Abstract
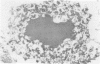
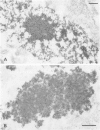
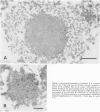
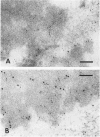
Ultrastructural immunoreactivities of alpha B-crystallin, glial fibrillary acidic protein (GFAP), ubiquitin, and vimentin in Rosenthal fibers (RFs) isolated from an Alexander's disease brain were investigated using nonosmium and low-temperature embedding technique. The morphology of RFs embedded in Lowicryl K4M resin was well preserved after treatment with 0.5% Triton X-100. alpha B-crystallin immunoreactivity was present in RFs of various sizes and was the strongest in loosely scattered deposits, which were considered to be the initial stage of RFs. Glial fibrillary acidic protein immunoreactivity in RFs was heavy, homogeneous throughout RFs, and equivalent to that in networks of glial filaments. Immunoreactivities of both alpha B-crystallin and GFAP were mainly restricted to the high electron-dense areas within RFs and were proved to exist close to each other by double immunolabeling. Rosenthal fibers were negative for vimentin. Ubiquitin immunoreactivity was relatively homogeneous in RFs with small diameters, but in RFs with large diameters, the immunoreactivity diminished in the center. Based on these observations, combined with the tendency of self-aggregation of alpha B-crystallin, it is conceivable that RFs are huge aggregation products of alpha B-crystallin involving GFAP, and that ubiquitination may be a consequent phenomenon, as it may be in other intracytoplasmic inclusions, such as neurofibrillary tangles and Lewy bodies.
Full text
