Abstract
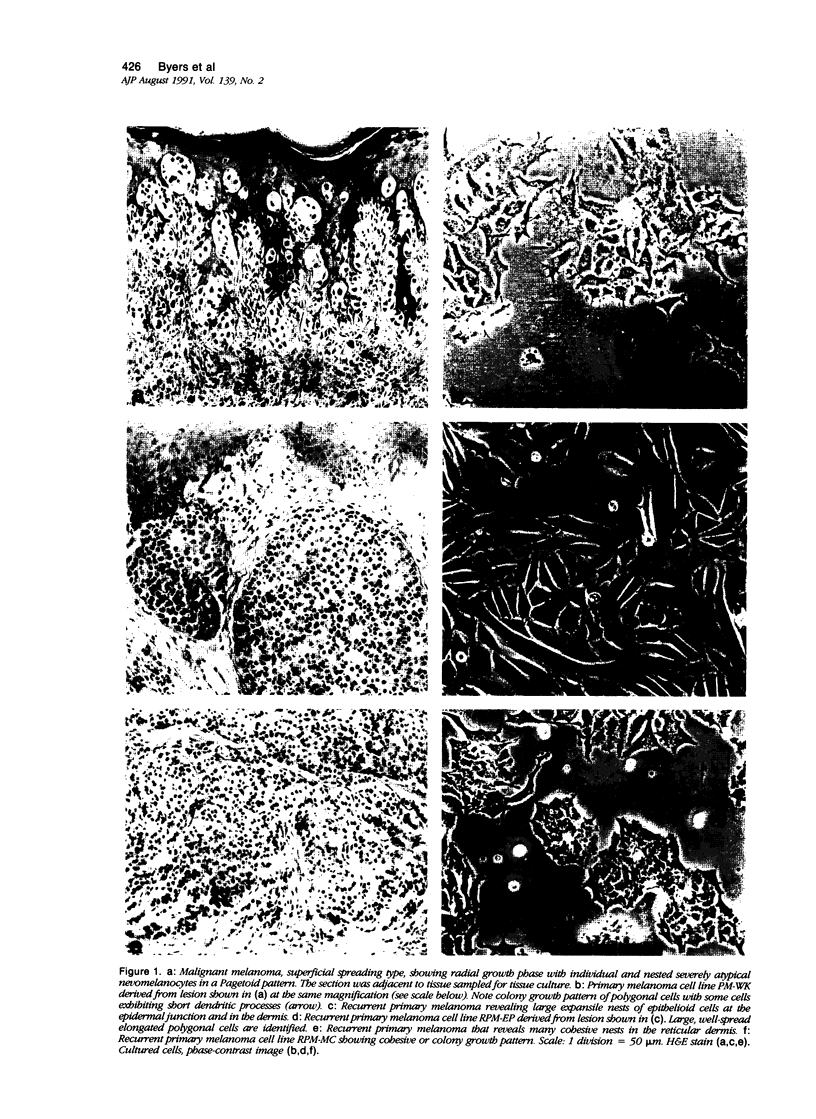
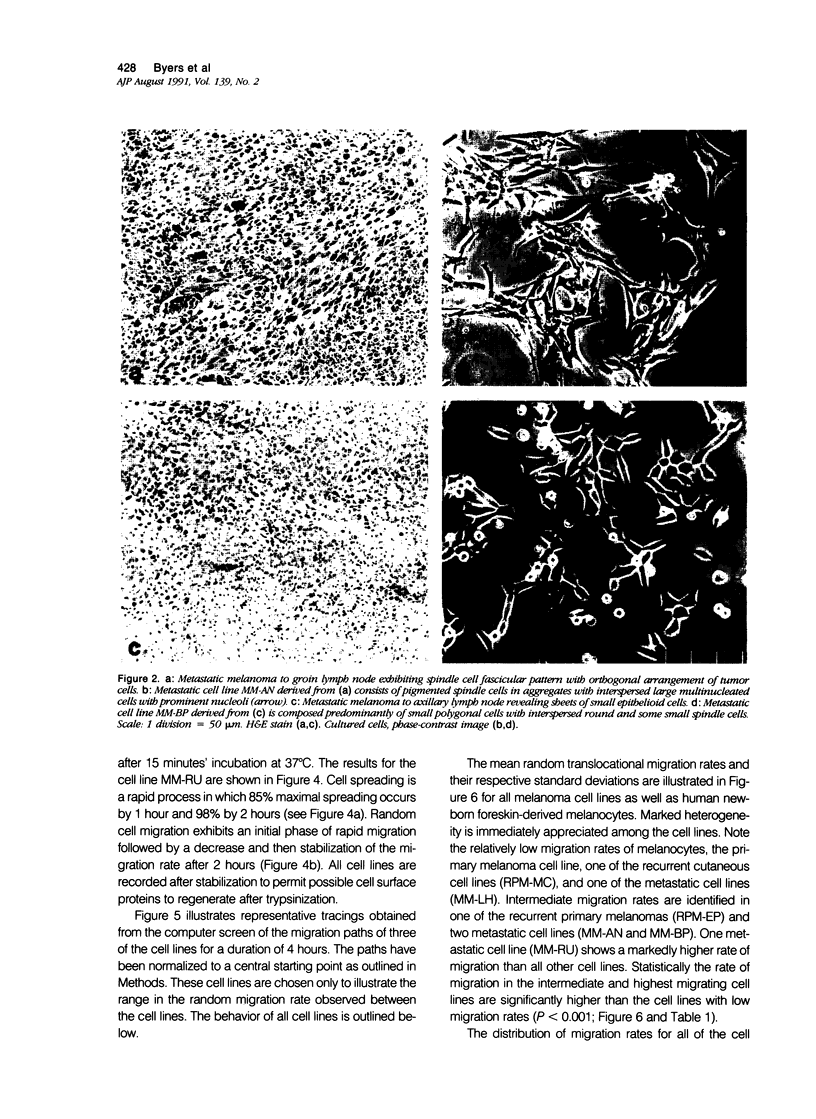
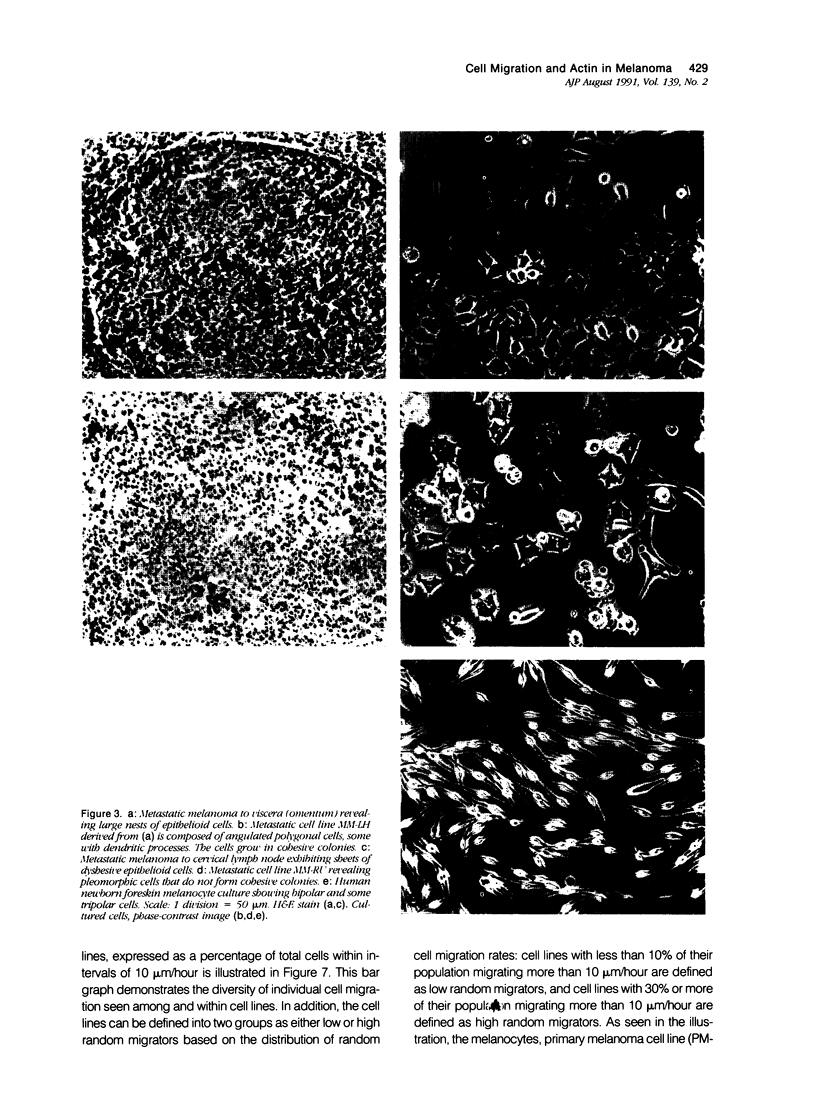
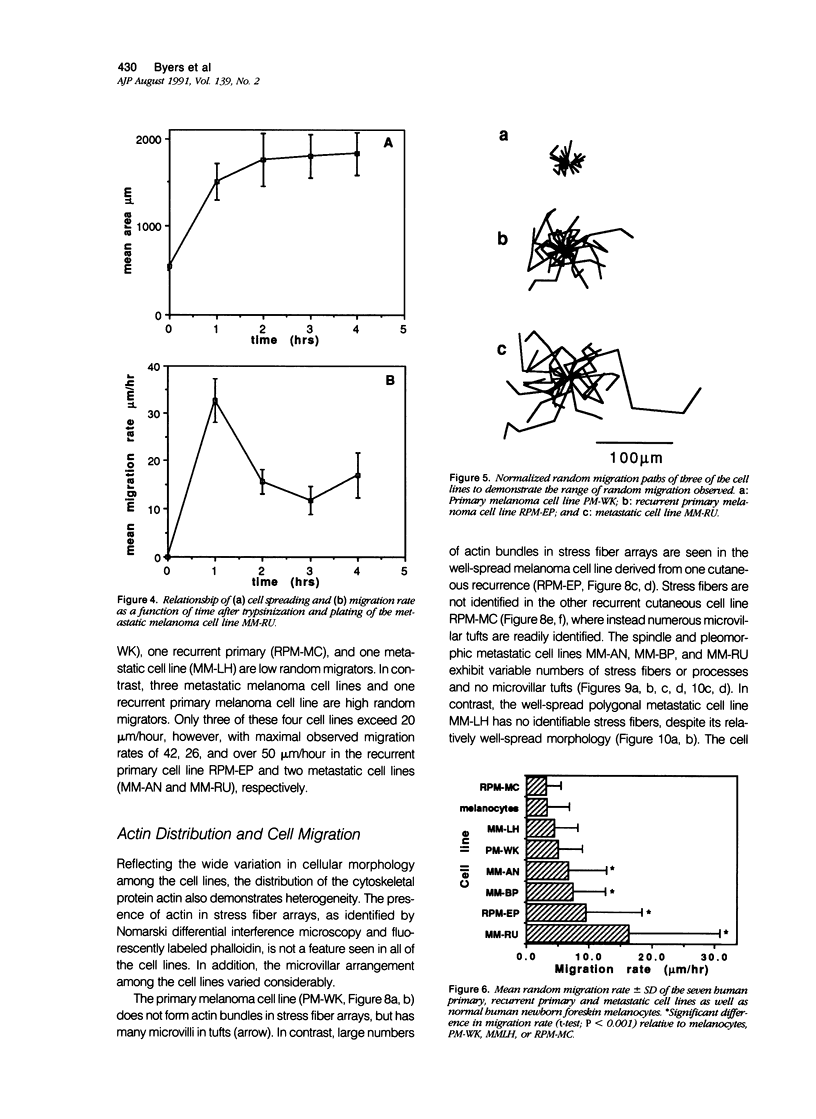
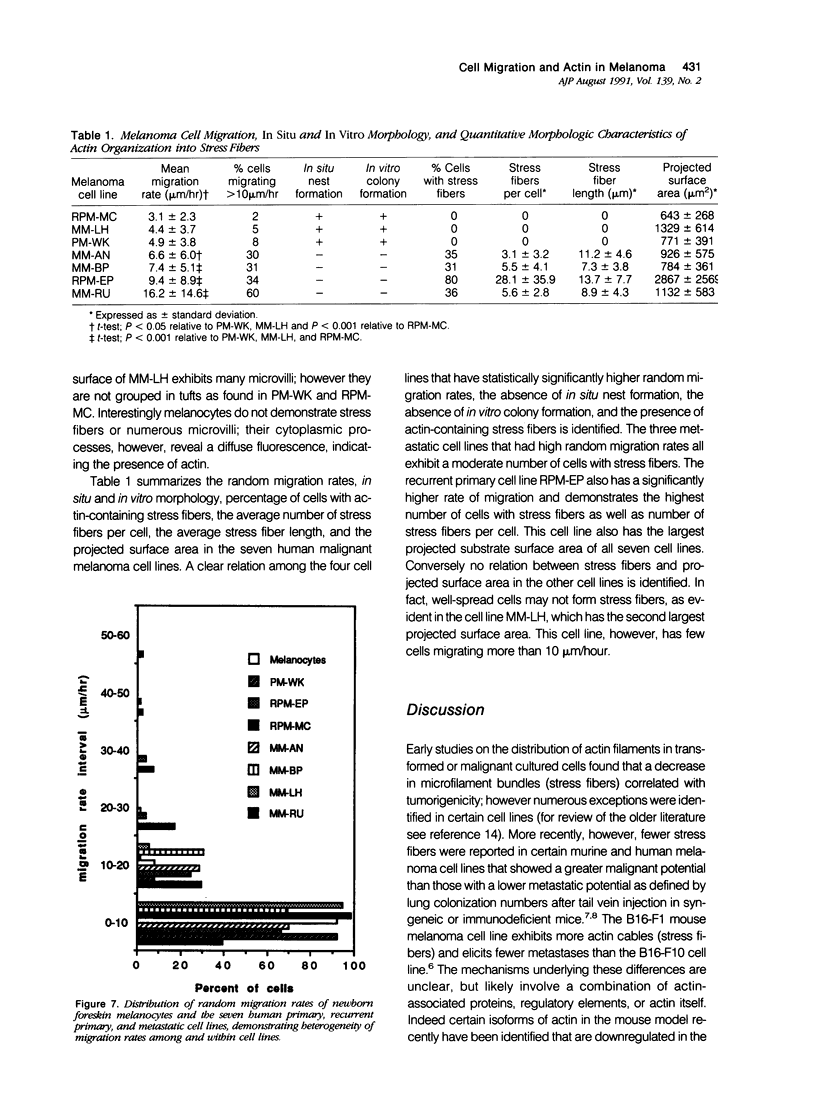
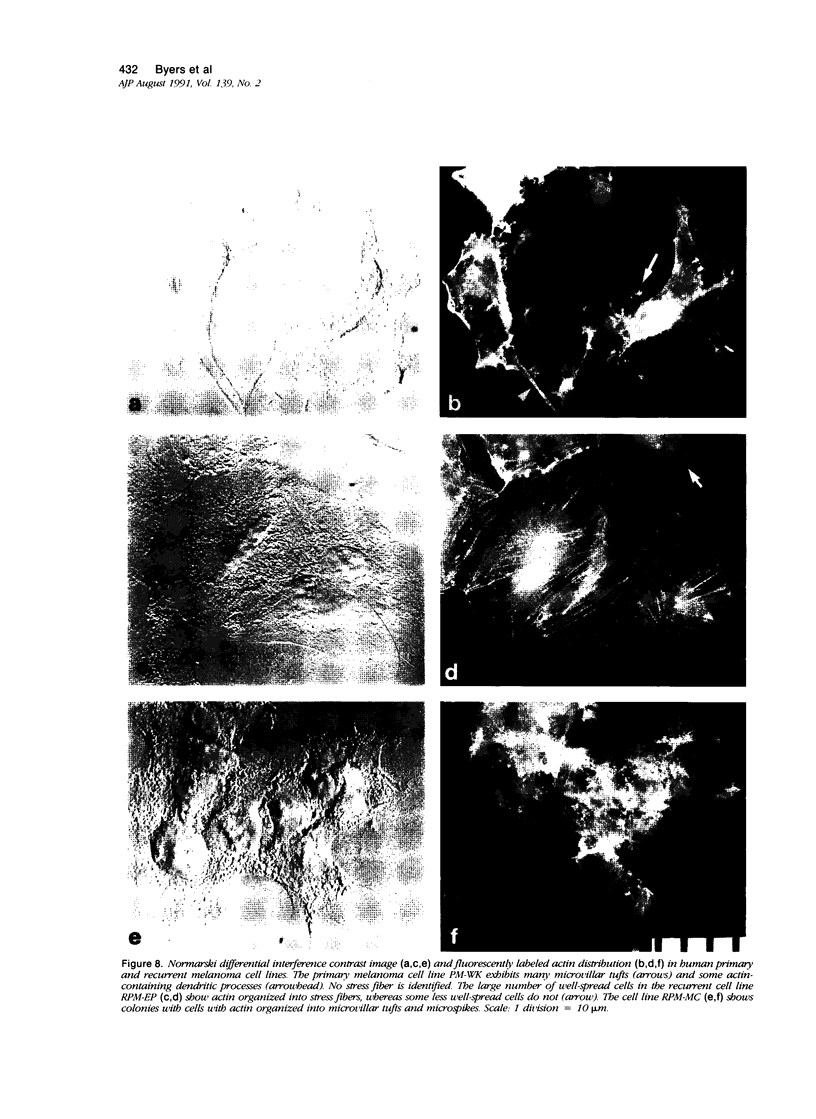
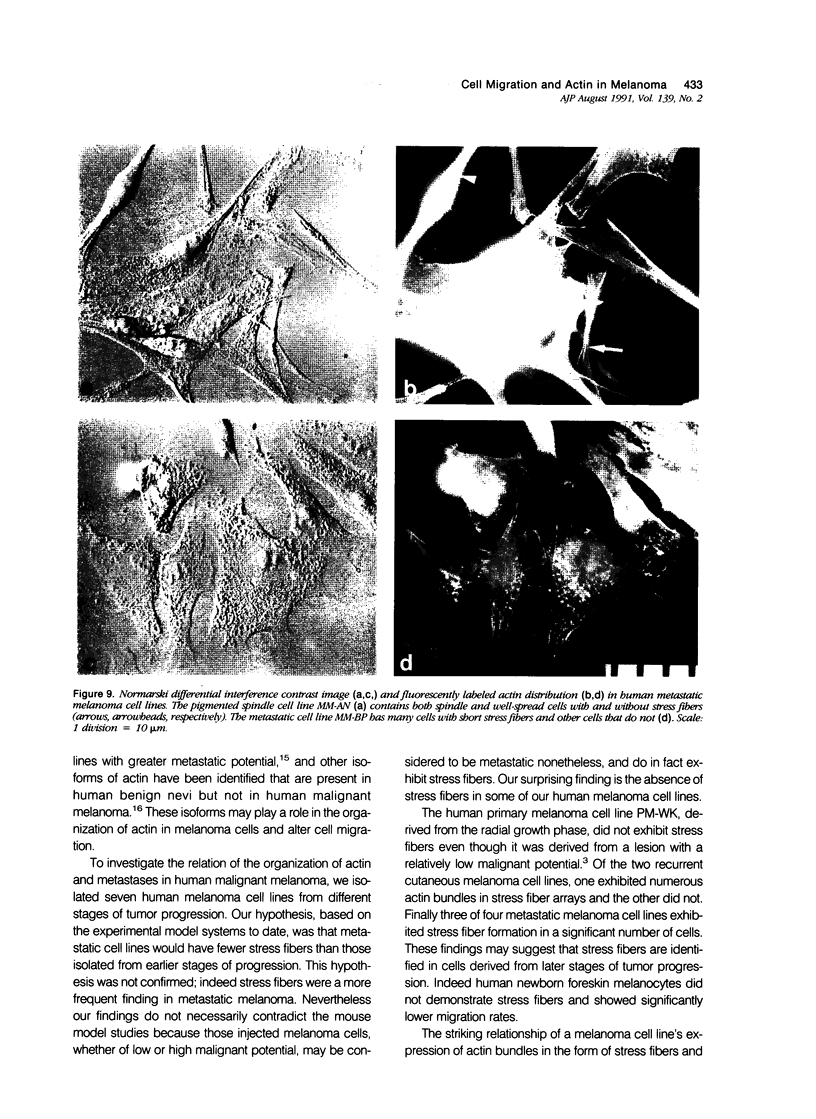
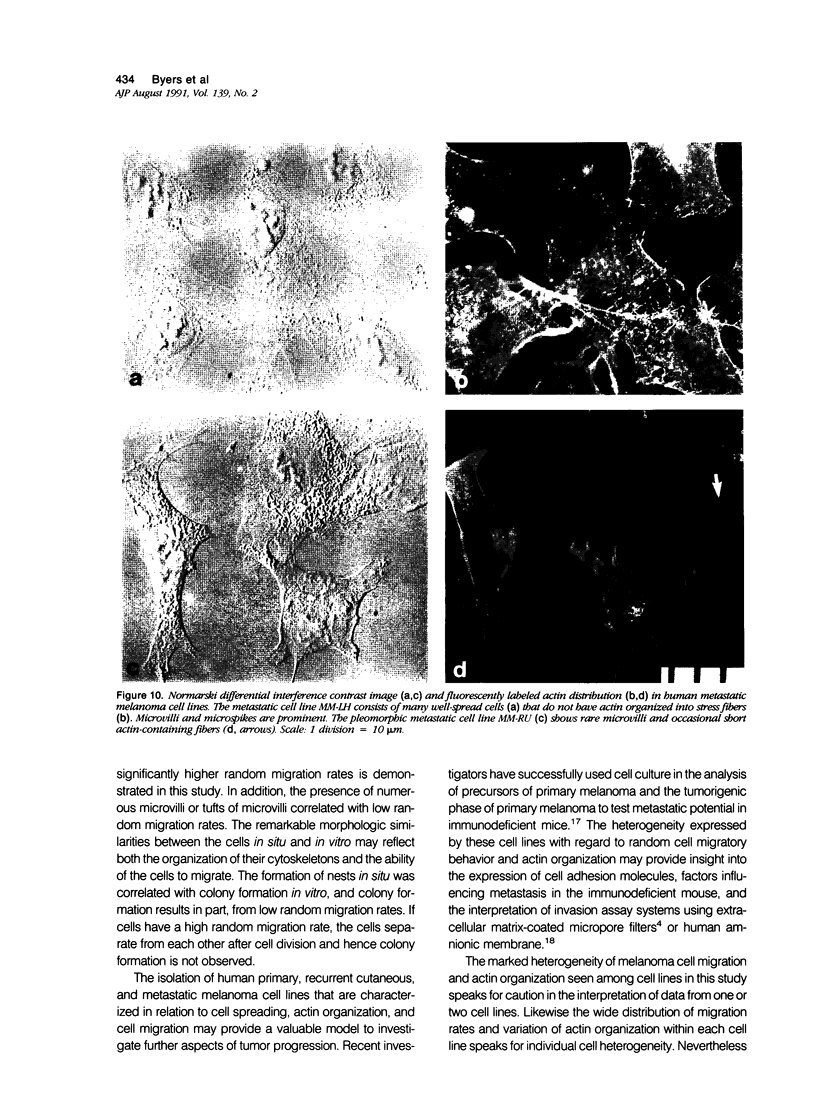
Random cell migration and actin organization in seven human primary, recurrent cutaneous, and metastatic melanoma cell lines were studied by time-lapse video recording and image analysis. The migration of over 800 randomly selected cells from the cell lines were recorded using an inverted microscope with an attached incubator housing. The fraction of cells with random migration rates greater than 10 microns/hour was 8% in an established primary melanoma cell line, 2% and 34% in two recurrent cutaneous melanoma cell lines, and 5%, 30%, 31%, and 60% in four metastatic cell lines. The three metastatic cell lines with