Abstract
Type II NaPi cotransporters are expressed in the apical membrane of Pi-(re)absorbing epithelia: the type IIa in renal proximal tubule and the type IIb in small intestine. Parathyroid hormone (PTH) leads to a retrieval from the apical membrane of the type IIa NaPi cotransporter. The type IIa cotransporter is also expressed in opossum kidney (OK) cells, and its expression is under the control of PTH. In the present study, we identified the molecular “domains” involved in the PTH-induced retrieval of the type IIa NaPi cotransporter. Wild-type mouse type IIa (mIIa) and type IIb (mIIb) as well as several mIIa-mIIb chimeras and site-directed mutants were fused to the enhanced green fluorescent protein and transfected into OK cells. We found that mIIa but not mIIb was internalized and degraded after incubation with 1–34 (or 3–34) PTH. Using chimeras, we found that the N and C termini were not required in this effect, whereas a “domain” located between residues 216 and 658 seemed to be necessary. This region contains two putative intracellular loops with highly conserved sequences between mIIa and mIIb; in the last intracellular loop, two charged amino acids of type IIa (K503R504) are replaced by uncharged residues in type IIb (N520I521). We generated two mutants in which these residues were interchanged: mIIaNI and mIIbKR. Similarly to mIIa, the mIIbKR mutant was endocytosed in response to 1–34 PTH; in contrast, mIIaNI behaved as mIIb and was not internalized. In conclusion, a dibasic amino acid motif (K503R504) located in the last intracellular loop of the type IIa NaPi cotransporter is essential for its PTH-induced retrieval.
Keywords: PTH, endocytosis
Regulated Pi reabsorption in the renal proximal tubule is an important element in overall Pi homeostasis. Its rate is mainly controlled by the amount of type IIa NaPi cotransporters expressed in the brush-border membrane of proximal tubular cells (1–4).
Parathyroid hormone (PTH) is the main phosphaturic hormone (5). PTH exerts its effect by an inhibition of proximal tubular brush-border NaPi cotransport associated with a membrane retrieval and lysosomal degradation of type IIa NaPi cotransporters (4, 6–9). These PTH-induced regulations are retained in cultured opossum kidney (OK) cells for both the endogenous (NaPi-4) and the transfected rat (NaPi-2) type IIa NaPi cotransporter (10–14). In proximal tubules and in OK cells, two signaling pathways are involved in PTH-induced down-regulation of Pi transport and of type IIa NaPi cotransporter: protein kinase A and protein kinase C (15–19). The mechanisms by which these kinase activities affect the cotransporter are not known.
The small intestinal brush-border membrane contains the type IIb NaPi cotransporter, identified in a procedure based on homology to IIa (20). Type IIa and type IIb NaPi cotransporters differ in several properties such as tissue distribution (20–22), pH sensitivity (1, 20, 23), and Na+ and Pi kinetics (1, 20, 24). Type IIa and type IIb NaPi cotransporters are differently regulated; the type IIa NaPi cotransporter shows a “dual” regulatory behavior, involving either fast de novo synthesis-independent (e.g., PTH and other peptide hormones; acute change in Pi intake) and/or slow mechanisms, which may require de novo synthesis of the transporter (e.g., growth factors) (4, 8). Less is known about regulation of the type IIb NaPi cotransporter. Recently, it was shown that its brush-border expression in rat duodenum is increased under conditions of chronic Pi deprivation and/or increased levels of 1,25-(OH)2 vitamin D3 (25). Rapid regulation involving fast membrane retrieval/insertion seems not to occur for the type IIb.
The renal type IIa and the small intestinal type IIb are about 75% homologous (20) and are suggested to have a similar topological structure including 8 to 10 transmembrane domains with cytoplasmic N- and C-terminal tails (26, 27). Major sequence differences between type IIa and type IIb cotransporters are located in the N and the C termini (20). The homology between type IIa and type IIb NaPi cotransporters offers the possibility to identify molecular “domains” within the two transporters potentially associated with the isoform-specific regulatory behavior. In the present study, we show that the type IIa but not the type IIb NaPi cotransporter transfected into OK cells is internalized and degraded upon treatment with PTH (analogues 1–34 and 3–34). Chimera constructions and subsequent site-directed mutagenesis indicated that the membrane retrieval of the type IIa transporter involves sequences within the putative last intracellular loop, notably two charged amino acids (K503R504) present in type IIa but not in type IIb.