PDF


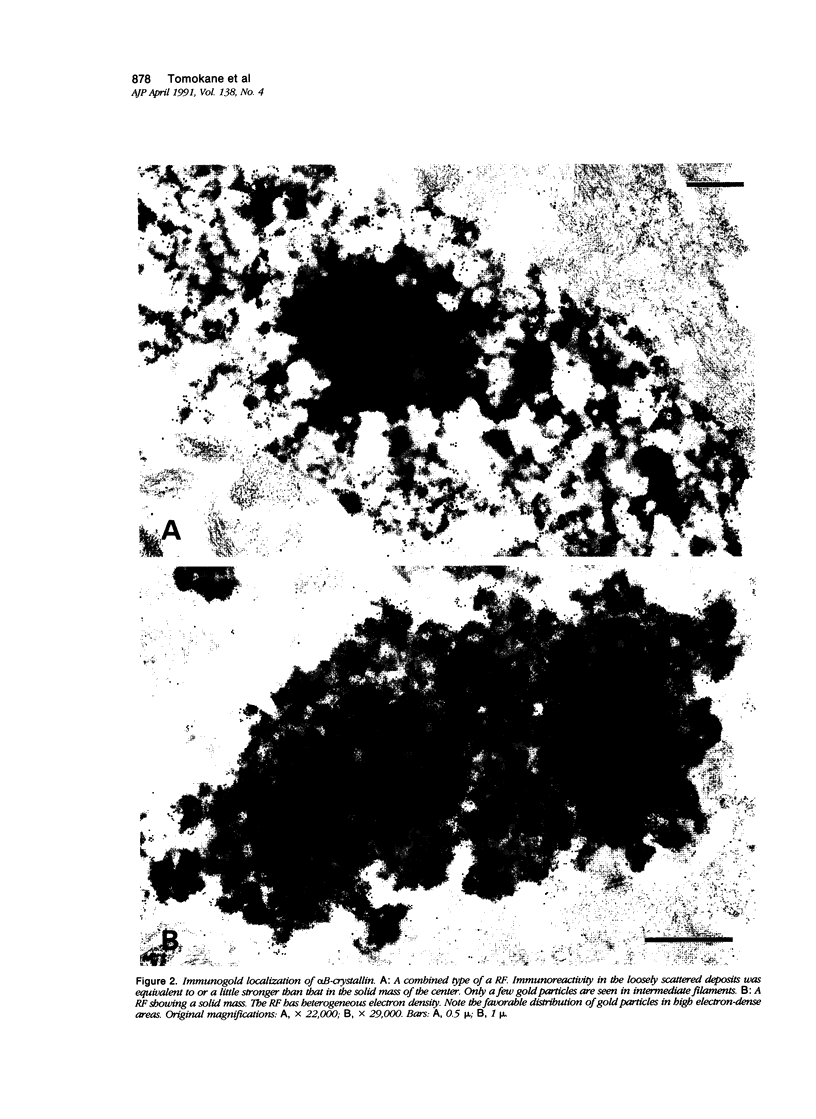

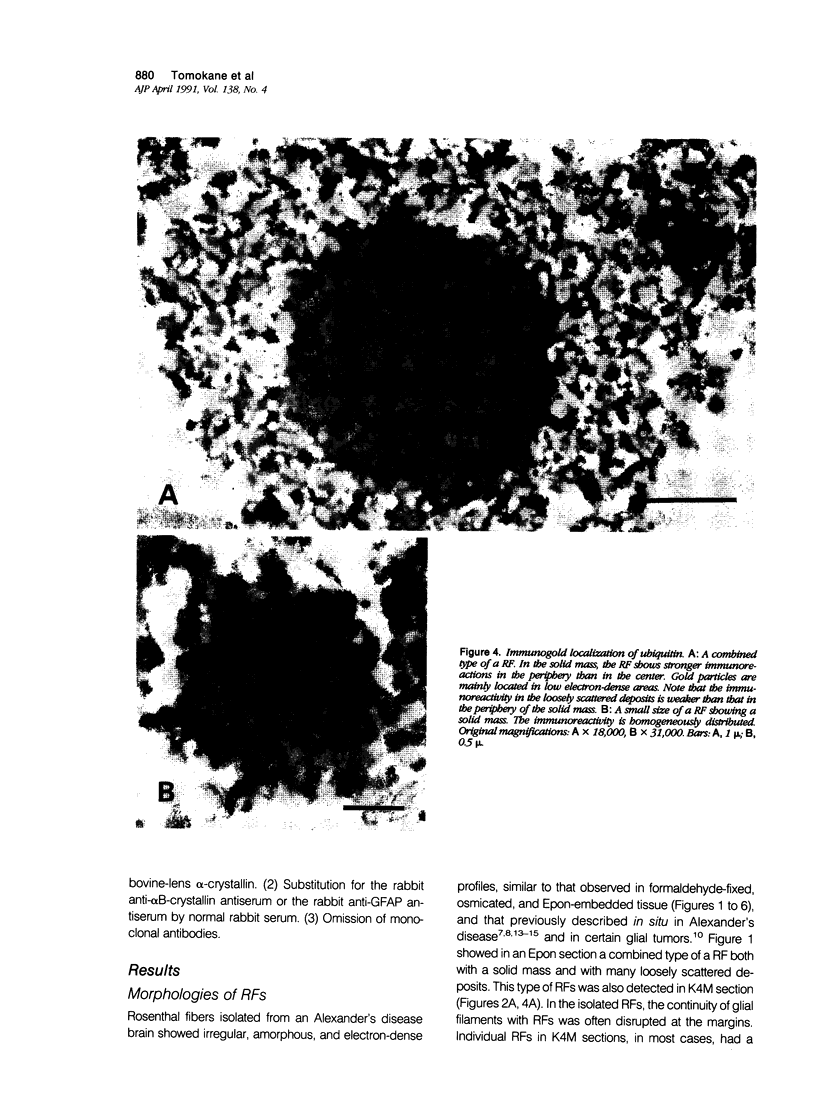
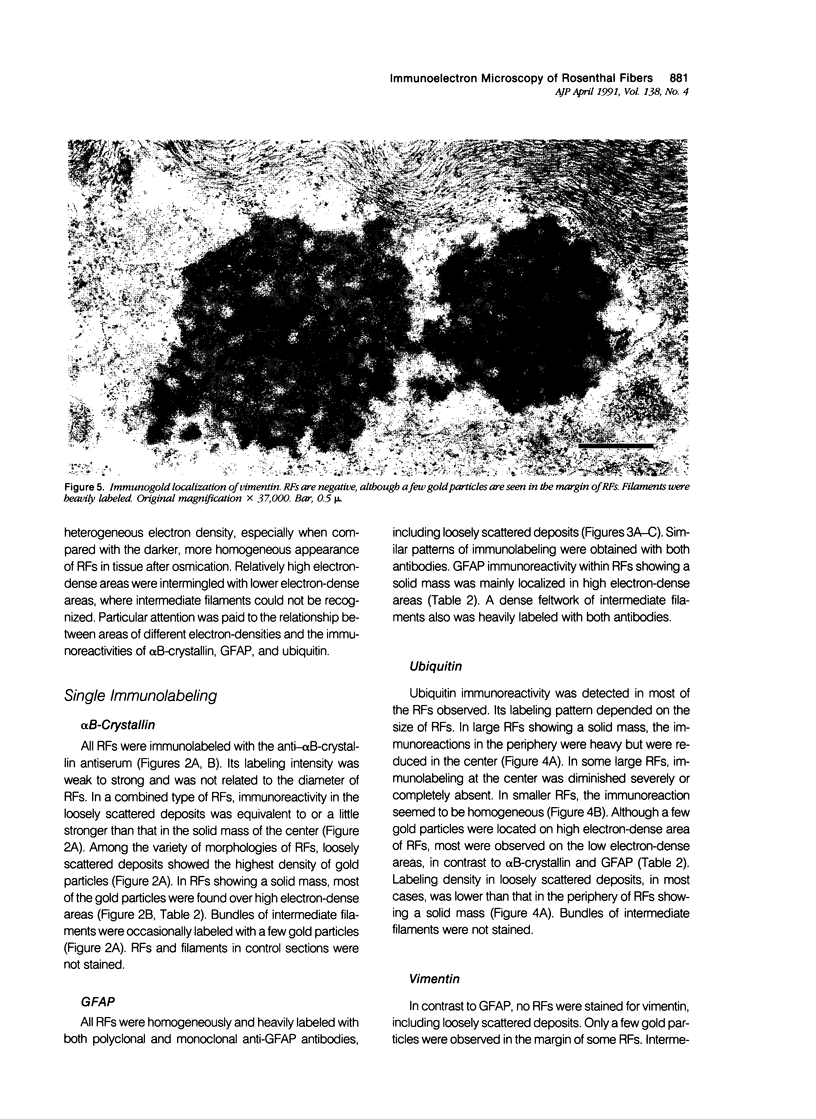

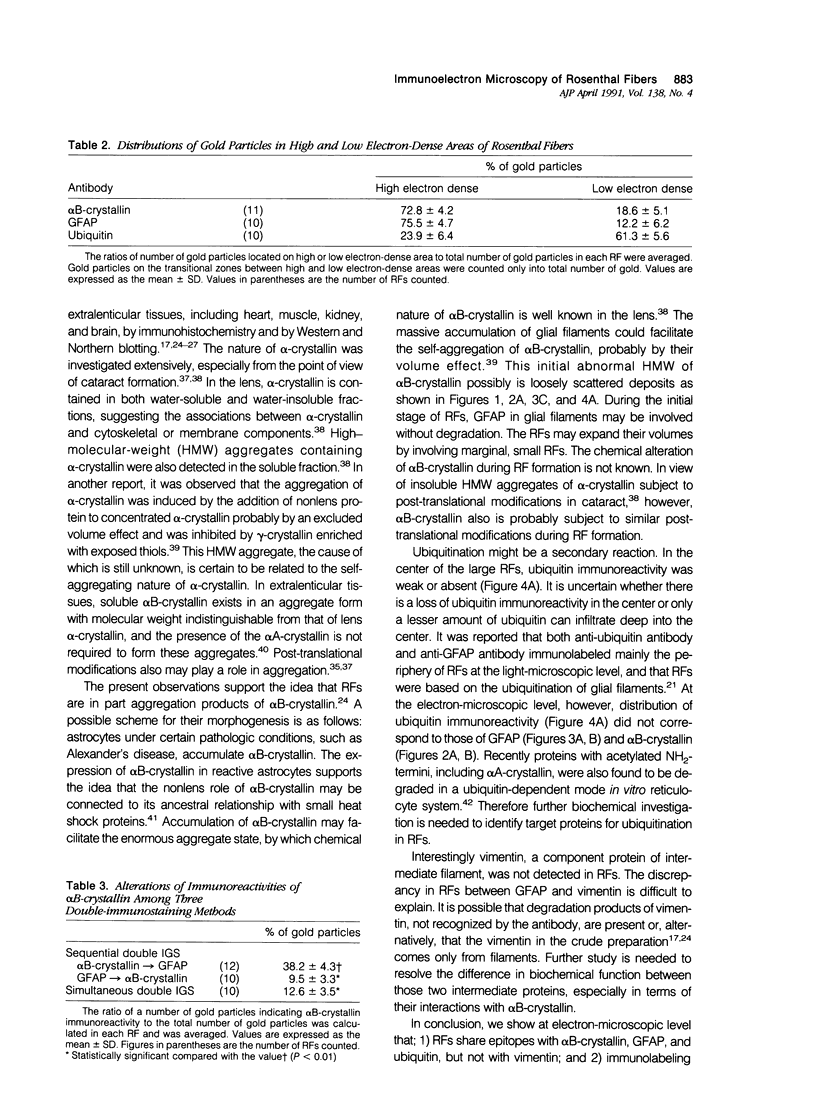


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER W. S. Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain. 1949 Sep;72(3):373-81, 3 pl. doi: 10.1093/brain/72.3.373. [DOI] [PubMed] [Google Scholar]
- Altman L. G., Schneider B. G., Papermaster D. S. Rapid embedding of tissues in Lowicryl K4M for immunoelectron microscopy. J Histochem Cytochem. 1984 Nov;32(11):1217–1223. doi: 10.1177/32.11.6436366. [DOI] [PubMed] [Google Scholar]
- Bettica A., Johnson A. B. Ultrastructural immunogold labeling of glial filaments in osmicated and unosmicated epoxy-embedded tissue. J Histochem Cytochem. 1990 Jan;38(1):103–109. doi: 10.1177/38.1.2152935. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Nagineni C. N. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989 Jan 16;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Bloemendal H. The vertebrate eye lens. Science. 1977 Jul 8;197(4299):127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Borrett D., Becker L. E. Alexander's disease. A disease of astrocytes. Brain. 1985 Jun;108(Pt 2):367–385. doi: 10.1093/brain/108.2.367. [DOI] [PubMed] [Google Scholar]
- CROME L. Megalencephaly associated with hyaline pan-neuropathy. Brain. 1953 Jun;76(2):215–228. doi: 10.1093/brain/76.2.215. [DOI] [PubMed] [Google Scholar]
