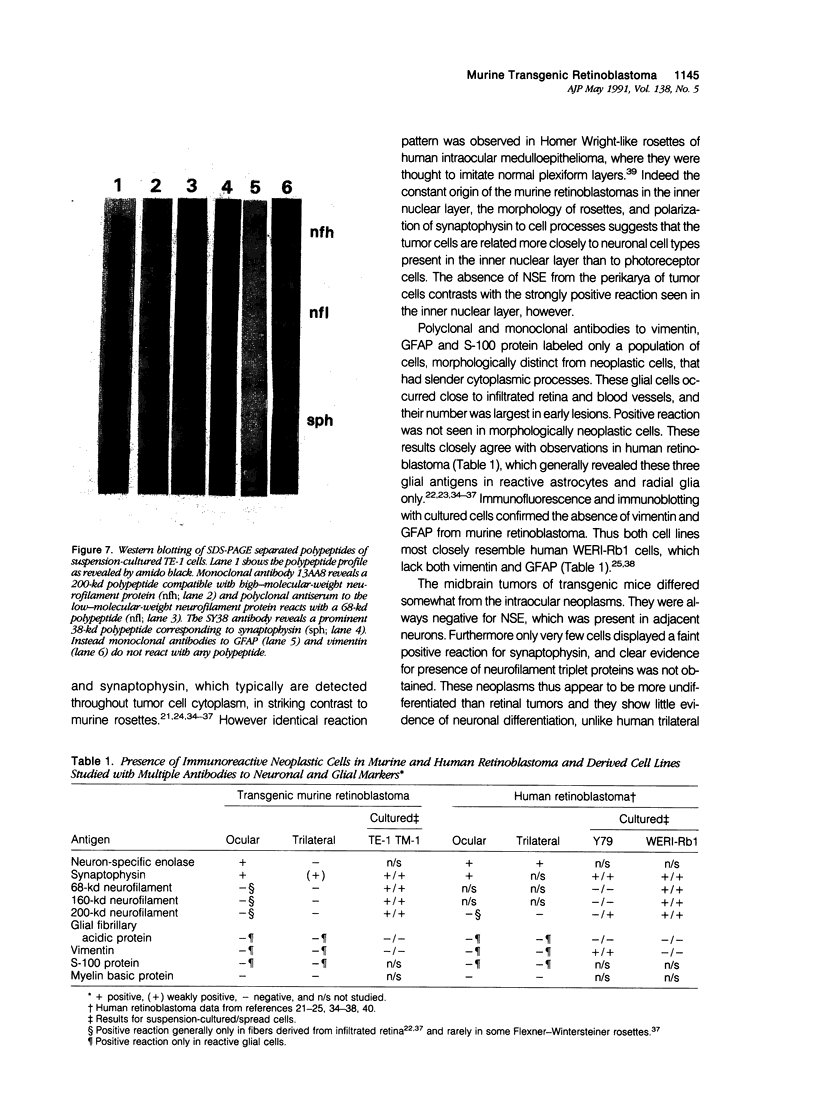
Antigenic properties of a murine transgenic model for hereditary retinoblastoma, induced by a chimeric gene coding for Simian virus 40 large T antigen, an oncogene that inactivates the retinoblastoma susceptibility gene product, were studied by immunohistochemistry. All transgenic mice develop bilateral intraocular retinal tumors in the inner nuclear layer with Homer Wright-like rosettes, and one quarter develop midbrain tumors resembling trilateral retinoblastoma. Cell lines TE-1 and TM-1 were established from intraocular and metastatic tumors, respectively. Intraocular tumors reacted with antibodies to neuron-specific enolase and synaptophysin, while vimentin, glial fibrillary acidic, and S-100 proteins were detected only in reactive glia derived from adjacent retina. The midbrain tumors showed weak reactivity to synaptophysin, and they blended with reactive astrocytes positive for glial markers. The tumors were negative for cytokeratins. Finally both derived cell lines expressed synaptophysin and individual neurofilament triplet proteins in immunofluorescence and Western blotting, supporting their essentially neuronal nature. The antigenic profile resembles human retinoblastoma, but differences in morphology and antigen distribution suggest a more close relationship to neurons of the inner nuclear layer than to photoreceptor cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. L., Meadows A. T., Zimmerman L. E., Rorke L. B., Voute P. A., Champion L. A., Miller R. W. Bilateral retinoblastoma with ectopic intracranial retinoblastoma: trilateral retinoblastoma. Cancer Genet Cytogenet. 1982 Mar;5(3):203–213. doi: 10.1016/0165-4608(82)90026-7. [DOI] [PubMed] [Google Scholar]
- Bernards R., Shackleford G. M., Schackleford G. M., Gerber M. R., Horowitz J. M., Friend S. H., Schartl M., Bogenmann E., Rapaport J. M., McGee T. Structure and expression of the murine retinoblastoma gene and characterization of its encoded protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6474–6478. doi: 10.1073/pnas.86.17.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D. Immunohistochemical differences between neurofilaments in perikarya, dendrites and axons. Immunofluorescence study with antisera raised to neurofilament polypeptides (200K, 150K, 70K) isolated by anion exchange chromatography. Exp Cell Res. 1983 Dec;149(2):397–408. doi: 10.1016/0014-4827(83)90352-x. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Draper G. J., Sanders B. M., Kingston J. E. Second primary neoplasms in patients with retinoblastoma. Br J Cancer. 1986 May;53(5):661–671. doi: 10.1038/bjc.1986.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Winter S., von Overbeck J., Gudat F., Heitz P. U., Stähli C. Identification of the conserved, conformation-dependent cytokeratin epitope recognized by monoclonal antibody (lu-5). Virchows Arch A Pathol Anat Histopathol. 1987;411(2):137–147. doi: 10.1007/BF00712737. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Gallie B. L., Squire J. A., Goddard A., Dunn J. M., Canton M., Hinton D., Zhu X. P., Phillips R. A. Mechanism of oncogenesis in retinoblastoma. Lab Invest. 1990 Apr;62(4):394–408. [PubMed] [Google Scholar]