Materials and Methods
Materials.
Fugene was obtained from Boehringer Mannheim, PTH peptides (1–34 and 3–34) and 1.4-diazabicyclo-(2.2.2) octane from Sigma, pEGFP-C1 vector from CLONTECH, and oligonucleotides from Microsynth (Balgach).
Mutagenesis.
NaPi-pEGFP constructs.
Mouse type IIa (mIIa) and type IIb (mIIb) NaPi cotransporters were fused to the C terminus of the enhanced green fluorescent protein (EGFP) by inserting their cDNAs into the pEGFP-C1 vector. For this purpose, new restriction sites were introduced by PCR at the 5′-end and 3′-end of both cDNAs: BglII–SacII for mIIa and SacI–SalI for mIIb. The cDNAs as well as the pEGFP plasmid were digested with the respective enzymes and ligated to generate the NaPi-pEGFP constructs.
mIIa-mIIb and mIIb-mIIa chimeras.
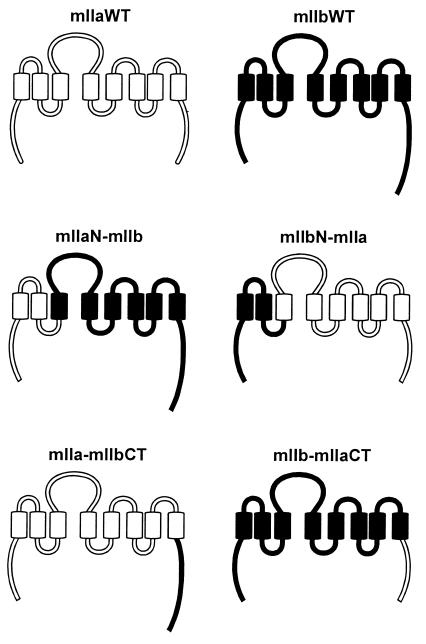
Several chimeras containing different portions of mIIa and mIIb were constructed (Fig. 1). To generate the mIIaN-mIIb and mIIbN-mIIa chimeras, an SpeI site was introduced by PCR into mIIa- and mIIb-pEGFP at position 216 and 213, respectively. The mutated plasmids were digested with SpeI and SacII. Each digestion generated two fragments. Both fragments were purified, and the mIIaN-mIIb and mIIbN-mIIa chimeras were constructed by interchanging the SpeI–SacII fragments. Similar procedures were followed to generate the mIIa-mIIbCT and mIIb-mIIaCT constructs (Fig. 1). In both cases, an SpeI site was introduced at positions 556 and 573 of the mIIa- and mIIb-pEGFP, respectively. All PCR reactions were carried out in the presence of complementary sense and antisense primers that contained the new restriction site in the middle of their sequences.
Figure 1.
Schematic representation of wt and chimeric NaPi cotransporters. The cytoplasmic tails and the extracellular and intracellular loops are indicated in open (for mIIa) and filled (for mIIb) bars. The transmembrane domains are shown in open (for mIIa) and filled (for mIIb) boxes. Chimeric transporters were generated by interchanging either an N-terminal domain (mIIaN-mIIb and mIIbN-mIIa) or the C-terminal tail (mIIa-mIIbCT and mIIb-mIIaCT) of mIIa and mIIb cotransporters.
mIIaNI, mIIbKR, mIIbN, and mIIbN-mIIaCT mutants.
All mutants were generated by PCR using complementary sense and antisense primers and mIIa-, mIIb-pEGFP, or the mIIb-mIIaCT chimeras as templates. In the mIIaNI mutant, we replaced the residues K503R504 by NI; in the mIIbKR mutant, the residues N520I521 were substituted by KR; in the mIIbN and mIIbN-mIIaCT mutants, the residue G388 was replaced by N.
All mutations were confirmed by sequencing, and two independent clones from each construct were used for the experiments.
Cell Culture and Transient Transfections.
OK cells were plated in 35-mm plastic dishes and maintained in the appropriate medium (28). Cultures at subconfluence were transfected with 3 μl of Fugene and 1-μg plasmids according to the manufacturer's instructions. Occasionally (e.g., for the transport experiments), cells were also transfected with Lipofectamine. All experiments were carried out with confluent cells 2 or 3 days after transfection. Four to ten independent experiments were performed on transiently transfected cells. We should emphasize that several attempts to obtain stably transfected cell lines expressing the EGFP-fused cotransporter have failed, although it was previously possible to establish cell lines containing the wild-type NaPi-2 cotransporter (10).