- Chiesa R., McDermott M. J., Mann E., Spector A. The apparent molecular size of native alpha-crystallin B in non-lenticular tissues. FEBS Lett. 1990 Jul 30;268(1):222–226. doi: 10.1016/0014-5793(90)81013-e. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25(2):193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Dinda A. K., Sarkar C., Roy S. Rosenthal fibres: an immunohistochemical, ultrastructural and immunoelectron microscopic study. Acta Neuropathol. 1990;79(4):456–460. doi: 10.1007/BF00308723. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDE R. L. ALEXANDER'S DISEASE. Arch Neurol. 1964 Oct;11:414–422. doi: 10.1001/archneur.1964.00460220076010. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Chiu F. C. Growth kinetics, cell shape, and the cytoskeleton of primary astrocyte cultures. J Neurochem. 1984 Jan;42(1):175–184. doi: 10.1111/j.1471-4159.1984.tb09714.x. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Corbin E. Isolation of a major protein component of Rosenthal fibers. Am J Pathol. 1988 Mar;130(3):569–578. [PMC free article] [PubMed] [Google Scholar]
- Gullotta F., Fliedner E. Spongioblastomas, astrocytomas and Rosenthal fibers. Ultrastructural, tissue culture and enzyme histochemical investigations. Acta Neuropathol. 1972;22(1):68–78. doi: 10.1007/BF00687551. [DOI] [PubMed] [Google Scholar]
- Herndon R. M., Rubinstein L. J., Freeman J. M., Mathieson G. Light and electron microscopic observations on Rosenthal fibers in Alexander's disease and in multiple sclerosis. J Neuropathol Exp Neurol. 1970 Oct;29(4):524–551. doi: 10.1097/00005072-197010000-00002. [DOI] [PubMed] [Google Scholar]
