Abstract
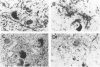
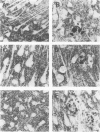
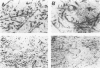
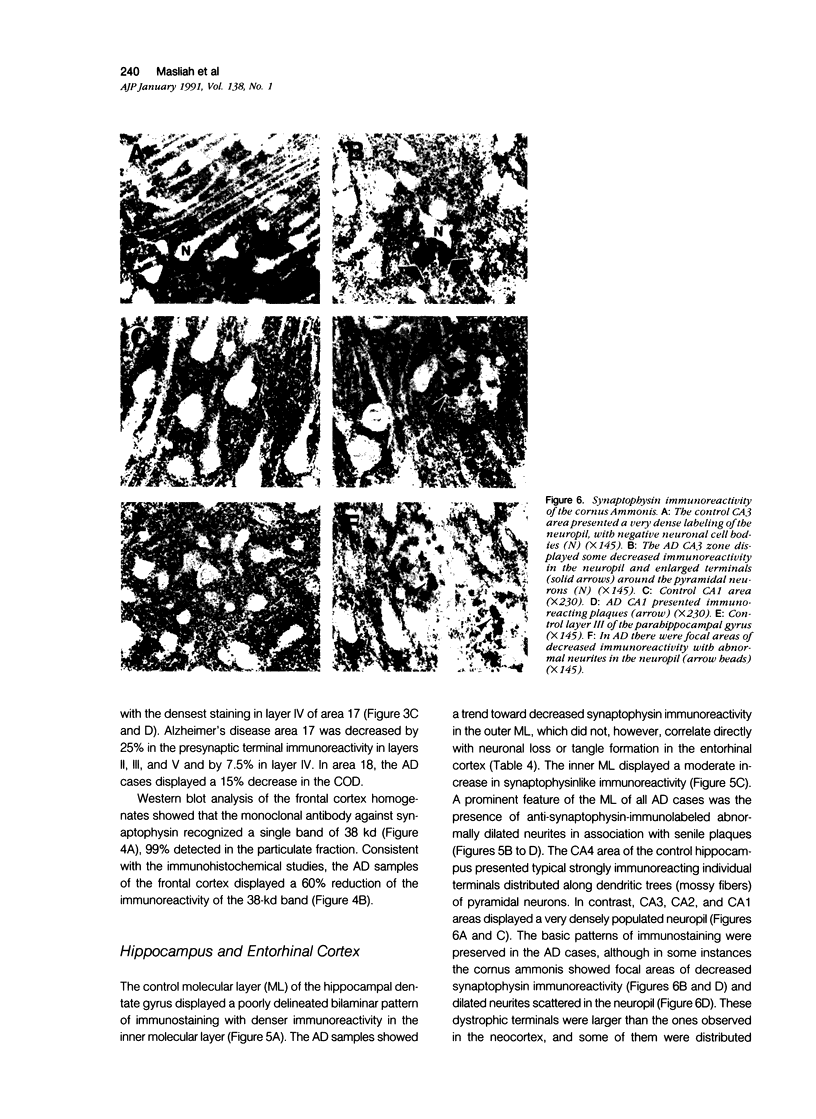
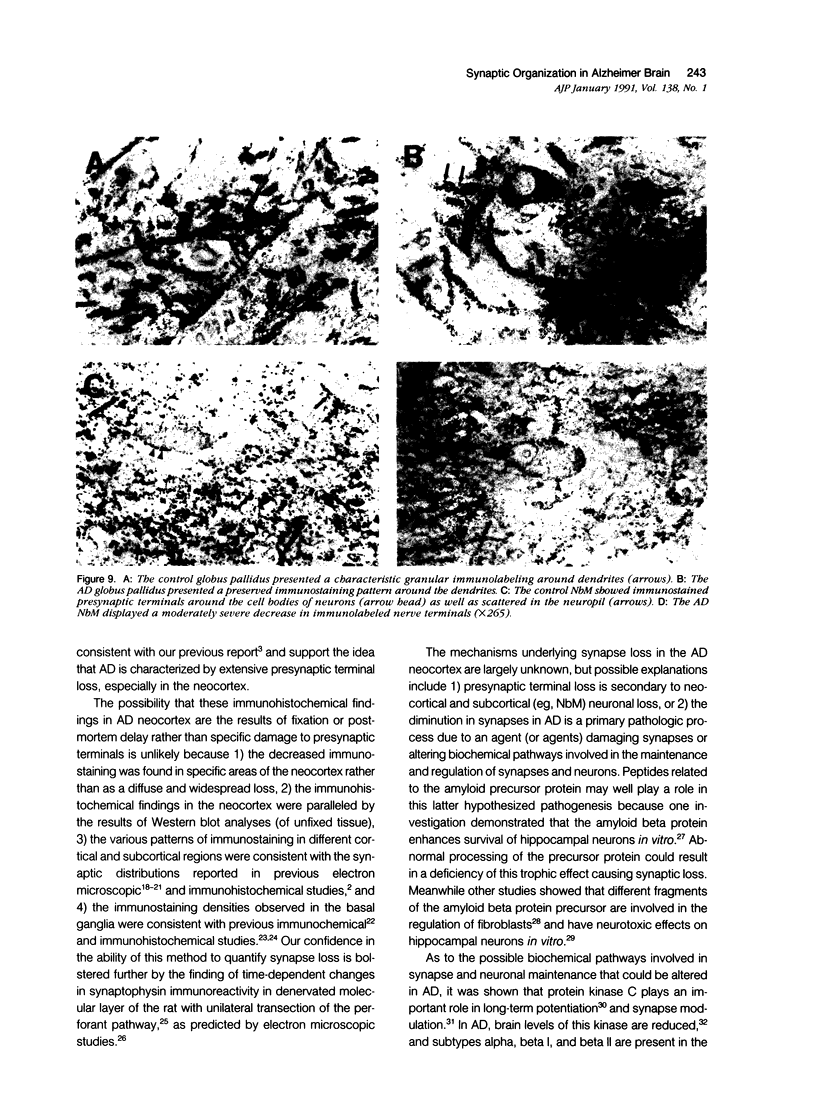
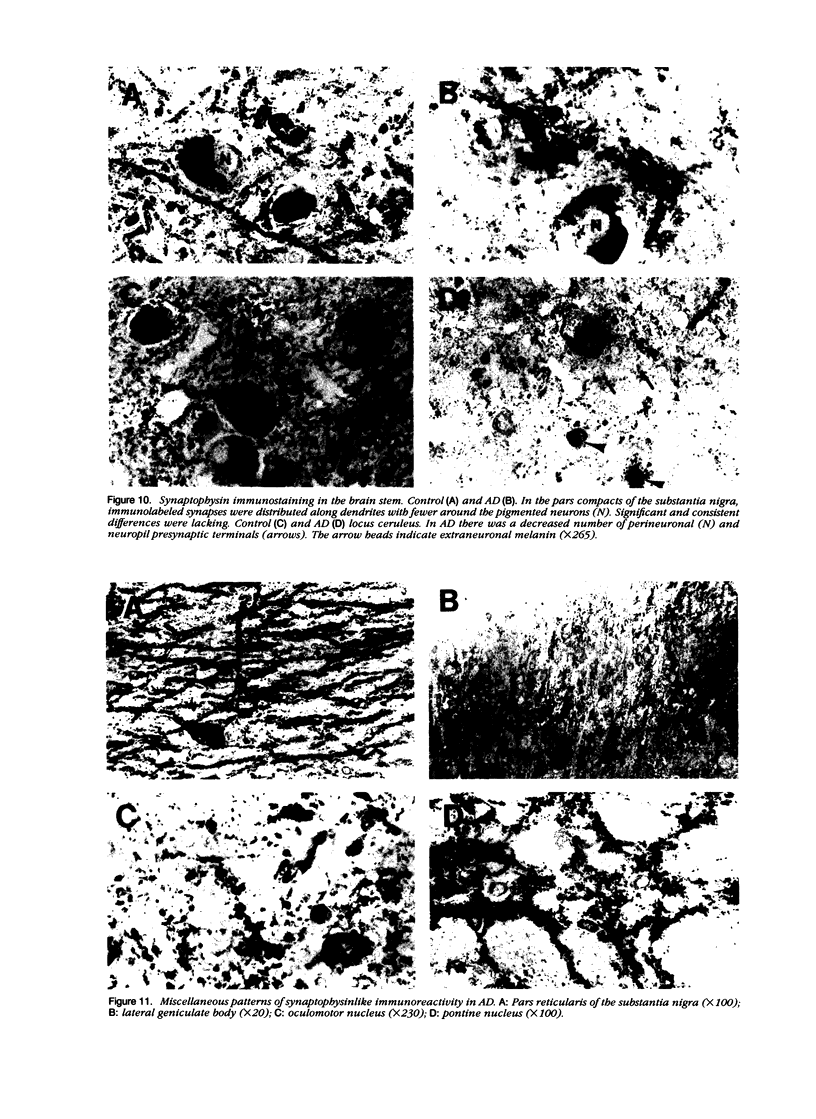
Quantification of synaptophysinlike immunoreactivity is a valuable method for studying the presynaptic terminals in the normal and damaged nervous system. The present report shows that in the control brain, the predominant pattern of synaptic immunostaining in the neocortex was that of an evenly distributed densely granular immunolabeling of the neuropil, while in the paleocortex and in subcortical areas of the brain most of the presynaptic terminals were distributed along the dendritic arborizations or around the neuronal somata. The immunochemical and the immunohistochemical analysis of the Alzheimer's disease tissue showed that the frontal and parietal cortex presented the most severe and widespread loss, with a 45% loss in synaptophysin immunoreactivity. These areas showed an average 35% loss of large neurons. The