Abstract
Thirty-one snap-frozen human prostate specimens containing examples of benign hyperplasia, prostatic intraepithelial neoplasia (PIN), and invasive carcinoma were analyzed using a panel of 24 antibodies and one lectin. Twenty-seven additional routinely processed radical prostatectomy specimens were studied using selected probes known to work on formalin-fixed paraffin-embedded material. Three probes, anticytokeratin KA4, anti-vimentin V9, and the lectin from Ulex europaeus (UEA-1), demonstrated phenotypic similarities between PIN and invasive carcinoma. Whereas the luminal cells of normal or hyperplastic prostatic epithelium are minimally reactive with KA4 (4%) or UEA-1 (0%) and strongly reactive with anti-vimentin (91%), both the PIN and invasive carcinoma are reactive with KA4 (89% and 93%, respectively) and UEA-1 (96% and 93%, respectively) and minimally reactive with anti-vimentin (15% and 0%, respectively). The increased KA4 staining was shown to be in part due to detection of cytokeratin 19, by using cytokeratin-19-specific antibodies, 4.62 and LP2K. The reasons for the increased expression of this cytokeratin and the decreased expression of vimentin are unclear but seem to indicate a phenotypic relationship between the PIN lesions and invasive carcinoma.
Full text
PDF

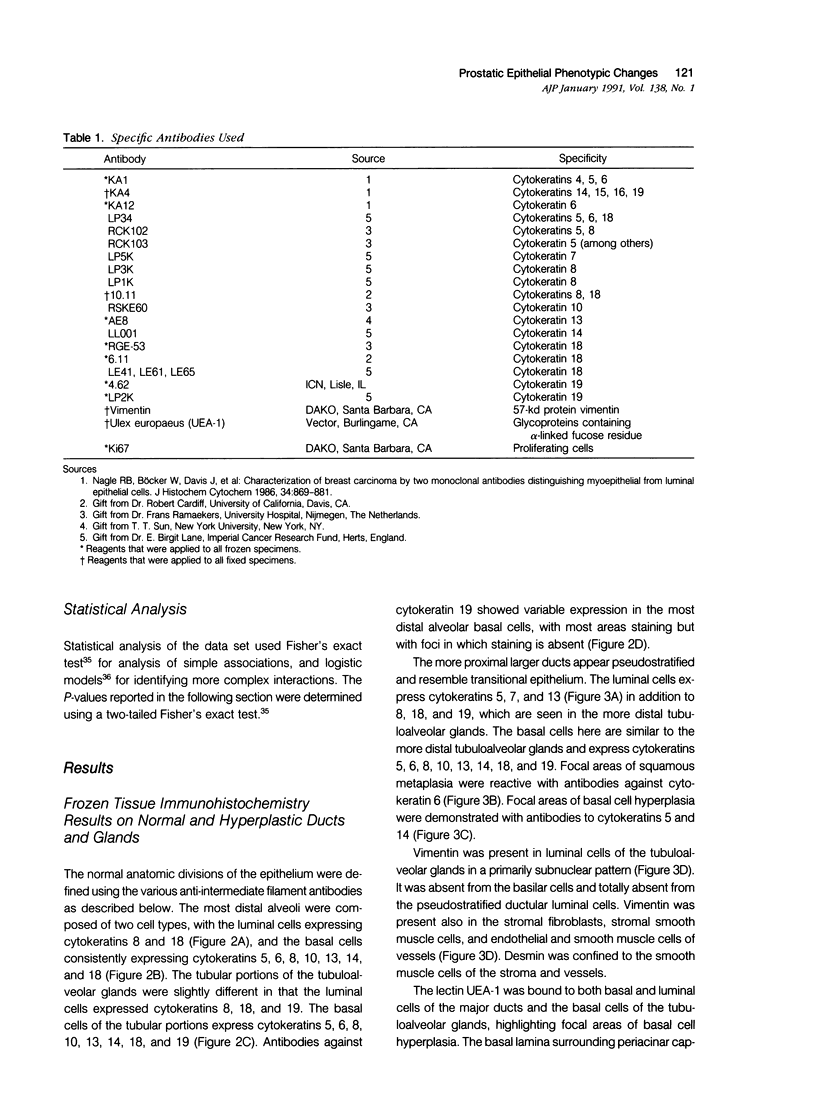






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews G. S. Latent Carcinoma of the Prostate. J Clin Pathol. 1949 Aug;2(3):197–208. doi: 10.1136/jcp.2.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawer M. K., Peehl D. M., Stamey T. A., Bostwick D. G. Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Res. 1985 Aug;45(8):3663–3667. [PubMed] [Google Scholar]
- Brawn P. N. Adenosis of the prostate: a dysplastic lesion that can be confused with prostate adenocarcinoma. Cancer. 1982 Feb 15;49(4):826–833. doi: 10.1002/1097-0142(19820215)49:4<826::aid-cncr2820490436>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Carney J. A. Differences between nonfamilial and familial cardiac myxoma. Am J Surg Pathol. 1985 Jan;9(1):53–55. doi: 10.1097/00000478-198501000-00009. [DOI] [PubMed] [Google Scholar]
- Ellis D. W., Leffers S., Davies J. S., Ng A. B. Multiple immunoperoxidase markers in benign hyperplasia and adenocarcinoma of the prostate. Am J Clin Pathol. 1984 Mar;81(3):279–284. doi: 10.1093/ajcp/81.3.279. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Harbitz T. B., Haugen O. A. Histology of the prostate in elderly men. A study in an autopsy series. Acta Pathol Microbiol Scand A. 1972;80(6):756–768. doi: 10.1111/j.1699-0463.1972.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Hedrick L., Epstein J. I. Use of keratin 903 as an adjunct in the diagnosis of prostate carcinoma. Am J Surg Pathol. 1989 May;13(5):389–396. doi: 10.1097/00000478-198905000-00006. [DOI] [PubMed] [Google Scholar]