- Hershko A., Eytan E., Ciechanover A., Haas A. L. Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. Relationship to the breakdown of abnormal proteins. J Biol Chem. 1982 Dec 10;257(23):13964–13970. [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Goldman J. E. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990 Jan;38(1):31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Janzer R. C., Friede R. L. Do Rosenthal fibers contain glial fibrillary acid protein? Acta Neuropathol. 1981;55(1):75–76. doi: 10.1007/BF00691535. [DOI] [PubMed] [Google Scholar]
- Kress Y., Gaskin F., Horoupian D. S., Brosnan C. Nickel induction of Rosenthal fibers in rat brain. Brain Res. 1981 Apr 6;210(1-2):419–425. doi: 10.1016/0006-8993(81)90920-3. [DOI] [PubMed] [Google Scholar]
- Lowe J., Blanchard A., Morrell K., Lennox G., Reynolds L., Billett M., Landon M., Mayer R. J. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease, and Alzheimer's disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988 May;155(1):9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- Lowe J., Morrell K., Lennox G., Landon M., Mayer R. J. Rosenthal fibres are based on the ubiquitination of glial filaments. Neuropathol Appl Neurobiol. 1989 Jan-Feb;15(1):45–53. doi: 10.1111/j.1365-2990.1989.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Mach H., Trautman P. A., Thomson J. A., Lewis R. V., Middaugh C. R. Inhibition of alpha-crystallin aggregation by gamma-crystallin. J Biol Chem. 1990 Mar 25;265(9):4844–4848. [PubMed] [Google Scholar]
- Manetto V., Perry G., Tabaton M., Mulvihill P., Fried V. A., Smith H. T., Gambetti P., Autilio-Gambetti L. Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative diseases. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4501–4505. doi: 10.1073/pnas.85.