Abstract
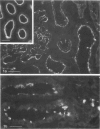
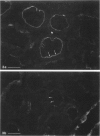
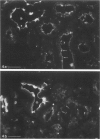
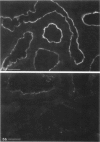
In addition to the glomerular lesions associated with Heymann nephritis, a rat model of human membranous nephritis, proximal tubule damage, and a perturbation of proximal tubule function also have been reported to occur in this disease. The aim of the present study was to examine in more detail the nature of the apical plasma membrane damage in proximal tubules using specific antibodies directed against clathrin, gp330, and a proton-pumping adenosine triphosphatase, all of which are components of the apical endocytotic apparatus of these epithelial cells. Immunocytochemical studies revealed a marked reduction in staining for all three antigens in proximal tubules from rats with active Heymann nephritis. Furthermore endocytotic uptake of intravenously injected FITC-dextran was considerably lower in diseased animals than in normal rats. Gp330 and rat IgG were identified as components of the luminal debris that accumulated during the course of Heymann nephritis. These results show that perturbation of proximal tubule endocytosis occurs in Heymann nephritis together with a loss of three apical antigens that are normally localized on membrane domains associated with the apical endocytotic pathway in these cells. The results also suggest that antibody-antigen complexes may be shed from the plasma membrane in both the glomerulus and the proximal tubule in this disease.
Full text
PDF


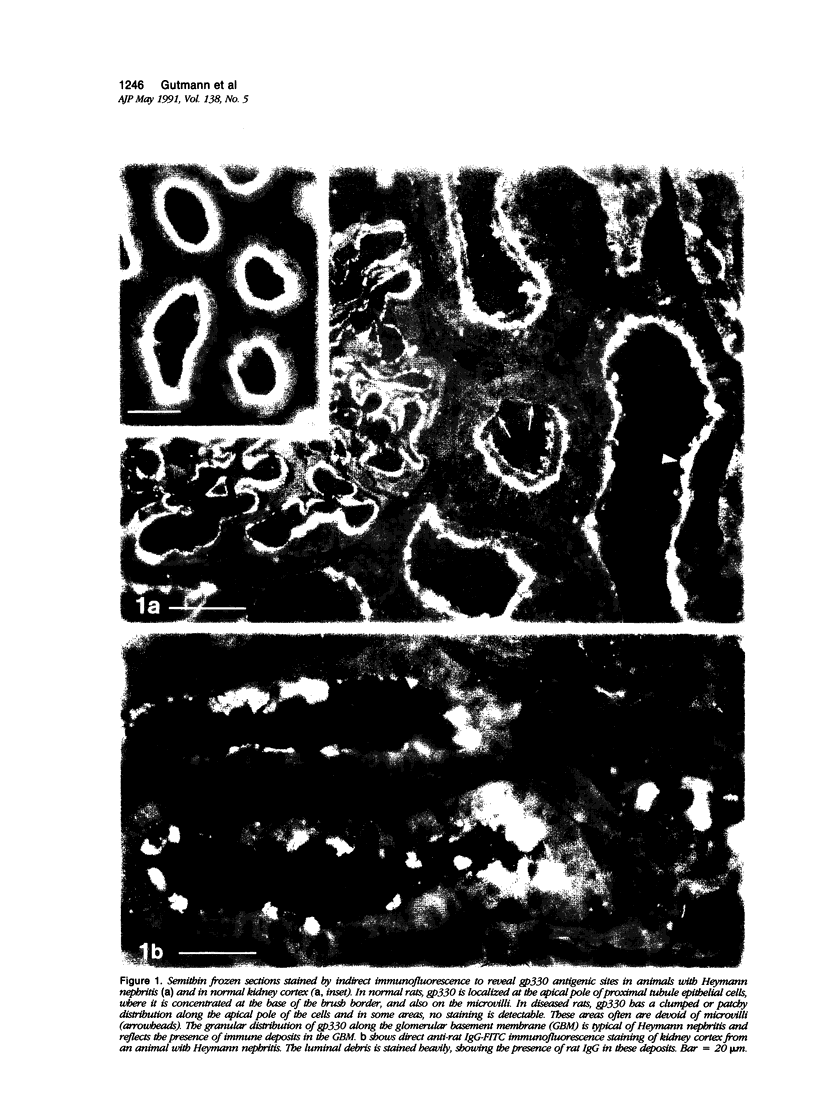
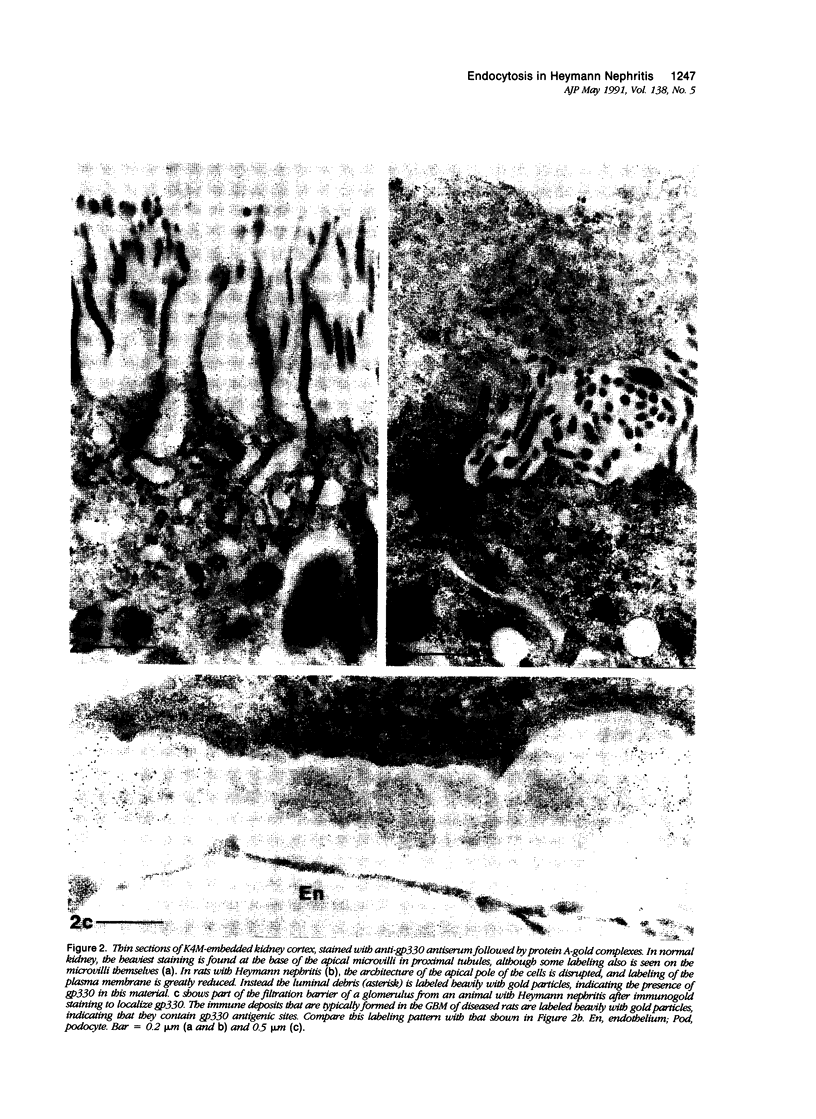

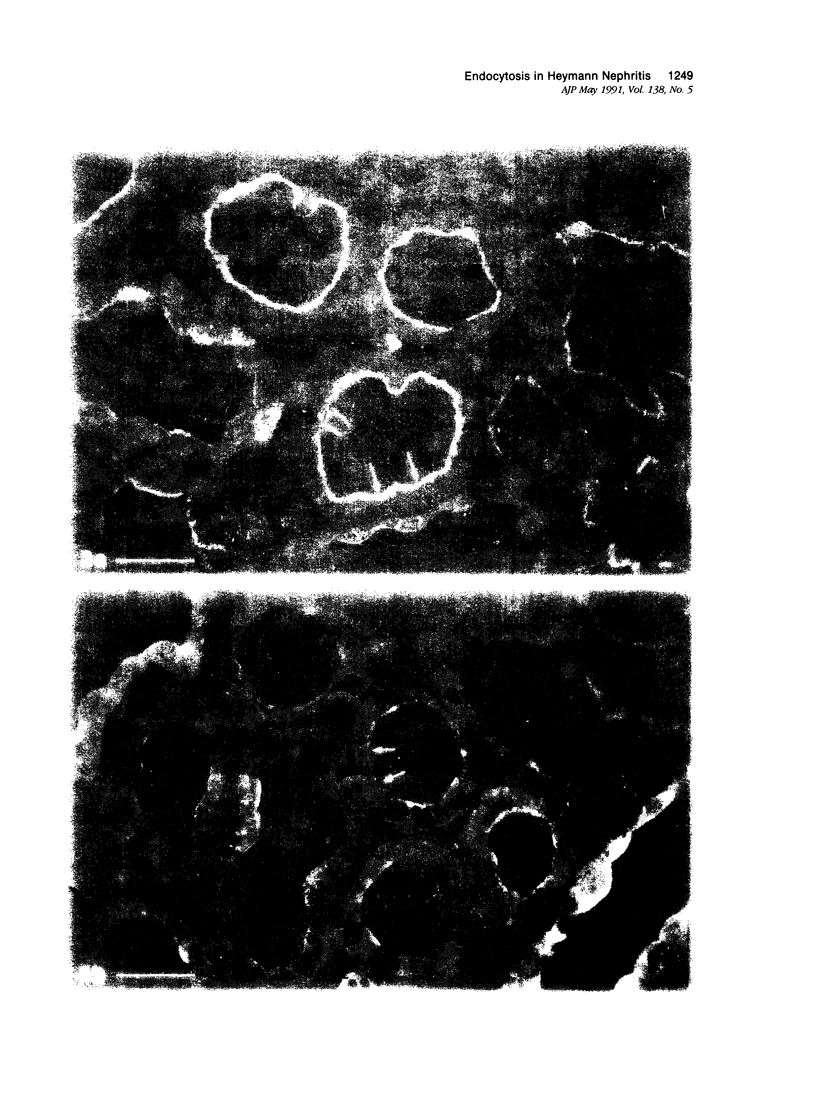

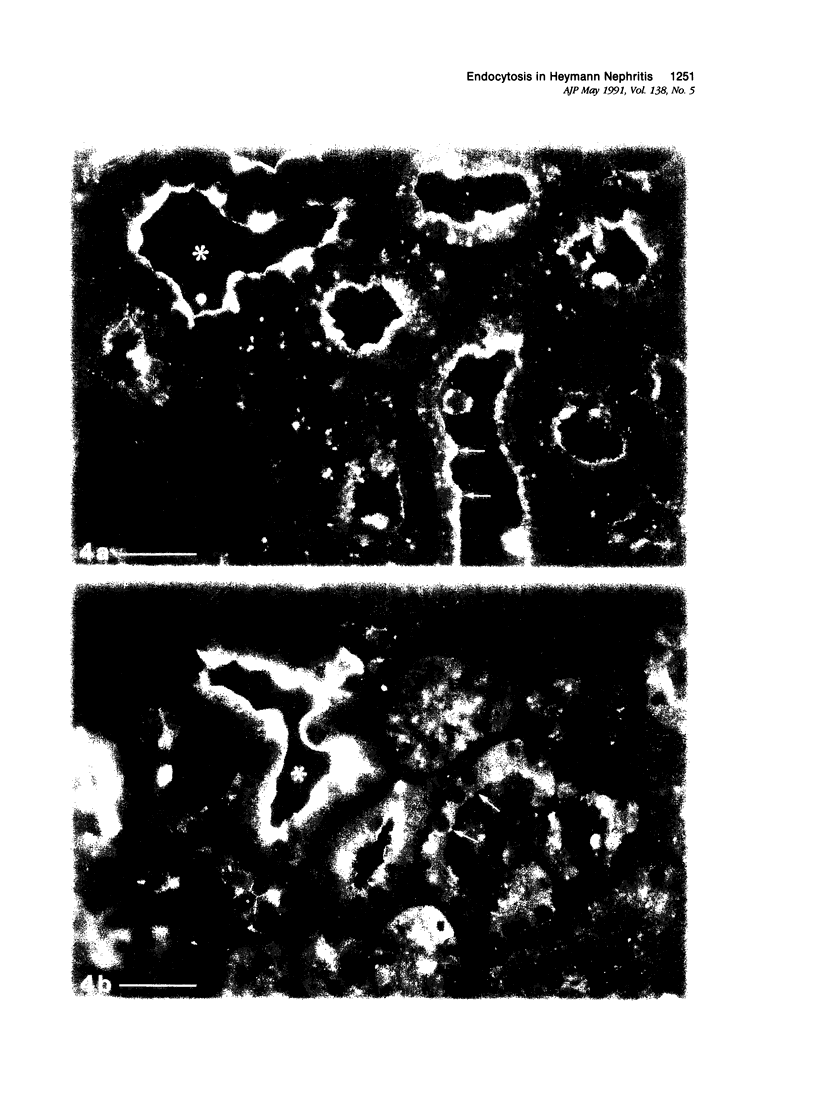



