Abstract
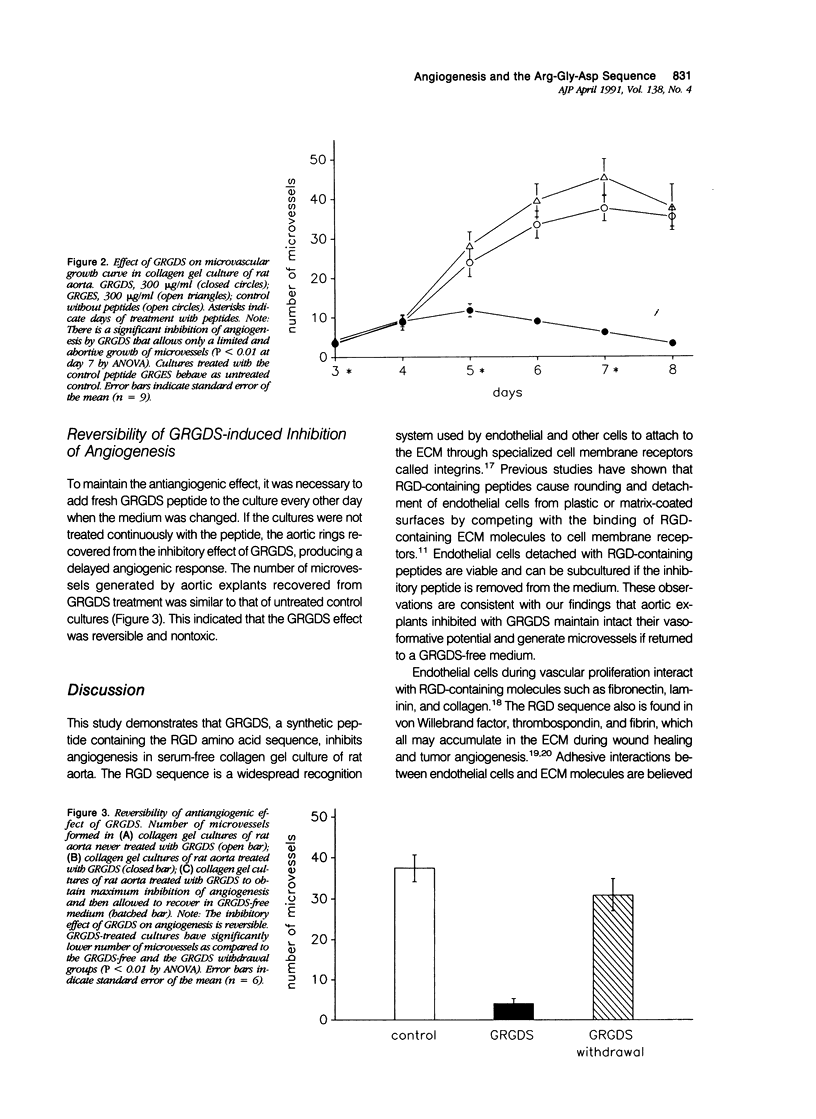
This study was designed to evaluate the effect of the synthetic peptide Gly-Arg-Gly-Asp-Ser (GRGDS) on angiogenesis in serum-free collagen gel culture of rat aorta. The GRGDS peptide contains the amino acid sequence Arg-Gly-Asp (RGD), which has been implicated as a recognition site in interactions between extracellular matrix (ECM) molecules and cell membrane receptors. RGD-containing synthetic peptides are known to inhibit attachment of endothelial cells to substrates, but their effect on angiogenesis has not been fully characterized. Aortic explants embedded in collagen gel in the absence of GRGDS generated branching microvessels through a process of endothelial migration and proliferation. Addition of GRGDS to the culture medium caused a marked inhibition of angiogenesis. In contrast, GRGES, a control peptide lacking the RGD sequence, failed to inhibit angiogenesis. The inhibitory effect of GRGDS was nontoxic and reversible. The angiogenic activity of aortic explants previously inhibited with GRGDS could be restored by incubating the cultures in GRGDS-free medium. These findings suggest that angiogenesis is an anchorage-dependent process that can be inhibited by interfering with the attachment of endothelial cells to the ECM. It also indicates that synthetic peptides can be used as probes to study the mechanisms by which the ECM regulates angiogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Daise M., Levine E. M., Buck C. A. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989 Jun;83(6):1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausprunk D. H. Synthesis of glycoproteins by endothelial cells in embryonic blood vessels. Dev Biol. 1982 Mar;90(1):79–90. doi: 10.1016/0012-1606(82)90213-5. [DOI] [PubMed] [Google Scholar]
- Basson C. T., Knowles W. J., Bell L., Albelda S. M., Castronovo V., Liotta L. A., Madri J. A. Spatiotemporal segregation of endothelial cell integrin and nonintegrin extracellular matrix-binding proteins during adhesion events. J Cell Biol. 1990 Mar;110(3):789–801. doi: 10.1083/jcb.110.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaut J. C., Darribère T., Poole T. J., Aoyama H., Yamada K. M., Thiery J. P. Biologically active synthetic peptides as probes of embryonic development: a competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984 Nov;99(5):1822–1830. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy M., Armstrong M. T., Armstrong P. B. Regulation of proliferation of embryonic heart mesenchyme: role of transforming growth factor-beta 1 and the interstitial matrix. Dev Biol. 1990 Oct;141(2):421–425. doi: 10.1016/0012-1606(90)90396-z. [DOI] [PubMed] [Google Scholar]