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Siegel N. R., Schwartz A. L., Ciechanover A. Degradation of proteins with acetylated amino termini by the ubiquitin system. Science. 1989 Jun 23;244(4911):1480–1483. doi: 10.1126/science.2544030. [DOI] [PubMed] [Google Scholar]
- Newman G. R., Jasani B., Williams E. D. A simple post-embedding system for the rapid demonstration of tissue antigens under the electron microscope. Histochem J. 1983 Jun;15(6):543–555. doi: 10.1007/BF01954145. [DOI] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Russo L. S., Jr, Aron A., Anderson P. J. Alexander's disease: a report and reappraisal. Neurology. 1976 Jul;26(7):607–614. doi: 10.1212/wnl.26.7.607. [DOI] [PubMed] [Google Scholar]
- Seil F. J., Schochet S. S., Jr, Earle K. M. Alexander's disease in an adult. Report of a case. Arch Neurol. 1968 Nov;19(5):494–502. doi: 10.1001/archneur.1968.00480050064006. [DOI] [PubMed] [Google Scholar]
- Tihen W. S. Central pontine myelinolysis and Rosenthal fibers of the brainstem. Association with emaciation and prolonged intravenous hyperalimentation. Neurology. 1972 Jul;22(7):710–716. doi: 10.1212/wnl.22.7.710. [DOI] [PubMed] [Google Scholar]
- Towfighi J., Young R., Sassani J., Ramer J., Horoupian D. S. Alexander's disease: further light-, and electron-microscopic observations. Acta Neuropathol. 1983;61(1):36–42. doi: 10.1007/BF00688384. [DOI] [PubMed] [Google Scholar]
- Walls T. J., Jones R. A., Cartlidge N., Saunders M. Alexander's disease with Rosenthal fibre formation in an adult. J Neurol Neurosurg Psychiatry. 1984 Apr;47(4):399–403. doi: 10.1136/jnnp.47.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]