Abstract
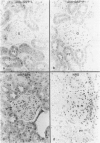
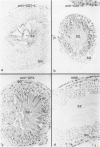
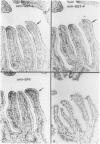
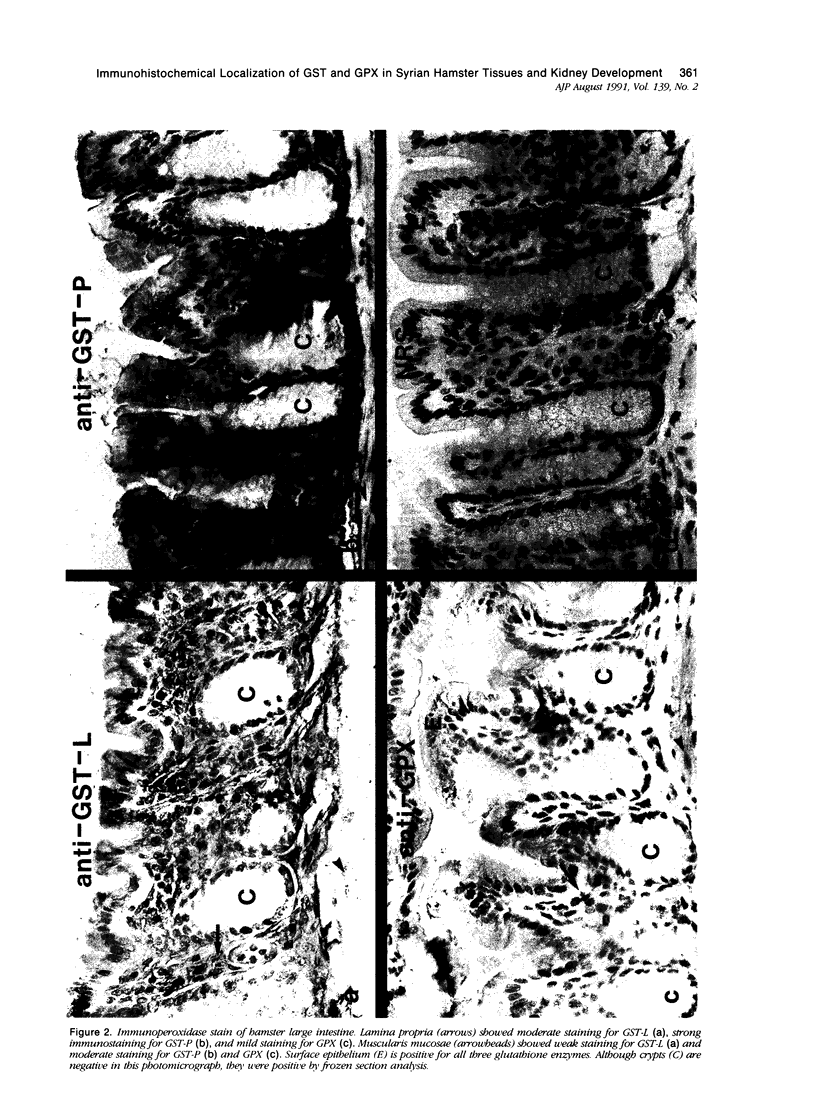
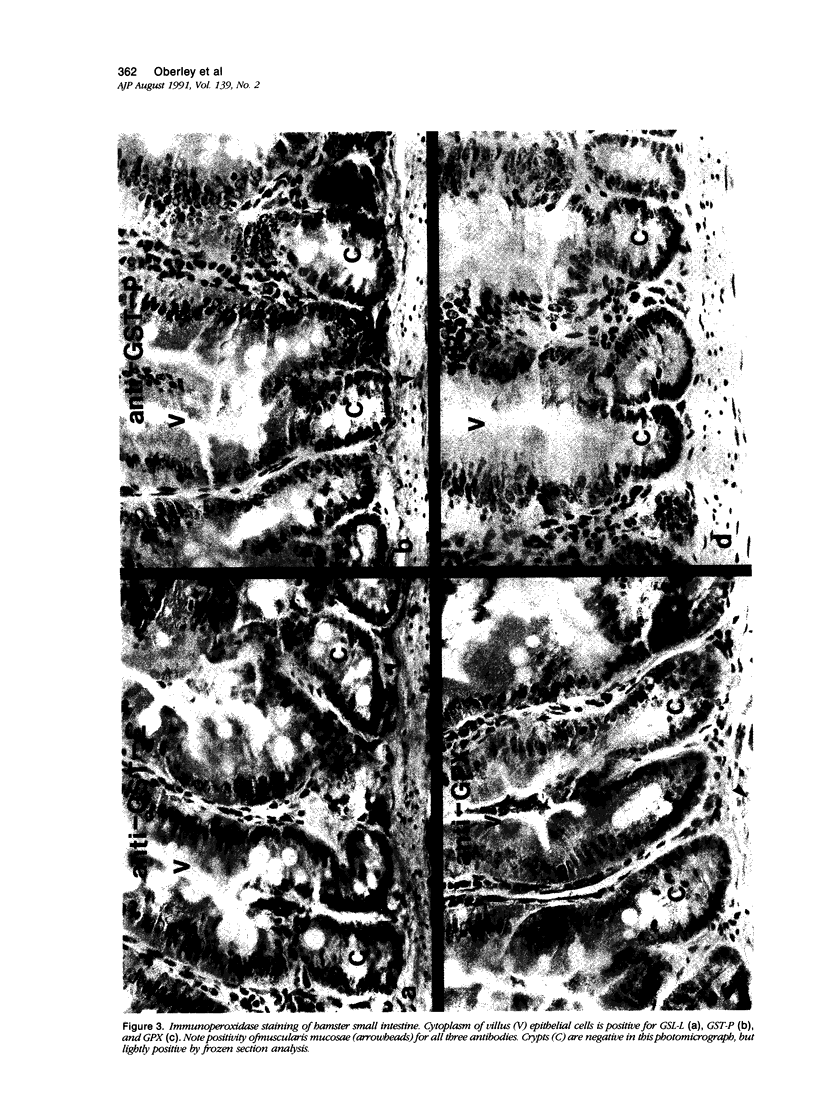
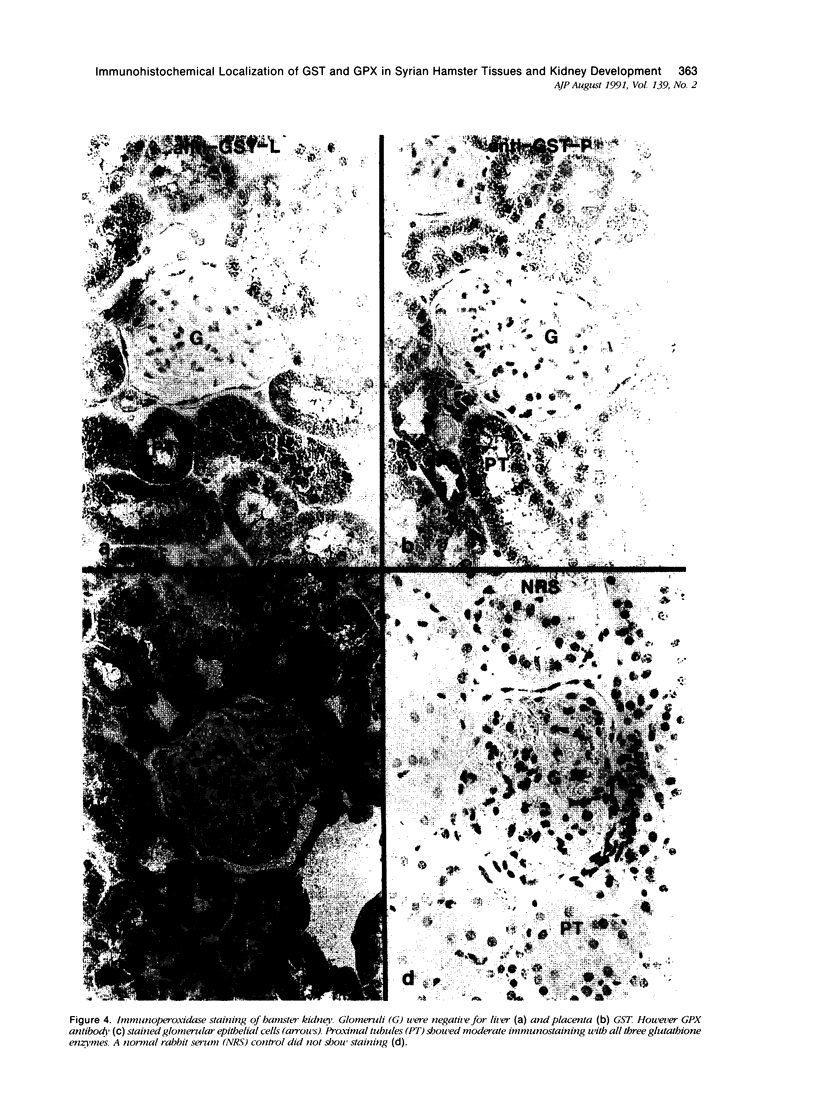
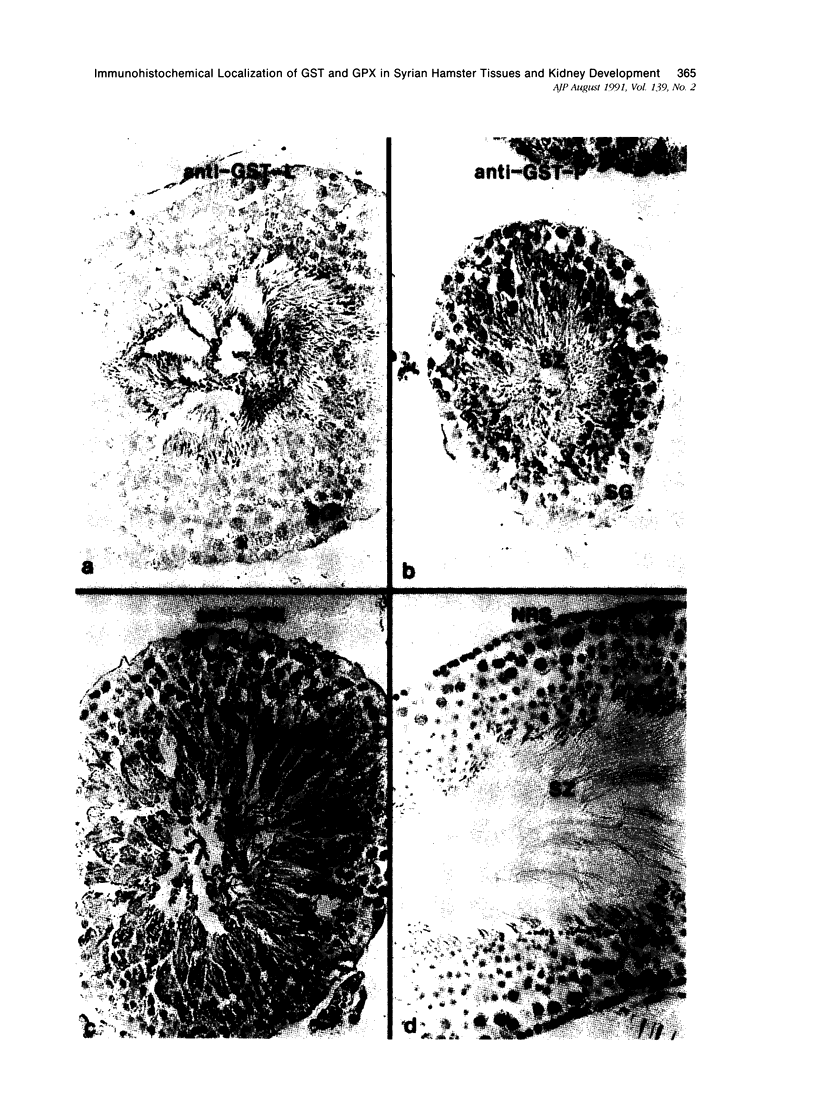
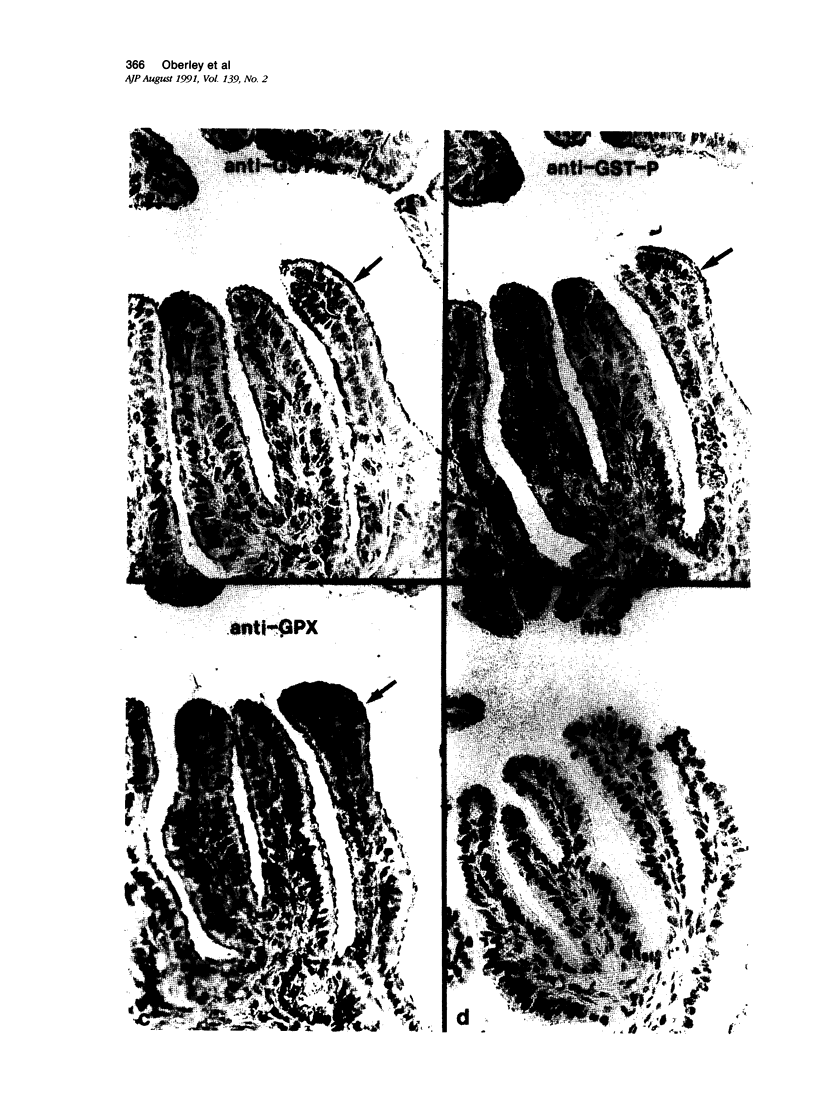
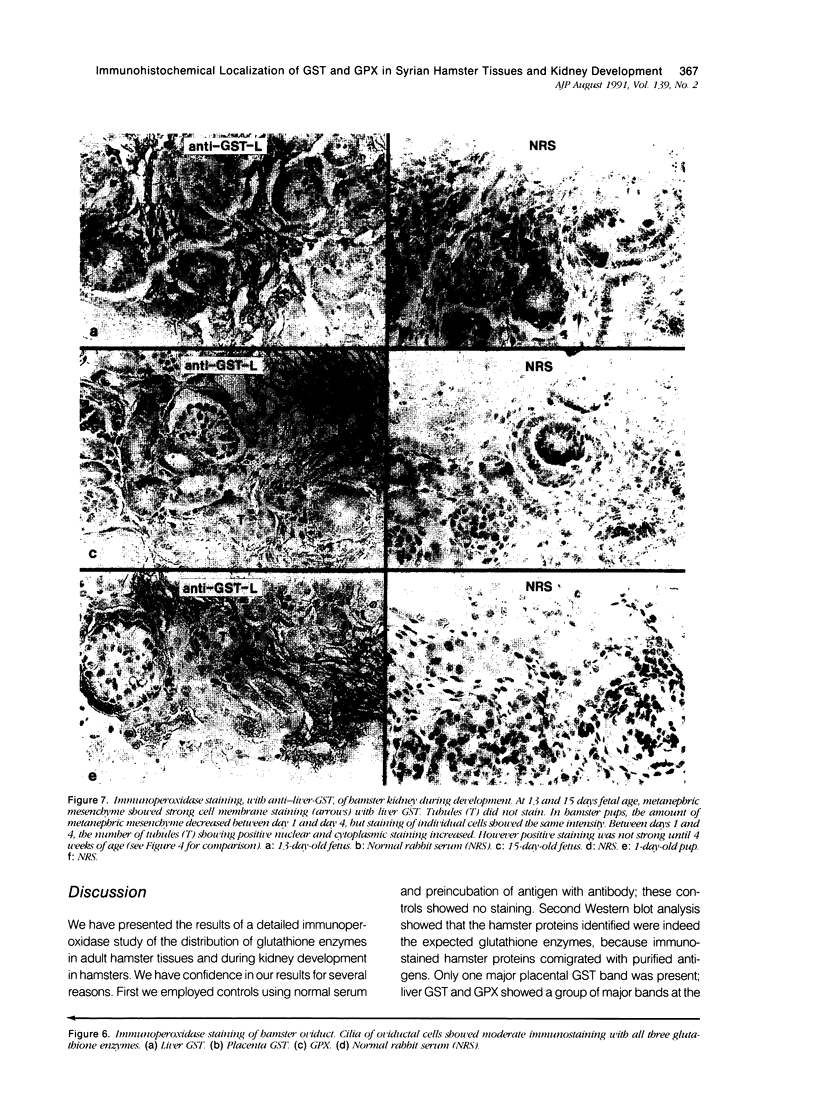
Tissues from adult Syrian hamsters were studied with immunoperoxidase techniques using polyclonal antibodies to glutathione-S-transferase (rat liver and human placental enzymes) and human erythrocyte glutathione peroxidase. Most tissues immunostained similarly with these antibodies. Most notable was the cytoplasmic staining of mesenchyme tissues, especially smooth muscle, by all three antibodies. Epithelial cells stained distinctively, but usually less intensely than mesenchyme. Epithelial cells from all levels of the gastrointestinal tract, respiratory epithelium, transitional