- Dejana E., Lampugnani M. G., Giorgi M., Gaboli M., Federici A. B., Ruggeri Z. M., Marchisio P. C. Von Willebrand factor promotes endothelial cell adhesion via an Arg-Gly-Asp-dependent mechanism. J Cell Biol. 1989 Jul;109(1):367–375. doi: 10.1083/jcb.109.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Fajardo L. F. The complexity of endothelial cells. A review. Am J Clin Pathol. 1989 Aug;92(2):241–250. doi: 10.1093/ajcp/92.2.241. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol. 1985 Jun;100(6):1948–1954. doi: 10.1083/jcb.100.6.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989 Sep 8;58(5):803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Knedler A., Ham R. G. Optimized medium for clonal growth of human microvascular endothelial cells with minimal serum. In Vitro Cell Dev Biol. 1987 Jul;23(7):481–491. doi: 10.1007/BF02628418. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988 Jul;132(1):18–27. [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Orci L., Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983 Nov;97(5 Pt 1):1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia R. F., Madri J. A. The microvascular extracellular matrix. Developmental changes during angiogenesis in the aortic ring-plasma clot model. Am J Pathol. 1987 Jul;128(1):78–90. [PMC free article] [PubMed] [Google Scholar]
- Nicosia R. F., Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990 Jul;63(1):115–122. [PubMed] [Google Scholar]
- Nicosia R. F., Tchao R., Leighton J. Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro. 1982 Jun;18(6):538–549. doi: 10.1007/BF02810077. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Chopek M., Chao S., McDonald T., Schwartz S. M. Injury induces increase of von Willebrand factor in rat endothelial cells. Am J Pathol. 1989 Apr;134(4):857–864. [PMC free article] [PubMed] [Google Scholar]
- Risau W., Lemmon V. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol. 1988 Feb;125(2):441–450. doi: 10.1016/0012-1606(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Taraboletti G., Roberts D., Liotta L. A., Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990 Aug;111(2):765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Peptide inhibitors of fibronectin, laminin, and other adhesion molecules: unique and shared features. J Cell Physiol. 1987 Jan;130(1):21–28. doi: 10.1002/jcp.1041300105. [DOI] [PubMed] [Google Scholar]
- Young W. C., Herman I. M. Extracellular matrix modulation of endothelial cell shape and motility following injury in vitro. J Cell Sci. 1985 Feb;73:19–32. doi: 10.1242/jcs.73.1.19. [DOI] [PubMed] [Google Scholar]