PTH Experiments.
Cultures of transfected OK cells were maintained at 37°C. The medium was changed 2 h prior to the experiment. Using a fluorescence microscope, living green fluorescent cells expressing either the wild type (wt) or the chimeric and mutated cotransporters were selected before the treatment. PTH peptides or vehicle (H2O) were then added to the cell medium as described previously (28). After 2 and 4 h of incubation at 37°C, the same selected cells were identified again, and the pattern of expression of the different cotransporters was compared with that before treatment. Four to ten experiments were carried out for each condition.
Confocal Microscopy Analysis.
OK cells were plated on glass coverslips and transfected as described above. After confluence, they were fixed in 3% paraformaldehyde and permeabilized with 0.1% saponine as described (11). They were then incubated 30 min in the presence of phalloidine-Texas Red to stain the actin. The coverslips were mounted using Dako-glycerol containing 2.5% 1.4-diazabicyclo-(2.2.2) octane. Confocal images were taken using a Leica TCSSP (Wetzlar, Germany) laser scan microscope equipped with a 63× oil immersion objective.
Phosphate Transport Measurements.
Sodium-dependent uptake of phosphate was measured in cells grown to confluency 2–3 days after transfection, as previously described (29).
Results and Discussion
Opossum kidney cells retain the proximal tubular characteristics of PTH-induced regulation of brush-border membrane NaPi cotransport, associated with membrane retrieval and lysosomal degradation of type IIa NaPi cotransporters (10, 11, 16–18, 30–33). As a similar regulatory behavior was observed for the intrinsic (NaPi-4) and transfected (NaPi-2) transporter (10, 11, 14, 19), OK cells represent an ideal in vitro system to study molecular determinants responsible for PTH-induced down-regulation of the type IIa NaPi cotransporter.
Expression of the Chimeric and Mutated Cotransporters in OK Cells.
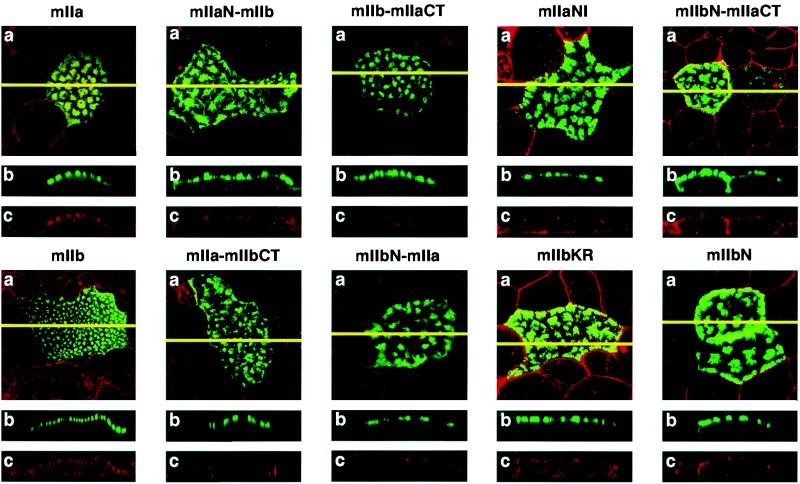
Wild type as well as chimeric and mutated NaPi cotransporters were fused to EGFP and transiently expressed in OK cells. We have previously shown that EGFP-fused wt cotransporters are fluorescent proteins correctly expressed in the apical membrane of OK cells. Their proper apical location can be identified by the appearance of fluorescence “patches” (refs. 34 and 35; Fig. 2). As shown by confocal microscopy (Fig. 2), all constructs used in the present study also exhibited “patches” of EGFP fluorescence that localized to the microvilli of OK cells. There was no difference among the expression pattern of wt mIIa, wt mIIb, and the different chimera and mutated cotransporters, indicating that all of these alterations did not interfere with the basic expression pattern. Only a few percent of cells were transfected to an extend resulting in clearly visible apical patches (data not shown). Parallel transport experiments on cells transfected with EGFP-fused type IIa or type IIb cotransporters resulted in an increase in NaPi cotransport activity as compared with nontransfected cells (about 1.5 and 4 times, respectively; data not shown). This increase was highly variable from transfection to transfection, which is explained by a variable proportion of transfected vs. nontransfected cells (see above). Although this variability precluded analysis of the PTH effect with the different constructs, it indicated that the EGFP-fused constructs are functionally active when expressed in OK cells. Thus, the analysis was entirely based on the morphological analysis of the same cells prior and after exposure to PTH, i.e., on the apical expression of the type IIa NaPi cotransporter. From previous expression on proximal tubules and on OK cells, we reached the conclusion that apical expression of this transporter crucially determines brush-border/apical NaPi cotransport activity (see Introduction and below).