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. G., Schneider B. G., Papermaster D. S. Rapid embedding of tissues in Lowicryl K4M for immunoelectron microscopy. J Histochem Cytochem. 1984 Nov;32(11):1217–1223. doi: 10.1177/32.11.6436366. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Baird L. G., Collins A. B., Kamata K., Bradford D., Erikson M. E., McCluskey R. T. Studies with monoclonal antibodies against brush border antigens in Heymann nephritis. Lab Invest. 1985 Oct;53(4):421–432. [PubMed] [Google Scholar]
- Brentjens J. R., Andres G. Interaction of antibodies with renal cell surface antigens. Kidney Int. 1989 Apr;35(4):954–968. doi: 10.1038/ki.1989.79. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Galloway C. J., Blank G. S., Jackson A. P., Seow H. F., Drickamer K., Parham P. Localization of clathrin light-chain sequences mediating heavy-chain binding and coated vesicle diversity. Nature. 1987 Mar 12;326(6109):203–205. doi: 10.1038/326203a0. [DOI] [PubMed] [Google Scholar]
- Brown D., Gluck S., Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol. 1987 Oct;105(4):1637–1648. doi: 10.1083/jcb.105.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest. 1988 Dec;82(6):2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Orci L. The "coat" of kidney intercalated cell tubulovesicles does not contain clathrin. Am J Physiol. 1986 Apr;250(4 Pt 1):C605–C608. doi: 10.1152/ajpcell.1986.250.4.C605. [DOI] [PubMed] [Google Scholar]
- Brown D., Weyer P., Orci L. Nonclathrin-coated vesicles are involved in endocytosis in kidney collecting duct intercalated cells. Anat Rec. 1987 Jul;218(3):237–242. doi: 10.1002/ar.1092180303. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Camussi G., Brentjens J. R., Noble B., Kerjaschki D., Malavasi F., Roholt O. A., Farquhar M. G., Andres G. Antibody-induced redistribution of Heymann antigen on the surface of cultured glomerular visceral epithelial cells: possible role in the pathogenesis of Heymann glomerulonephritis. J Immunol. 