epithelium, and epidermis all showed strong staining with these antibodies. Other epithelial cell types were usually positive but showed less dramatic staining. Most epithelial tissues showed both nuclear and cytoplasmic staining; some also showed cell-surface (eg, cilia) staining. The role of these enzymes in cell differentiation of a stable organ was studied by immunostaining the kidney during its development. Early stroma (13- and 15-day fetuses) of the kidney (metanephric mesenchyme) showed strong cell-surface staining for glutathione transferases and moderate staining for glutathione peroxidase; renal tubules (which are epithelial cells) at this stage were negative for these markers. As renal tubules differentiated, first cytoplasm and then nuclei stained moderately, suggesting that glutathione-S-transferases and glutathione peroxidase are markers of both mesenchymal cells, including embryonic mesenchyme, and terminal differentiation of at least some epithelial cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Balin A. K. Oxidative influence on development and differentiation: an overview of a free radical theory of development. Free Radic Biol Med. 1989;6(6):631–661. doi: 10.1016/0891-5849(89)90071-3. [DOI] [PubMed] [Google Scholar]
- Bennett C. F., Spector D. L., Yeoman L. C. Nonhistone protein BA is a glutathione S-transferase localized to interchromatinic regions of the cell nucleus. J Cell Biol. 1986 Feb;102(2):600–609. doi: 10.1083/jcb.102.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fryer A. A., Hume R., Strange R. C. The development of glutathione S-transferase and glutathione peroxidase activities in human lung. Biochim Biophys Acta. 1986 Oct 1;883(3):448–453. doi: 10.1016/0304-4165(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Oberley T. D., Li J. J. Morphological and immunohistochemical studies of the estrogen-induced Syrian hamster renal tumor: probable cell of origin. Cancer Res. 1989 Feb 15;49(4):1020–1028. [PubMed] [Google Scholar]
