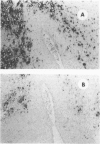
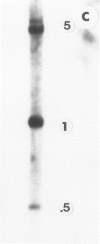
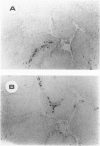
Abstract
More than 22 types of human papillomavirus (HPV) have been detected in genital tract squamous cell intraepithelial lesions. Seven of two hundred eighty-six (2.4%) genital tract tissues in which HPV DNA was detected by in situ hybridization contained two or more different HPV types. When analyzed by site, 5 of 204 (2.4%) of cervical intraepithelial lesions were infected by more than one type, compared with 2 of 82 (2.4%) of vulvar lesions. The rate for low-grade lesions was similar (5/218; 2.3%) to that for high-grade lesions (2/68; 2.9%). In contrast, two different HPV types were detected in 6/33 (18%) of tissues by the polymerase chain reaction (PCR) using type-specific primers for eight HPV types. It is concluded that infection by one HPV type is rarely associated with concurrent 'active' infection by a second HPV type, even though DNA of a different viral type can be detected by PCR in about one fifth of such cases. Further study is required to determine if an existing HPV infection can inhibit replication by a different HPV type.
Full text
PDF

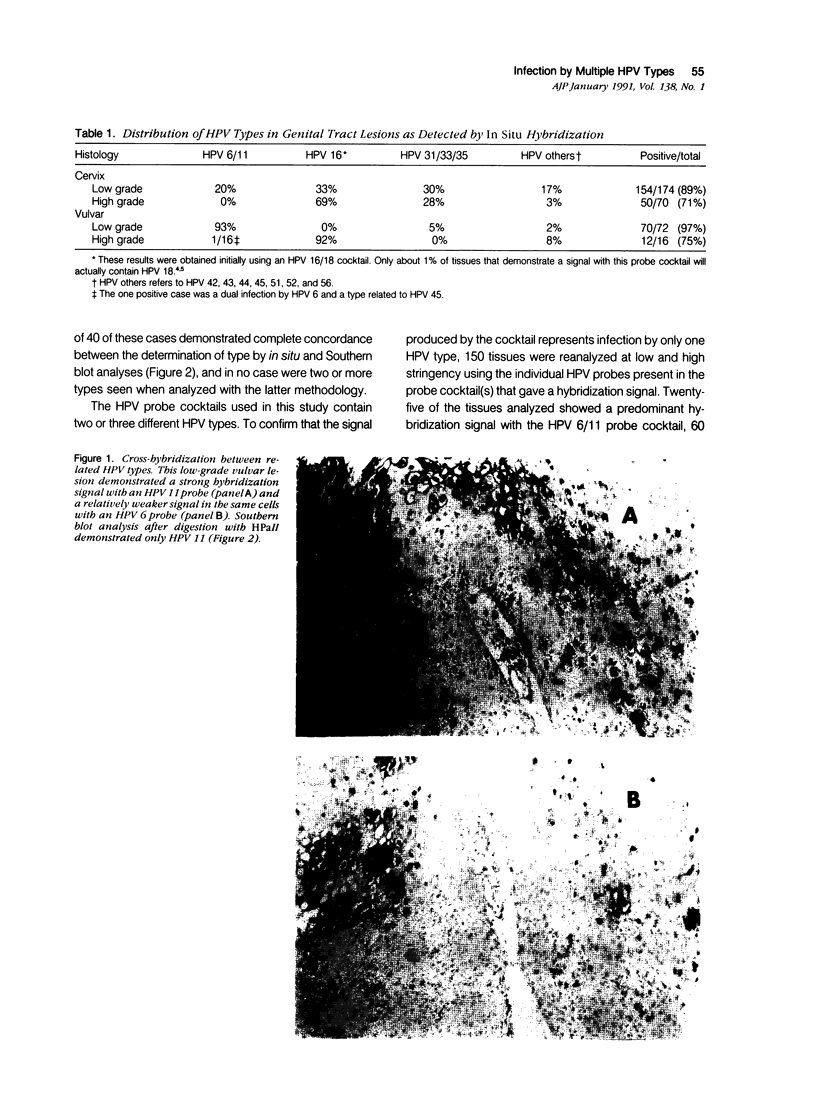



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron C., Ferenczy A., Shah K. V., Naghashfar Z. Multicentric human papillomavirus infections of the female genital tract: correlation of viral types with abnormal mitotic figures, colposcopic presentation, and location. Obstet Gynecol. 1987 May;69(5):736–742. [PubMed] [Google Scholar]
- Branch A. D., Benenfeld B. J., Franck E. R., Shaw J. F., Varban M. L., Willis K. K., Rosen D. L., Robertson H. D. Interference between coinoculated viroids. Virology. 1988 Apr;163(2):538–546. doi: 10.1016/0042-6822(88)90294-2. [DOI] [PubMed] [Google Scholar]
- Buscema J., Naghashfar Z., Sawada E., Daniel R., Woodruff J. D., Shah K. The predominance of human papillomavirus type 16 in vulvar neoplasia. Obstet Gynecol. 1988 Apr;71(4):601–606. [PubMed] [Google Scholar]
- Crum C. P., Mitao M., Levine R. U., Silverstein S. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol. 1985 Jun;54(3):675–681. doi: 10.1128/jvi.54.3.675-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A. T., Temple G. F., Kurman R. J., Jenson A. B., Lancaster W. D. Oncogenic association of specific human papillomavirus types with cervical neoplasia. J Natl Cancer Inst. 1987 Oct;79(4):671–677. [PubMed] [Google Scholar]