1985 Oct;135(4):2409–2416. [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Gluck S., Caldwell J. Immunoaffinity purification and characterization of vacuolar H+ATPase from bovine kidney. J Biol Chem. 1987 Nov 15;262(32):15780–15789. [PubMed] [Google Scholar]
- Gutmann E. J., Niles J. L., McCluskey R. T., Brown D. Colchicine-induced redistribution of an apical membrane glycoprotein (gp330) in proximal tubules. Am J Physiol. 1989 Aug;257(2 Pt 1):C397–C407. doi: 10.1152/ajpcell.1989.257.2.C397. [DOI] [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Hori M. T., Abrass C. K. Isolation and characterization of circulating immune complexes from rats with experimental membranous nephropathy. J Immunol. 1990 May 15;144(10):3849–3855. [PubMed] [Google Scholar]
- Kamata K., Baird L. G., Erikson M. E., Collins A. B., McCluskey R. T. Characterization of antigens and antibody specificities involved in Heymann nephritis. J Immunol. 1985 Oct;135(4):2400–2408. [PubMed] [Google Scholar]
- Kanalas J. J., Makker S. P. A possible ligand of serum origin for the kidney autoantigen of Heymann nephritis. J Immunol. 1988 Dec 15;141(12):4152–4157. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa K., Gorfien S., Brentjens J. R., Andres G., Noble B. Reabsorption of horseradish peroxidase by proximal tubules in rats with Heymann nephritis. Am J Kidney Dis. 1986 Jan;7(1):58–68. doi: 10.1016/s0272-6386(86)80057-9. [DOI] [PubMed] [Google Scholar]
- Klassen J., Sugisaki T., Milgrom F., McCluskey R. T. Studies on multiple renal lesions in Heymann nephritis. Lab Invest. 1971 Dec;25(6):577–585. [PubMed] [Google Scholar]