- Herman M. M., Perentes E., Katsetos C. D., Darcel F., Frankfurter A., Collins V. P., Donoso L. A., Eng L. F., Marangos P. J., Wiechmann A. F. Neuroblastic differentiation potential of the human retinoblastoma cell lines Y-79 and WERI-Rb1 maintained in an organ culture system. An immunohistochemical, electron microscopic, and biochemical study. Am J Pathol. 1989 Jan;134(1):115–132. [PMC free article] [PubMed] [Google Scholar]
- Kingston J. E., Plowman P. N., Hungerford J. L. Ectopic intracranial retinoblastoma in childhood. Br J Ophthalmol. 1985 Oct;69(10):742–748. doi: 10.1136/bjo.69.10.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelä T. Neuron-specific enolase in retinoblastoma. An immunohistochemical study. Acta Ophthalmol (Copenh) 1986 Feb;64(1):19–25. doi: 10.1111/j.1755-3768.1986.tb06866.x. [DOI] [PubMed] [Google Scholar]
- Kivelä T., Tarkkanen A. Recurrent medulloepithelioma of the ciliary body. Immunohistochemical characteristics. Ophthalmology. 1988 Nov;95(11):1565–1575. doi: 10.1016/s0161-6420(88)32972-6. [DOI] [PubMed] [Google Scholar]
- Kivelä T., Tarkkanen A. S-100 protein in retinoblastoma revisited. An immunohistochemical study. Acta Ophthalmol (Copenh) 1986 Dec;64(6):664–673. doi: 10.1111/j.1755-3768.1986.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Kivelä T., Tarkkanen A., Virtanen I. Intermediate filaments in the human retina and retinoblastoma. An immunohistochemical study of vimentin, glial fibrillary acidic protein, and neurofilaments. Invest Ophthalmol Vis Sci. 1986 Jul;27(7):1075–1084. [PubMed] [Google Scholar]
- Kivelä T., Tarkkanen A., Virtanen I. Synaptophysin in the human retina and retinoblastoma. An immunohistochemical and Western blotting study. Invest Ophthalmol Vis Sci. 1989 Feb;30(2):212–219. [PubMed] [Google Scholar]
- Kobayashi M., Mukai N., Solish S. P., Pomeroy M. E. A highly predictable animal model of retinoblastoma. Acta Neuropathol. 1982;57(2-3):203–208. doi: 10.1007/BF00685390. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Mukai N. Retinoblastoma-like tumors induced by human adenovirus type 12 in rats. Cancer Res. 1974 Jul;34(7):1646–1651. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- Messmer E. P., Font R. L., Kirkpatrick J. B., Höpping W. Immunohistochemical demonstration of neuronal and astrocytic differentiation in retinoblastoma. Ophthalmology. 1985 Jan;92(1):167–173. doi: 10.1016/s0161-6420(85)34076-9. [DOI] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Mukai N., Nakajima T., Freddo T., Jacobson M., Dunn M. Retinoblastoma-like neoplasm induced in C3H/BifB/Ki strain mice by human adenovirus serotype 12. Acta Neuropathol. 1977 Aug 16;39(2):147–155. doi: 10.1007/BF00703321. [DOI] [PubMed] [Google Scholar]
- O'Brien J. M., Marcus D. M., Niffenegger A. S., Bernards R., Carpenter J. L., Windle J. J., Mellon P., Albert D. M. Trilateral retinoblastoma in transgenic mice. Trans Am Ophthalmol Soc. 1989;87:301–326. [PMC free article] [PubMed] [Google Scholar]