- Helpap B. The biological significance of atypical hyperplasia of the prostate. Virchows Arch A Pathol Anat Histol. 1980;387(3):307–317. doi: 10.1007/BF00454834. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- JOHNSON J. A. Histological diagnosis and prognosis in patients operated upon for prostatic obstruction. Acta Chir Scand Suppl. 1962;Suppl 286:1–25. [PubMed] [Google Scholar]
- Kastendieck H., Altenähr E., Hüsselmann H., Bressel M. Carcinoma and dysplastic lesions of the prostate. A histomorphological analysis of 50 total prostatectomies by step-section technique. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1976 Dec 20;88(1):33–54. doi: 10.1007/BF00284745. [DOI] [PubMed] [Google Scholar]
- Kastendieck H. Correlations between atypical primary hyperplasia and carcinoma of the prostate. A histological study of 180 total prostatectomies. Pathol Res Pract. 1980 Nov;169(3-4):366–387. doi: 10.1016/S0344-0338(80)80014-8. [DOI] [PubMed] [Google Scholar]
- Kovi J. Microscopic differential diagnosis of small acinar adenocarcinoma of prostate. Pathol Annu. 1985;20(Pt 1):157–196. [PubMed] [Google Scholar]
- Kovi J., Mostofi F. K. Atypical hyperplasia of prostate. Urology. 1989 Dec;34(6 Suppl):23–27. [PubMed] [Google Scholar]
- Kovi J., Mostofi F. K., Heshmat M. Y., Enterline J. P. Large acinar atypical hyperplasia and carcinoma of the prostate. Cancer. 1988 Feb 1;61(3):555–561. doi: 10.1002/1097-0142(19880201)61:3<555::aid-cncr2820610322>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kuwahara T., Nagayama T. Distribution of keratin protein in normal prostate and prostatic tumors. An immunohistochemical study. Acta Pathol Jpn. 1987 Feb;37(2):339–342. doi: 10.1111/j.1440-1827.1987.tb03070.x. [DOI] [PubMed] [Google Scholar]
- Liavåg I. Atrophy and regeneration in the pathogenesis of prostatic carcinoma. Acta Pathol Microbiol Scand. 1968;73(3):338–350. doi: 10.1111/j.1699-0463.1968.tb04602.x. [DOI] [PubMed] [Google Scholar]
- McNeal J. E., Alroy J., Leav I., Redwine E. A., Freiha F. S., Stamey T. A. Immunohistochemical evidence for impaired cell differentiation in the premalignant phase of prostate carcinogenesis. Am J Clin Pathol. 1988 Jul;90(1):23–32. doi: 10.1093/ajcp/90.1.23. [DOI] [PubMed] [Google Scholar]
- McNeal J. E., Bostwick D. G. Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol. 1986 Jan;17(1):64–71. doi: 10.1016/s0046-8177(86)80156-3. [DOI] [PubMed] [Google Scholar]
- McNeal J. E., Leav I., Alroy J., Skutelsky E. Differential lectin staining of central and peripheral zones of the prostate and alterations in dysplasia. Am J Clin Pathol. 1988 Jan;89(1):41–48. doi: 10.1093/ajcp/89.1.41. [DOI] [PubMed] [Google Scholar]