- Laguens R., Segal A. Experimental autologous immune-complex nephritis: an electron microscope and immunohistochemical study. Exp Mol Pathol. 1969 Aug;11(1):89–98. doi: 10.1016/0014-4800(69)90073-2. [DOI] [PubMed] [Google Scholar]
- Larkin J. M., Brown M. S., Goldstein J. L., Anderson R. G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983 May;33(1):273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Lencer W. I., Weyer P., Verkman A. S., Ausiello D. A., Brown D. FITC-dextran as a probe for endosome function and localization in kidney. Am J Physiol. 1990 Feb;258(2 Pt 1):C309–C317. doi: 10.1152/ajpcell.1990.258.2.C309. [DOI] [PubMed] [Google Scholar]
- Madsen K. M., Tisher C. C. Structural-functional relationships along the distal nephron. Am J Physiol. 1986 Jan;250(1 Pt 2):F1–15. doi: 10.1152/ajprenal.1986.250.1.F1. [DOI] [PubMed] [Google Scholar]
- Maxwell M. H. Two rapid and simple methods used for the removal of resins from 1.0 micron thick epoxy sections. J Microsc. 1978 Mar;112(2):253–255. doi: 10.1111/j.1365-2818.1978.tb01174.x. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mendrick D. L., Noble B., Brentjens J. R., Andres G. A. Antibody-mediated injury to proximal tubules in Heymann nephritis. Kidney Int. 1980 Sep;18(3):328–343. doi: 10.1038/ki.1980.143. [DOI] [PubMed] [Google Scholar]
- Natori Y., Hayakawa I., Shibata S. Identification of gp108, a pathogenic antigen of passive Heymann nephritis, as dipeptidyl peptidase IV. Clin Exp Immunol. 1987 Nov;70(2):434–439. [PMC free article] [PubMed] [Google Scholar]
- Noble B., Andres G. A., Brentjens J. R. Passively transferred anti-brush border antibodies induce injury of proximal tubules in the absence of complement. Clin Exp Immunol. 1984 May;56(2):281–288. [PMC free article] [PubMed] [Google Scholar]