- Perentes E., Herbort C. P., Rubinstein L. J., Herman M. M., Uffer S., Donoso L. A., Collins V. P. Immunohistochemical characterization of human retinoblastomas in situ with multiple markers. Am J Ophthalmol. 1987 May 15;103(5):647–658. doi: 10.1016/s0002-9394(14)74324-7. [DOI] [PubMed] [Google Scholar]
- Pesin S. R., Shields J. A. Seven cases of trilateral retinoblastoma. Am J Ophthalmol. 1989 Feb 15;107(2):121–126. doi: 10.1016/0002-9394(89)90209-2. [DOI] [PubMed] [Google Scholar]
- Roarty J. D., McLean I. W., Zimmerman L. E. Incidence of second neoplasms in patients with bilateral retinoblastoma. Ophthalmology. 1988 Nov;95(11):1583–1587. doi: 10.1016/s0161-6420(88)32971-4. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Bardenstein D. S., Donoso L. A., Rajagopalan S., Brownstein S. An immunohistopathologic study of trilateral retinoblastoma. Am J Ophthalmol. 1987 Jun 15;103(6):776–781. doi: 10.1016/s0002-9394(14)74393-4. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Wiggert B., Shields J., Donoso L., Bardenstein D., Katz N., Friendly D., Chader G. Retinoblastoma. Immunohistochemistry and cell differentiation. Ophthalmology. 1987 Apr;94(4):378–387. doi: 10.1016/s0161-6420(87)33448-7. [DOI] [PubMed] [Google Scholar]
- Tarkkanen A., Tuovinen E. Retinoblastoma in Finland 1912-1964. Acta Ophthalmol (Copenh) 1971;49(2):293–300. doi: 10.1111/j.1755-3768.1971.tb00953.x. [DOI] [PubMed] [Google Scholar]
- Terenghi G., Polak J. M., Ballesta J., Cocchia D., Michetti F., Dahl D., Marangos P. J., Garner A. Immunocytochemistry of neuronal and glial markers in retinoblastoma. Virchows Arch A Pathol Anat Histopathol. 1984;404(1):61–73. doi: 10.1007/BF00704251. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso M. O. Clues to the cells of origin in retinoblastoma. Int Ophthalmol Clin. 1980 Summer;20(2):191–210. [PubMed] [Google Scholar]
- Virtanen I., Kallajoki M., Närvänen O., Paranko J., Thornell L. E., Miettinen M., Lehto V. P. Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec. 1986 May;215(1):10–20. doi: 10.1002/ar.1092150103. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Kivelä T., Bugnoli M., Mencarelli C., Pallini V., Albert D. M., Tarkkanen A. Expression of intermediate filaments and synaptophysin show neuronal properties and lack of glial characteristics in Y79 retinoblastoma cells. Lab Invest. 1988 Nov;59(5):649–656. [PubMed] [Google Scholar]
- Virtanen I., Miettinen M., Lehto V. P., Kariniemi A. L., Paasivuo R. Diagnostic application of monoclonal antibodies to intermediate filaments. Ann N Y Acad Sci. 1985;455:635–648. doi: 10.1111/j.1749-6632.1985.tb50441.x. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Windle J. J., Albert D. M., O'Brien J. M., Marcus D. M., Disteche C. M., Bernards R., Mellon P. L. Retinoblastoma in transgenic mice. Nature. 1990 Feb 15;343(6259):665–669. doi: 10.1038/343665a0. [DOI] [PubMed] [Google Scholar]
- Winther J., Jensen O. A., Prause J. U., Tommerup N. Characterization of an intraocular retinoblastoma-like tumour. Acta Ophthalmol (Copenh) 1987 Aug;65(4):491–502. doi: 10.1111/j.1755-3768.1987.tb07029.x. [DOI] [PubMed] [Google Scholar]