significantly higher mean migration rates (P less than 0.001) were derived from lymph node metastases, whereas the fourth metastatic cell line was derived from a visceral metastasis. The cellular morphology and presence of cell nests in the original tissue correlated with in vitro cell morphology and the formation of colonies. The ability of cells to organize actin into stress fibers directly correlated with significantly higher random migration rates and lack of colony formation. Characterization of random migration rates and actin organization of human melanoma cells that are isolated from different stages of tumor progression may lend insight into metastasis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badenoch-Jones P., Ramshaw I. A. Spontaneous capillary tube migration of metastatic rat mammary adenocarcinoma cells. Invasion Metastasis. 1984;4(2):98–110. [PubMed] [Google Scholar]
- Byers H. R., White G. E., Fujiwara K. Organization and function of stress fibers in cells in vitro and in situ. A review. Cell Muscle Motil. 1984;5:83–137. doi: 10.1007/978-1-4684-4592-3_2. [DOI] [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Guerry D., 4th, Braitman L. E., Trock B. J., Schultz D., Synnestvedt M., Halpern A. C. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989 Dec 20;81(24):1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Guerry D., 4th, Epstein M. N., Greene M. H., Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984 Dec;15(12):1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Van Horn M. The biologic forms of malignant melanoma. Hum Pathol. 1986 May;17(5):443–450. doi: 10.1016/s0046-8177(86)80032-6. [DOI] [PubMed] [Google Scholar]
- Halaban R., Alfano F. D. Selective elimination of fibroblasts from cultures of normal human melanocytes. In Vitro. 1984 May;20(5):447–450. doi: 10.1007/BF02619590. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Seftor R. E., Misiorowski R. L., Saba P. Z., Sundareshan P., Welch D. R. Comparison of tumor cell invasion assays: human amnion versus reconstituted basement membrane barriers. Invasion Metastasis. 1989;9(5):278–297. [PubMed] [Google Scholar]
- Herlyn M., Thurin J., Balaban G., Bennicelli J. L., Herlyn D., Elder D. E., Bondi E., Guerry D., Nowell P., Clark W. H. Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res. 1985 Nov;45(11 Pt 2):5670–5676. [PubMed] [Google Scholar]
- Herman I. M., Crisona N. J., Pollard T. D. Relation between cell activity and the distribution of cytoplasmic actin and myosin. J Cell Biol. 1981 Jul;90(1):84–91. doi: 10.1083/jcb.90.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L., Verna J. M., Levinstone D., Sher S., Marek L., Bell E. The relationship of fibroblast translocations to cell morphology and stress fibre density. J Cell Sci. 1982 Feb;53:21–36. doi: 10.1242/jcs.53.1.21. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Wewer U., Rao N. C., Schiffmann E., Stracke M., Guirguis R., Thorgeirsson U., Muschel R., Sobel M. Biochemical mechanisms of tumor invasion and metastases. Adv Exp Med Biol. 1988;233:161–169. doi: 10.1007/978-1-4899-5037-6_18. [DOI] [PubMed] [Google Scholar]
- Nabi I. R., Raz A. Loss of metastatic responsiveness to cell shape modulation in a newly characterized B16 melanoma adhesive cell variant. Cancer Res. 1988 Mar 1;48(5):1258–1264. [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Riser B. L., Dixit V. M., Varani J. Modulation of keratinocyte motility. Correlation with production of extracellular matrix molecules in response to growth promoting and antiproliferative factors. Am J Pathol. 1988 Sep;132(3):543–551. [PMC free article] [PubMed] [Google Scholar]
- Raz A. Actin organization, cell motility, and metastasis. Adv Exp Med Biol. 1988;233:227–233. doi: 10.1007/978-1-4899-5037-6_25. [DOI] [PubMed] [Google Scholar]