- Little C., O'Brien P. J. An intracellular GSH-peroxidase with a lipid peroxide substrate. Biochem Biophys Res Commun. 1968 Apr 19;31(2):145–150. doi: 10.1016/0006-291x(68)90721-3. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., Ridnour L. A., Sierra-Rivera E., Oberley T. D., Guernsey D. L. Superoxide dismutase activities of differentiating clones from an immortal cell line. J Cell Physiol. 1989 Jan;138(1):50–60. doi: 10.1002/jcp.1041380109. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Oberley L. W., Slattery A. F., Lauchner L. J., Elwell J. H. Immunohistochemical localization of antioxidant enzymes in adult Syrian hamster tissues and during kidney development. Am J Pathol. 1990 Jul;137(1):199–214. [PMC free article] [PubMed] [Google Scholar]
- Rotruck J. T., Pope A. L., Ganther H. E., Swanson A. B., Hafeman D. G., Hoekstra W. G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973 Feb 9;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Siegers C. P., Böse-Younes H., Thies E., Hoppenkamps R., Younes M. Glutathione and GSH-dependent enzymes in the tumorous and nontumorous mucosa of the human colon and rectum. J Cancer Res Clin Oncol. 1984;107(3):238–241. doi: 10.1007/BF01032615. [DOI] [PubMed] [Google Scholar]
- Strange R. C., Davis B. A., Faulder C. G., Cotton W., Bain A. D., Hopkinson D. A., Hume R. The human glutathione S-transferases: developmental aspects of the GST1, GST2, and GST3 loci. Biochem Genet. 1985 Dec;23(11-12):1011–1028. doi: 10.1007/BF00499944. [DOI] [PubMed] [Google Scholar]
- Sun Y., Elwell J. H., Oberley L. W. A simultaneous visualization of the antioxidant enzymes glutathione peroxidase and catalase on polyacrylamide gels. Free Radic Res Commun. 1988;5(2):67–75. doi: 10.3109/10715768809066913. [DOI] [PubMed] [Google Scholar]
- Tateoka N., Tsuchida S., Soma Y., Sato K. Purification and characterization of glutathione S-transferases in human kidney. Clin Chim Acta. 1987 Jul 15;166(2-3):207–218. doi: 10.1016/0009-8981(87)90423-2. [DOI] [PubMed] [Google Scholar]
- Terrier P., Townsend A. J., Coindre J. M., Triche T. J., Cowan K. H. An immunohistochemical study of pi class glutathione S-transferase expression in normal human tissue. Am J Pathol. 1990 Oct;137(4):845–853. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A. H., Oberley T. D., Oberley L. W., Ramanathan R. Effect of cell substrate on antioxidant enzyme activities in cultured renal glomerular epithelium. Am J Pathol. 1988 Mar;130(3):616–628. [PMC free article] [PubMed] [Google Scholar]
- Yang A. H., Oberley T. D., Oberley L. W., Schmid S. M., Cummings K. B. In vitro modulation of antioxidant enzymes in normal and malignant renal epithelium. In Vitro Cell Dev Biol. 1987 Aug;23(8):546–558. doi: 10.1007/BF02620972. [DOI] [PubMed] [Google Scholar]