- Noble B., Mendrick D. L., Brentjens J. R., Andres G. A. Antibody-mediated injury to proximal tubules in the rat kidney induced by passive transfer of homologous anti-brush border serum. Clin Immunol Immunopathol. 1981 May;19(2):289–301. doi: 10.1016/0090-1229(81)90071-4. [DOI] [PubMed] [Google Scholar]
- Pietromonaco S., Kerjaschki D., Binder S., Ullrich R., Farquhar M. G. Molecular cloning of a cDNA encoding a major pathogenic domain of the Heymann nephritis antigen gp330. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1811–1815. doi: 10.1073/pnas.87.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychowdhury R., Niles J. L., McCluskey R. T., Smith J. A. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989 Jun 9;244(4909):1163–1165. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- Rodman J. S., Kerjaschki D., Merisko E., Farquhar M. G. Presence of an extensive clathrin coat on the apical plasmalemma of the rat kidney proximal tubule cell. J Cell Biol. 1984 May;98(5):1630–1636. doi: 10.1083/jcb.98.5.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman J. S., Seidman L., Farquhar M. G. The membrane composition of coated pits, microvilli, endosomes, and lysosomes is distinctive in the rat kidney proximal tubule cell. J Cell Biol. 1986 Jan;102(1):77–87. doi: 10.1083/jcb.102.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salant D. J., Belok S., Madaio M. P., Couser W. G. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest. 1980 Dec;66(6):1339–1350. doi: 10.1172/JCI109987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest. 1985 May;75(5):1638–1644. doi: 10.1172/JCI111871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Zamlauski-Tucker M. J., Van Liew J. B., Noble B. Pathophysiology of the kidney in rats with Heymann nephritis. Kidney Int. 1985 Sep;28(3):504–512. doi: 10.1038/ki.1985.157. [DOI] [PubMed] [Google Scholar]