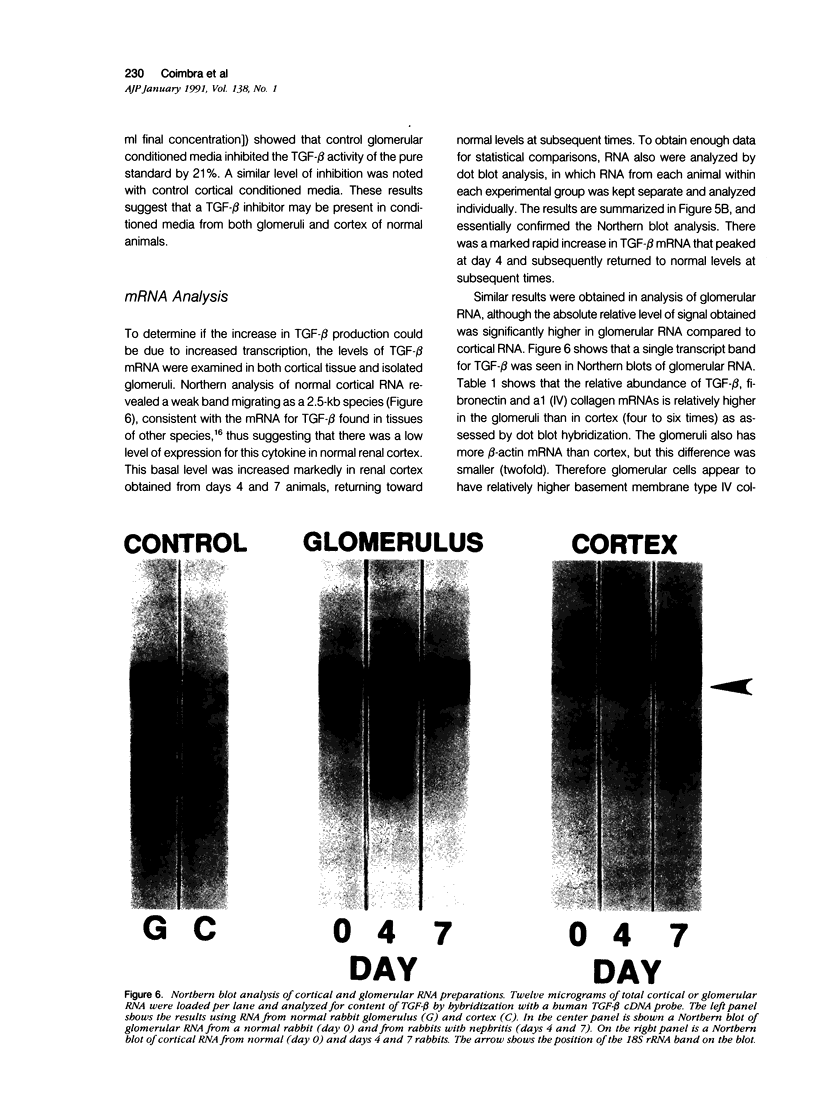
Abstract
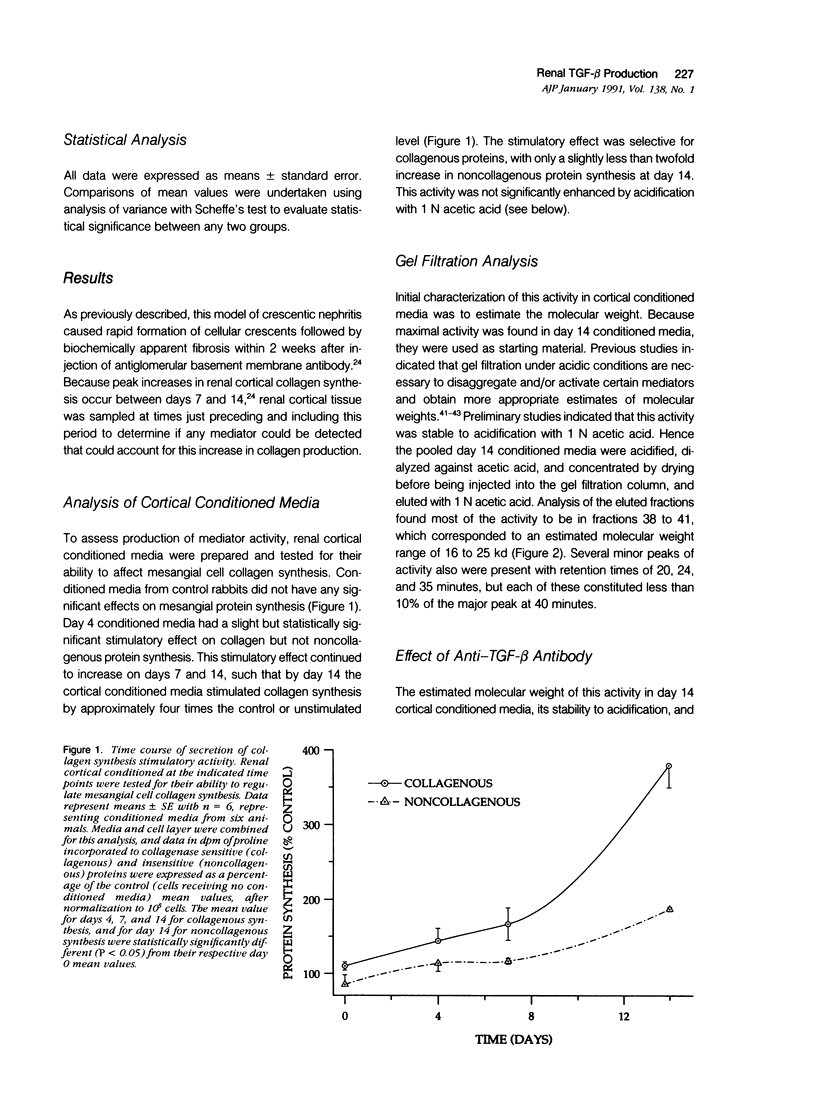
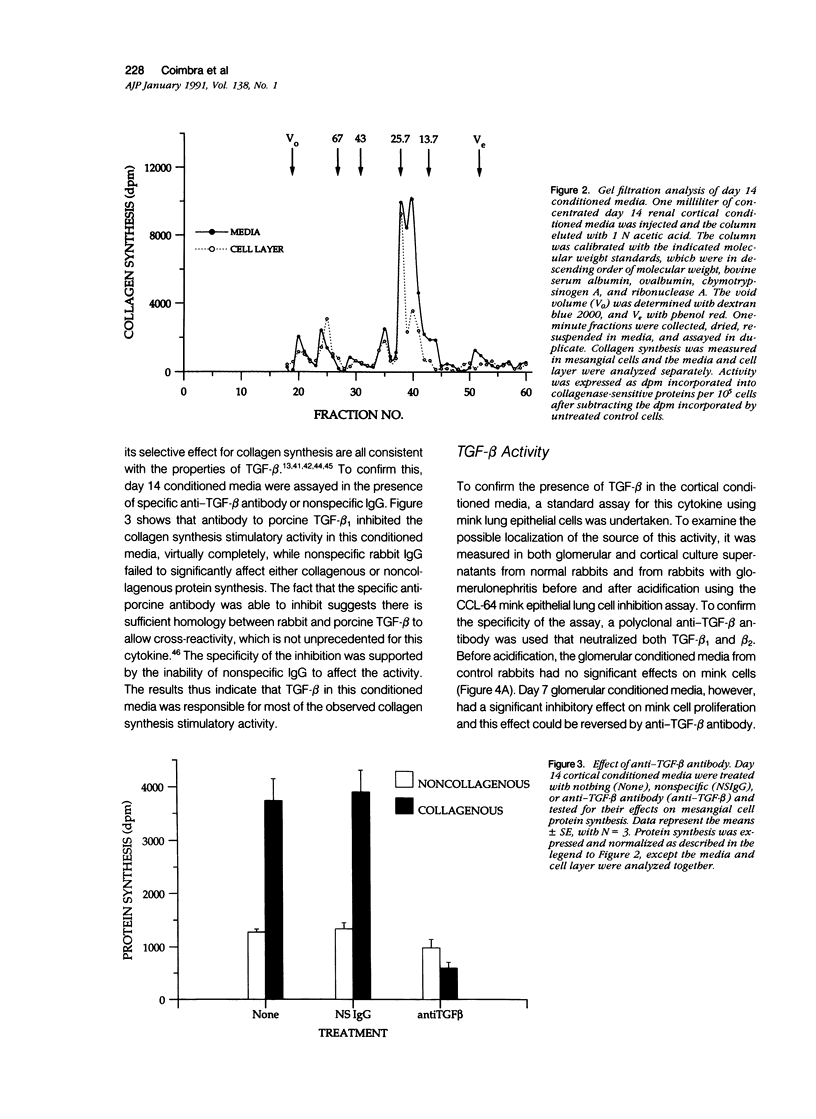
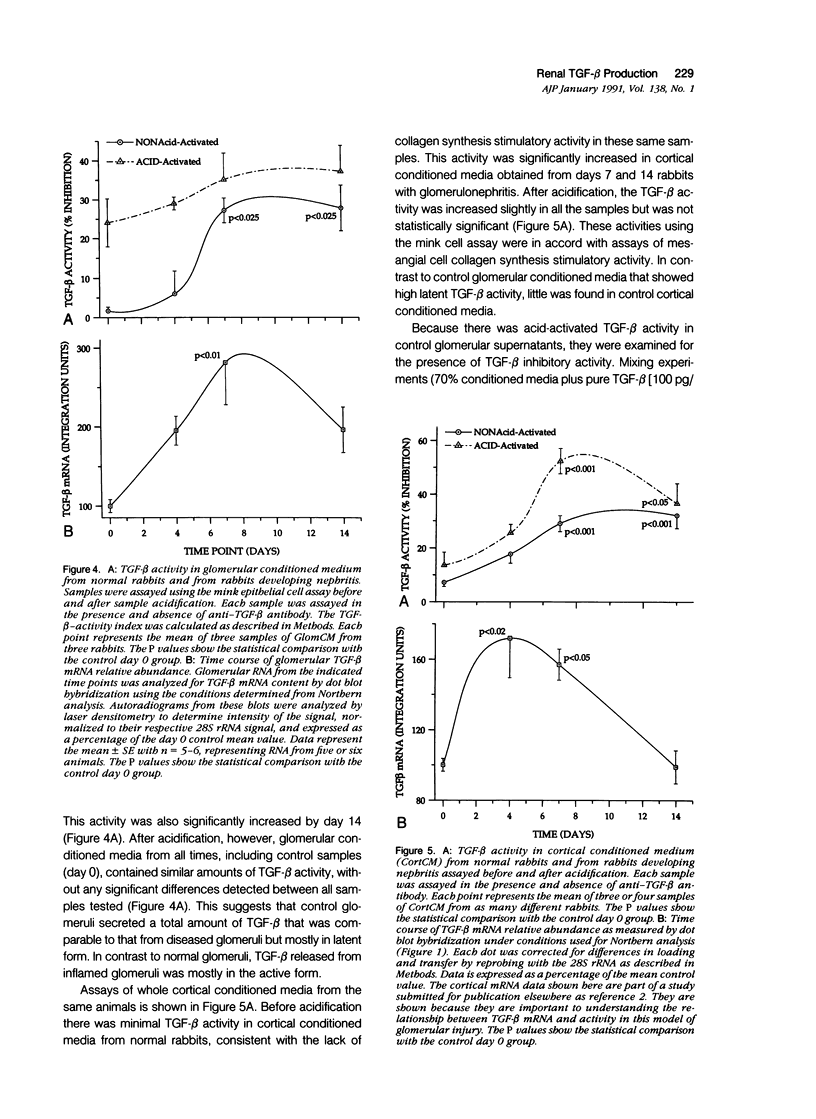
The purpose of this study was to assay for the presence of collagen synthesis stimulatory activity in the kidney during immune-induced renal injury that results in severe fibrosis in both glomerular and interstitial compartments. A model of antiglomerular basement (anti-GBM) disease in the rabbit was induced on day 0 by the injection of anti-GBM antibody and renal cortex tissues were then sampled at various time points. Only conditioned media prepared from diseased renal cortical samples showed collagen synthesis stimulatory activity when tested on rabbit mesangial cells. The activity had an estimated molecular weight range of 16 to 25 kd and was neutralized by antibody to transforming growth factor-beta (TGF-beta). A standard assay for TGF-beta using a mink lung epithelial cell line confirmed the increase in TGF-beta activity in conditioned media of diseased cortex from day 7 and day 14 animals, which was not significantly activated by previous acidification. This suggests that most of the TGF-beta present in renal conditioned media was in the active form. The increase in renal cortical secretion of active TGF-beta was accompanied by increases in renal cortical TGF-beta mRNA content on days 4 and 7 after induction, with subsequent return to control levels. A similar increase in TGF-beta activity was present in nonacidified conditioned media of purified glomeruli from diseased