- Raz A., Geiger B. Altered organization of cell-substrate contacts and membrane-associated cytoskeleton in tumor cell variants exhibiting different metastatic capabilities. Cancer Res. 1982 Dec;42(12):5183–5190. [PubMed] [Google Scholar]
- Raz A., Zöller M., Ben-Ze'ev Cell configuration and adhesive properties of metastasizing and non-metastasizing BSp73 rat adenocarcinoma cells. Exp Cell Res. 1986 Jan;162(1):127–141. doi: 10.1016/0014-4827(86)90431-3. [DOI] [PubMed] [Google Scholar]
- Sasase A., Mishima Y., Ichihashi M., Taniguchi S. Biochemical analysis of metastasis-related Ax actin in B16 mouse melanoma cells after chemical reversional modulation and of tumor progression-related A' actin in the ontogeny of human malignant melanoma. Pigment Cell Res. 1989 Nov-Dec;2(6):493–501. doi: 10.1111/j.1600-0749.1989.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Sharpe R. J., Byers H. R., Scott C. F., Bauer S. I., Maione T. E. Growth inhibition of murine melanoma and human colon carcinoma by recombinant human platelet factor 4. J Natl Cancer Inst. 1990 May 16;82(10):848–853. doi: 10.1093/jnci/82.10.848. [DOI] [PubMed] [Google Scholar]
- Taniguchi S., Sadano H., Kakunaga T., Baba T. Altered expression of a third actin accompanying malignant progression in mouse B16 melanoma cells. Jpn J Cancer Res. 1989 Jan;80(1):31–40. doi: 10.1111/j.1349-7006.1989.tb02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T., Geiger B., Raz A. Motility and adhesive properties of high- and low-metastatic murine neoplastic cells. Cancer Res. 1984 Feb;44(2):811–824. [PubMed] [Google Scholar]
- Zachary J. M., Cleveland G., Kwock L., Lawrence T., Weissman R. M., Nabell L., Fried F. A., Staab E. V., Risinger M. A., Lin S. Actin filament organization of the Dunning R3327 rat prostatic adenocarcinoma system: correlation with metastatic potential. Cancer Res. 1986 Feb;46(2):926–932. [PubMed] [Google Scholar]