- McNeal J. E. Morphogenesis of prostatic carcinoma. Cancer. 1965 Dec;18(12):1659–1666. doi: 10.1002/1097-0142(196512)18:12<1659::aid-cncr2820181223>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- McNeal J. E. Normal histology of the prostate. Am J Surg Pathol. 1988 Aug;12(8):619–633. doi: 10.1097/00000478-198808000-00003. [DOI] [PubMed] [Google Scholar]
- McNeal J. E. Significance of duct-acinar dysplasia in prostatic carcinogenesis. Urology. 1989 Dec;34(6 Suppl):9–15. [PubMed] [Google Scholar]
- Miller A., Seljelid R. Cellular atypia in the prostate. Scand J Urol Nephrol. 1971;5(1):17–21. doi: 10.3109/00365597109133567. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Mostofi F. K., Sesterhenn I. A., Davis C. J., Jr Malignant change in hyperplastic prostate glands. The AFIP experience. Urology. 1989 Dec;34(6 Suppl):49–51. [PubMed] [Google Scholar]
- Nagle R. B., Ahmann F. R., McDaniel K. M., Paquin M. L., Clark V. A., Celniker A. Cytokeratin characterization of human prostatic carcinoma and its derived cell lines. Cancer Res. 1987 Jan 1;47(1):281–286. [PubMed] [Google Scholar]
- Nagle R. B., Böcker W., Davis J. R., Heid H. W., Kaufmann M., Lucas D. O., Jarasch E. D. Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem. 1986 Jul;34(7):869–881. doi: 10.1177/34.7.2423579. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Clark V. A., McDaniel K. M., Davis J. R. Immunohistochemical demonstration of keratins in human ovarian neoplasms. A comparison of methods. J Histochem Cytochem. 1983 Aug;31(8):1010–1014. doi: 10.1177/31.8.6190854. [DOI] [PubMed] [Google Scholar]
- Nagle R. B. Intermediate filaments: a review of the basic biology. Am J Surg Pathol. 1988;12 (Suppl 1):4–16. [PubMed] [Google Scholar]
- Oertel H. An Address on Involutionary Changes in Prostate and Female Breast in Relation to Cancer Development. Can Med Assoc J. 1926 Mar;16(3):237–241. [PMC free article] [PubMed] [Google Scholar]
- Reese J. H., McNeal J. E., Redwine E. A., Stamey T. A., Freiha F. S. Tissue type plasminogen activator as a marker for functional zones, within the human prostate gland. Prostate. 1988;12(1):47–53. doi: 10.1002/pros.2990120107. [DOI] [PubMed] [Google Scholar]
- Troncoso P., Babaian R. J., Ro J. Y., Grignon D. J., von Eschenbach A. C., Ayala A. G. Prostatic intraepithelial neoplasia and invasive prostatic adenocarcinoma in cystoprostatectomy specimens. Urology. 1989 Dec;34(6 Suppl):52–56. [PubMed] [Google Scholar]
- Verhagen A. P., Aalders T. W., Ramaekers F. C., Debruyne F. M., Schalken J. A. Differential expression of keratins in the basal and luminal compartments of rat prostatic epithelium during degeneration and regeneration. Prostate. 1988;13(1):25–38. doi: 10.1002/pros.2990130104. [DOI] [PubMed] [Google Scholar]
- Wernert N., Seitz G., Achtstätter T. Immunohistochemical investigation of different cytokeratins and vimentin in the prostate from the fetal period up to adulthood and in prostate carcinoma. Pathol Res Pract. 1987 Oct;182(5):617–626. [PubMed] [Google Scholar]