days 7 and 14 animals, which was also accompanied by significant increases in TGF-beta mRNA. However with acidification no significant differences were noted between control and diseased samples, suggesting the presence of substantial latent TGF-beta activity in control glomerular conditioned media. These same control-conditioned media contained inhibitor activity for added exogenous TGF-beta. These results support the conclusion that the association between increased TGF-beta secretion and increased renal cortical collagen synthesis in this model is consistent with a role for this cytokine in directing fibrogenesis in the kidney.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990 Feb;37(2):689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Cattell V., Jamieson S. W. The origin of glomerular crescents in experimental nephrotoxic serum nephritis in the rabbit. Lab Invest. 1978 Dec;39(6):584–590. [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987 Feb 25;262(6):2869–2874. [PubMed] [Google Scholar]
- Chan Y. L., Olvera J., Wool I. G. The structure of rat 28S ribosomal ribonucleic acid inferred from the sequence of nucleotides in a gene. Nucleic Acids Res. 1983 Nov 25;11(22):7819–7831. doi: 10.1093/nar/11.22.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. K., Hoshi H., McKeehan W. L. Transforming growth factor type beta specifically stimulates synthesis of proteoglycan in human adult arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5287–5291. doi: 10.1073/pnas.84.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. E., Ham K. N., Tange J. D., Ryan G. B. Origin of glomerular crescents in rabbit nephrotoxic nephritis. J Pathol. 1983 Mar;139(3):247–258. doi: 10.1002/path.1711390303. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Downer G., Phan S. H., Wiggins R. C. Analysis of renal fibrosis in a rabbit model of crescentic nephritis. J Clin Invest. 1988 Sep;82(3):998–1006. doi: 10.1172/JCI113710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fine A., Goldstein R. H. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987 Mar 15;262(8):3897–3902. [PubMed] [Google Scholar]
- Foidart J. B., Dechenne C. A., Mahieu P., Creutz C. E., de Mey J. Tissue culture of normal rat glomeruli. Isolation and morphological characterization of two homogeneous cell lines. Invest Cell Pathol. 1979 Jan-Mar;2(1):15–26. [PubMed] [Google Scholar]
- Hill D. J., Strain A. J., Elstow S. F., Swenne I., Milner R. D. Bi-functional action of transforming growth factor-beta on DNA synthesis in early passage human fetal fibroblasts. J Cell Physiol. 1986 Aug;128(2):322–328. doi: 10.1002/jcp.1041280226. [DOI] [PubMed] [Google Scholar]
- Hooke D. H., Gee D. C., Atkins R. C. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987 Apr;31(4):964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Jaffer F., Saunders C., Shultz P., Throckmorton D., Weinshell E., Abboud H. E. Regulation of mesangial cell growth by polypeptide mitogens. Inhibitory role of transforming growth factor beta. Am J Pathol. 1989 Aug;135(2):261–269. [PMC free article] [PubMed] [Google Scholar]