visual cortex, hippocampus, entorhinal cortex, nucleus basalis of Meynert, and locus ceruleus displayed some degree of loss, but to a lesser extent. In addition to this loss, the basic patterns of organization of the presynaptic terminals were altered, with the presence of abundant, enlarged synaptophysin-labeled terminals. This study further supports the role of synaptic pathology in Alzheimer's disease.
Full text
PDF
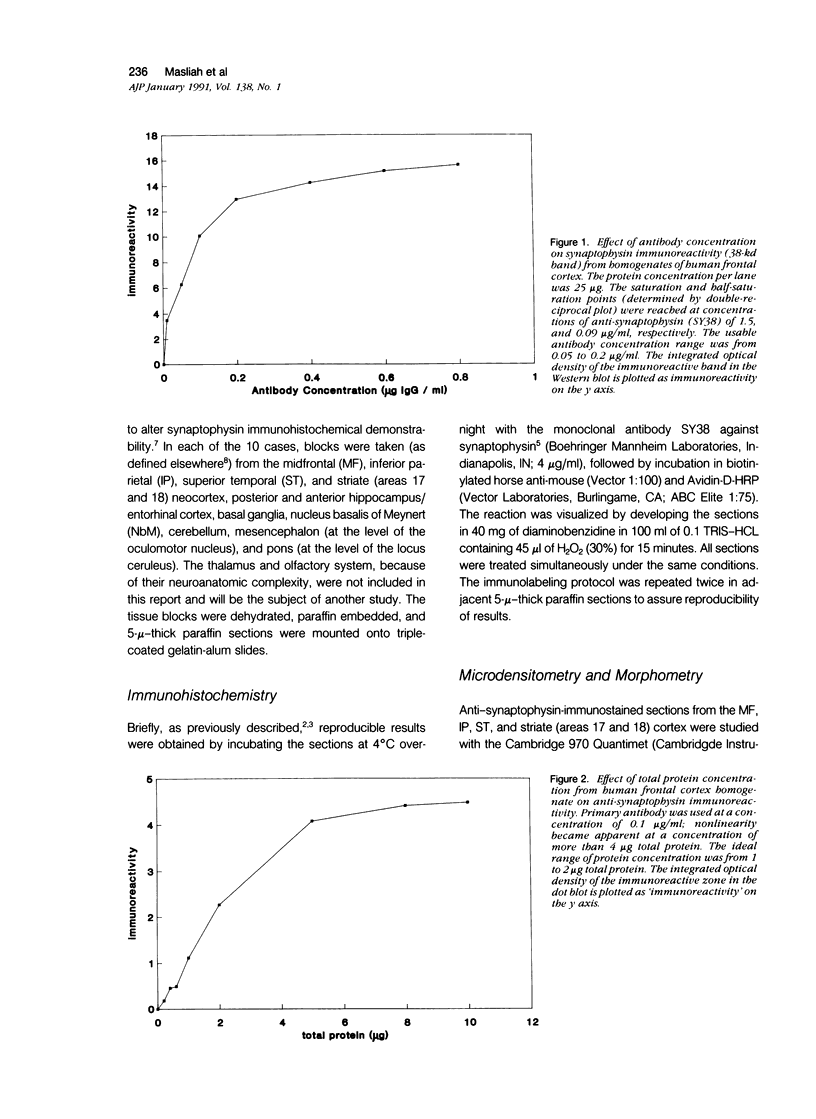
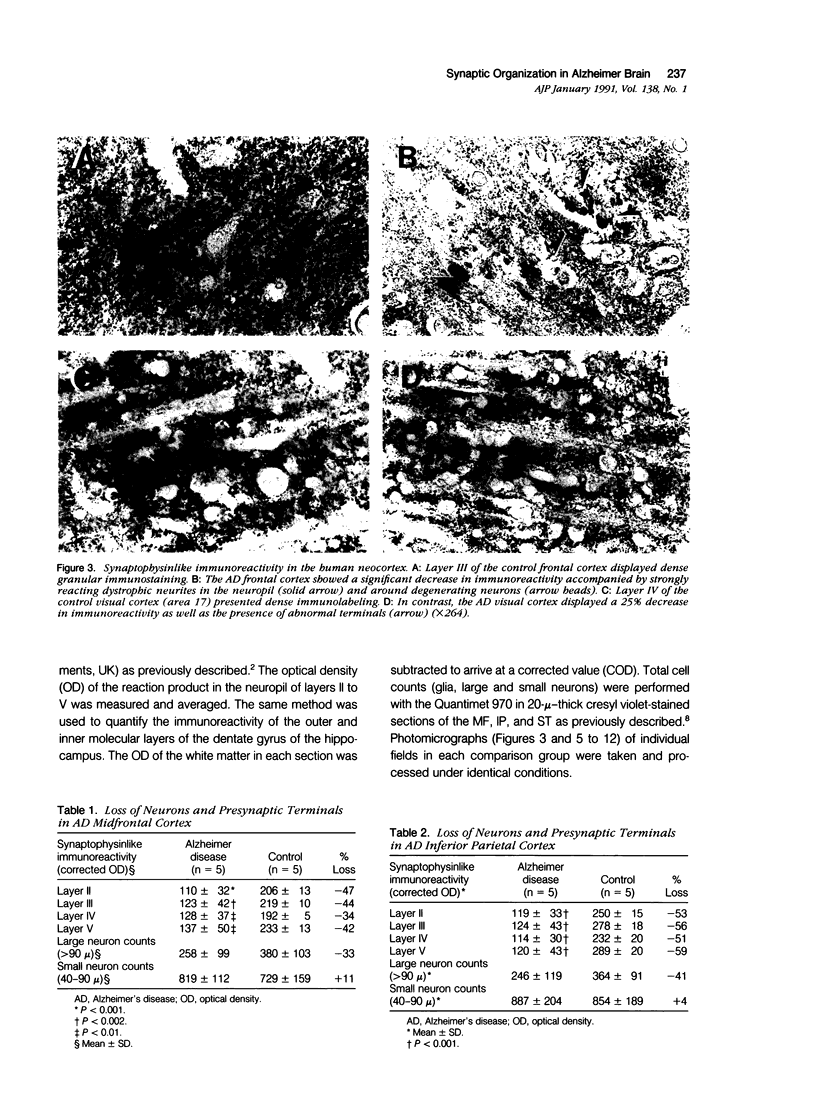
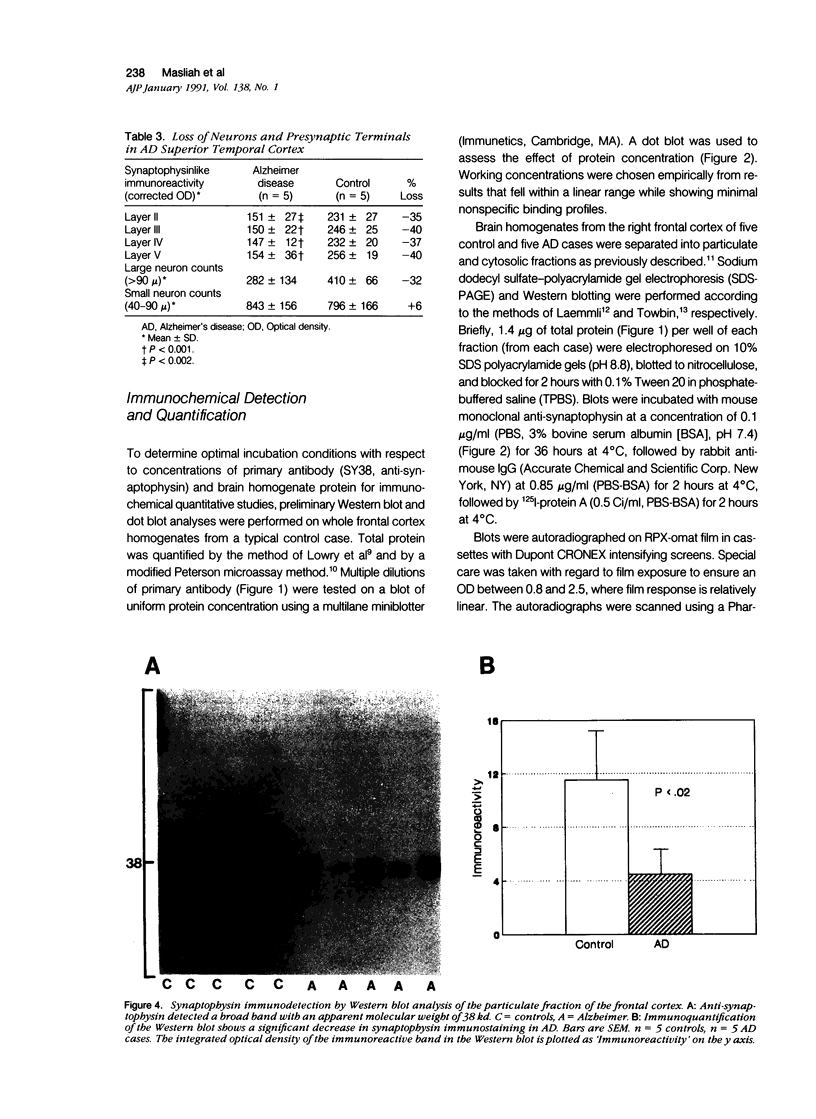
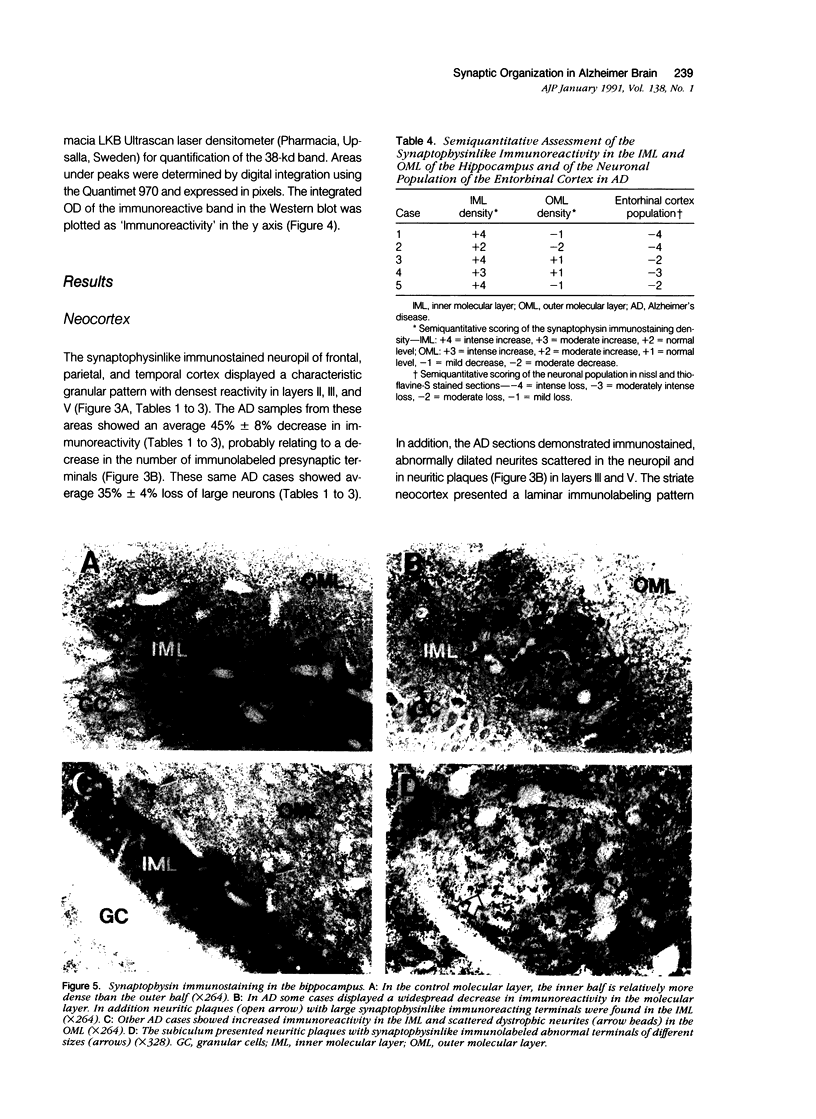




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendt T., Bigl V., Tennstedt A., Arendt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer's disease. Neuroscience. 1985 Jan;14(1):1–14. doi: 10.1016/0306-4522(85)90160-5. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Benzing W. C., Evans J., Terry R. D., Shields D., Hansen L. A. Substance P and somatostatin coexist within neuritic plaques: implications for the pathogenesis of Alzheimer's disease. Neuroscience. 1989;31(3):663–671. doi: 10.1016/0306-4522(89)90431-4. [DOI] [PubMed] [Google Scholar]