- Nuovo G. J., Friedman D., Richart R. M. In situ hybridization analysis of human papillomavirus DNA segregation patterns in lesions of the female genital tract. Gynecol Oncol. 1990 Feb;36(2):256–262. doi: 10.1016/0090-8258(90)90184-m. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J. Human papillomavirus DNA in genital tract lesions histologically negative for condylomata. Analysis by in situ, Southern blot hybridization and the polymerase chain reaction. Am J Surg Pathol. 1990 Jul;14(7):643–651. doi: 10.1097/00000478-199007000-00005. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Richart R. M. A comparison of biotin- and 35S-based in situ hybridization methodologies for detection of human papillomavirus DNA. Lab Invest. 1989 Oct;61(4):471–476. [PubMed] [Google Scholar]
- Nuovo G. J., Richart R. M. A comparison of slot blot, southern blot, and in situ hybridization analyses for human papillomavirus DNA in genital tract lesions. Obstet Gynecol. 1989 Oct;74(4):673–678. [PubMed] [Google Scholar]
- Nuovo G., Crum C. P., Silverstein S. Papillomavirus infection of the uterine cervix. Microb Pathog. 1987 Aug;3(2):71–78. doi: 10.1016/0882-4010(87)90065-9. [DOI] [PubMed] [Google Scholar]
- Reid R., Greenberg M., Jenson A. B., Husain M., Willett J., Daoud Y., Temple G., Stanhope C. R., Sherman A. I., Phibbs G. D. Sexually transmitted papillomaviral infections. I. The anatomic distribution and pathologic grade of neoplastic lesions associated with different viral types. Am J Obstet Gynecol. 1987 Jan;156(1):212–222. doi: 10.1016/0002-9378(87)90241-9. [DOI] [PubMed] [Google Scholar]
- Schneider A., Kraus H., Schuhmann R., Gissmann L. Papillomavirus infection of the lower genital tract: detection of viral DNA in gynecological swabs. Int J Cancer. 1985 Apr 15;35(4):443–448. doi: 10.1002/ijc.2910350405. [DOI] [PubMed] [Google Scholar]
- Syrjänen K., Mäntyjärvi R., Väyrynen M., Syrjänen S., Parkkinen S., Yliskoski M., Saarikoski S., Castrén O. Human papillomavirus (HPV) infections involved in the neoplastic process of the uterine cervix as established by prospective follow-up of 513 women for two years. Eur J Gynaecol Oncol. 1987;8(1):5–16. [PubMed] [Google Scholar]
- Twiggs L. B., Okagaki T., Clark B., Fukushima M., Ostrow R., Faras A. A clinical, histopathologic, and molecular biologic investigation of vulvar intraepithelial neoplasia. Int J Gynecol Pathol. 1988;7(1):48–55. doi: 10.1097/00004347-198803000-00005. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Melchers W. J., Mullink H., Meijer C. J., Struyk A., Quint W. G., van der Noordaa J., ter Schegget J. Sensitivity of in situ detection with biotinylated probes of human papilloma virus type 16 DNA in frozen tissue sections of squamous cell carcinomas of the cervix. Am J Pathol. 1988 Jun;131(3):587–594. [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M. Heterogeneity of the human papillomavirus group. J Virol. 1989 Nov;63(11):4898–4903. doi: 10.1128/jvi.63.11.4898-4903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]