- Khalil N., Bereznay O., Sporn M., Greenberg A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989 Sep 1;170(3):727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1026–1034. doi: 10.1016/0006-291x(85)91239-2. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. Stimulation of rat mesangial cell proliferation by macrophage interleukin 1. J Immunol. 1983 Dec;131(6):2830–2836. [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay K., Striker L. J., Stauffer J. W., Doi T., Agodoa L. Y., Striker G. E. Transforming growth factor-beta. Murine glomerular receptors and responses of isolated glomerular cells. J Clin Invest. 1989 Apr;83(4):1160–1167. doi: 10.1172/JCI113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcion C., Lachman L., Killen P. D., Morel-Maroger L., Striker G. E. Mesangial cells, effect of monocyte products on proliferation and matrix synthesis. Transplant Proc. 1982 Sep;14(3):559–564. [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- O'Connor-McCourt M. D., Wakefield L. M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J Biol Chem. 1987 Oct 15;262(29):14090–14099. [PubMed] [Google Scholar]
- Oberbäumer I., Laurent M., Schwarz U., Sakurai Y., Yamada Y., Vogeli G., Voss T., Siebold B., Glanville R. W., Kühn K. Amino acid sequence of the non-collagenous globular domain (NC1) of the alpha 1(IV) chain of basement membrane collagen as derived from complementary DNA. Eur J Biochem. 1985 Mar 1;147(2):217–224. doi: 10.1111/j.1432-1033.1985.tb08739.x. [DOI] [PubMed] [Google Scholar]