- Cole G., Dobkins K. R., Hansen L. A., Terry R. D., Saitoh T. Decreased levels of protein kinase C in Alzheimer brain. Brain Res. 1988 Jun 14;452(1-2):165–174. doi: 10.1016/0006-8993(88)90021-2. [DOI] [PubMed] [Google Scholar]
- Davies C. A., Mann D. M., Sumpter P. Q., Yates P. O. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987 Apr;78(2):151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Difiglia M., Rafols J. A. Synaptic organization of the globus pallidus. J Electron Microsc Tech. 1988 Nov;10(3):247–263. doi: 10.1002/jemt.1060100304. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. Synaptic organization of the striatum. J Electron Microsc Tech. 1988 Nov;10(3):265–281. doi: 10.1002/jemt.1060100305. [DOI] [PubMed] [Google Scholar]
- Girault J. A., Raisman-Vozari R., Agid Y., Greengard P. Striatal phosphoproteins in Parkinson disease and progressive supranuclear palsy. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2493–2497. doi: 10.1073/pnas.86.7.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S., Hirano A., Rojas-Corona R. R. Immunohistochemical visualization of afferent nerve terminals in human globus pallidus and its alteration in neostriatal neurodegenerative disorders. Acta Neuropathol. 1989;78(5):543–550. doi: 10.1007/BF00687717. [DOI] [PubMed] [Google Scholar]
- Hamos J. E., DeGennaro L. J., Drachman D. A. Synaptic loss in Alzheimer's disease and other dementias. Neurology. 1989 Mar;39(3):355–361. doi: 10.1212/wnl.39.3.355. [DOI] [PubMed] [Google Scholar]
- Hoog A., Gould V. E., Grimelius L., Franke W. W., Falkmer S., Chejfec G. Tissue fixation methods alter the immunohistochemical demonstrability of synaptophysin. Ultrastruct Pathol. 1988 Nov-Dec;12(6):673–678. doi: 10.3109/01913128809056493. [DOI] [PubMed] [Google Scholar]
- Iimoto D. S., Masliah E., DeTeresa R., Terry R. D., Saitoh T. Aberrant casein kinase II in Alzheimer's disease. Brain Res. 1990 Jan 22;507(2):273–280. doi: 10.1016/0006-8993(90)90282-g. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclerc N., Beesley P. W., Brown I., Colonnier M., Gurd J. W., Paladino T., Hawkes R. Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J Comp Neurol. 1989 Feb 8;280(2):197–212. doi: 10.1002/cne.902800204. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. The role of protein kinase C in long-term potentiation: a testable model. Brain Res Brain Res Rev. 1989 Jul-Sep;14(3):279–296. doi: 10.1016/0165-0173(89)90004-0. [DOI] [PubMed] [Google Scholar]
- Lovinger D. M., Routtenberg A. Synapse-specific protein kinase C activation enhances maintenance of long-term potentiation in rat hippocampus. J Physiol. 1988 Jun;400:321–333. doi: 10.1113/jphysiol.1988.sp017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Cole G., Shimohama S., Hansen L., DeTeresa R., Terry R. D., Saitoh T. Differential involvement of protein kinase C isozymes in Alzheimer's disease. J Neurosci. 1990 Jul;10(7):2113–2124. doi: 10.1523/JNEUROSCI.10-07-02113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., Alford M., DeTeresa R. Quantitative immunohistochemistry of synaptophysin in human neocortex: an alternative method to estimate density of presynaptic terminals in paraffin sections. J Histochem Cytochem. 1990 Jun;38(6):837–844. doi: 10.1177/38.6.2110586. [DOI] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., DeTeresa R. M., Hansen L. A. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989 Aug 28;103(2):234–239. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- Masliah E., Terry R., Buzsáki G. Thalamic nuclei in Alzheimer disease: evidence against the cholinergic hypothesis of plaque formation. Brain Res. 1989 Jul 31;493(2):240–246. doi: 10.1016/0006-8993(89)91159-1. [DOI] [PubMed] [Google Scholar]
- O'Kusky J., Colonnier M. A laminar analysis of the number of neurons, glia, and synapses in the adult cortex (area 17) of adult macaque monkeys. J Comp Neurol. 1982 Sep 20;210(3):278–290. doi: 10.1002/cne.902100307. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rudelli R. D., Ambler M. W., Wisniewski H. M. Morphology and distribution of Alzheimer neuritic (senile) and amyloid plaques in striatum and diencephalon. Acta Neuropathol. 1984;64(4):273–281. doi: 10.1007/BF00690393. [DOI] [PubMed] [Google Scholar]
- Scheff S. W., DeKosky S. T., Price D. A. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990 Jan-Feb;11(1):29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Peck A., DeTeresa R., Schechter R., Horoupian D. S. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981 Aug;10(2):184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas S. I., Jahn R., Greengard P. Quantitation of nerve terminal populations: synaptic vesicle-associated proteins as markers for synaptic density in the rat neostriatum. Synapse. 1988;2(5):516–520. doi: 10.1002/syn.890020507. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Ouimet C. C. The ventral striatopallidal complex: an immunocytochemical analysis of medium-sized striatal neurons and striatopallidal fibers in the basal forebrain of the rat. Neuroscience. 1989;28(3):663–672. doi: 10.1016/0306-4522(89)90013-4. [DOI] [PubMed] [Google Scholar]
- Whitson J. S., Selkoe D. J., Cotman C. W. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989 Mar 17;243(4897):1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]