- Okada F., Yamaguchi K., Ichihara A., Nakamura T. One of two subunits of masking protein in latent TGF-beta is a part of pro-TGF-beta. FEBS Lett. 1989 Jan 2;242(2):240–244. doi: 10.1016/0014-5793(89)80477-6. [DOI] [PubMed] [Google Scholar]
- Okuda S., Languino L. R., Ruoslahti E., Border W. A. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest. 1990 Aug;86(2):453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi Y. M., Weiss M. A., Hsu A., Ooi B. S. Mechanisms of suppression of mouse mesangial cell proliferation by macrophage supernatants. J Immunol. 1983 Apr;130(4):1790–1795. [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Meyers C. A., Wideman J., Blacher R., Pan Y. C., Stein S., Lehrman S. R., Smith J. M., Lamb L. C. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983 Dec 6;22(25):5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Striker L. J. Glomerular cell culture. Lab Invest. 1985 Aug;53(2):122–131. [PubMed] [Google Scholar]
- Van den Eijnden-Van Raaij A. J., Koornneef I., Van Oostwaard T. M., Feyen A., Kruijer W., De Laat S. W., Van Zoelen E. J. Purification of a growth factor related to platelet-derived growth factor and a type beta transforming growth factor secreted by mouse neuroblastoma cells. A general strategy for the purification of basic polypeptide growth factors. Biochem J. 1989 Jan 15;257(2):375–382. doi: 10.1042/bj2570375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins R. C., Glatfelter A., Brukman J. Procoagulant activity in glomeruli and urine of rabbits with nephrotoxic nephritis. Lab Invest. 1985 Aug;53(2):156–165. [PubMed] [Google Scholar]