Figure 2.
Expression of wild types, chimeric, and mutated NaPi cotransporters in OK cells. Cells were transfected with wt mIIa and mIIb, with the chimeras mIIaN-mIIb, mIIbN-mIIa, mIIa-mIIbCT, and mIIb-mIIaCT or with the mutants mIIaNI and mIIbKR. They were then processed for confocal microscopy. (a) Focal planes. (b and c) The xy cross-sections. The endogenous fluorescence of EGFP is shown in green (b) and the actin staining in red (c); a merge of the fluorescence signals is shown in a.
Effect of PTH on wt Type IIa and wt Type IIb NaPi Cotransporters.
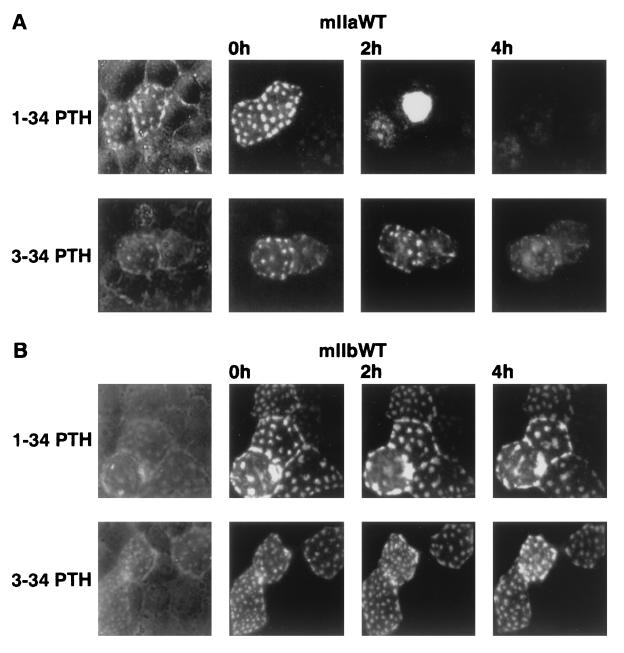
The PTH fragments 1–34 and 3–34 lead to inhibition of OK-cell NaPi cotransport associated with internalization and lysosomal degradation of the transporter protein (10, 11, 18). The maximal effect induced by 3–34 PTH is about 50% of that induced by 1–34 PTH (16–18). Also, transiently transfected EGFP-coupled rat type IIa is internalized upon treatment of OK cells with 1–34 PTH (14).
Fig. 3 shows that EGFP-coupled mIIa (Fig. 3A) but not mIIb (Fig. 3B) is internalized upon treatment with 1–34 PTH; also, 3–34 PTH led to a retrieval of mIIa but not of mIIb from the apical surface, although as expected (see above) the effect on mIIa was less efficient (Fig. 3A).
Figure 3.
mIIa but not mIIb is down-regulated by PTH. OK cells were transfected with mIIa (A) or mIIb (B) and processed for PTH experiment (see Materials and Methods). Cells were treated with 10−8 M 1–34 PTH (Upper) or with 10−6 M 3–34 PTH (Lower) for 2 and 4 h. Down-regulation of mIIa is indicated by the disappearance of its specific “patches.” Results are representative of four independent experiments.
Regulation of mIIa-mIIb Chimeras by 1–34 PTH.
The above observations on the differential PTH-induced internalization of the homologous (75%) type IIa and type IIb isoforms (Fig. 3) offered the possibility to identify structural “domains” (motifs) potentially involved in these effects.
For the regulation of different transporters, channels, and receptors, specific roles of domains located in cytoplasmic tails have been suggested (36–43). As the cytoplasmic N- and C-termini of type IIa and IIb cotransporters show little homology (20), we first investigated their involvement in the PTH regulation of the type IIa transporter.
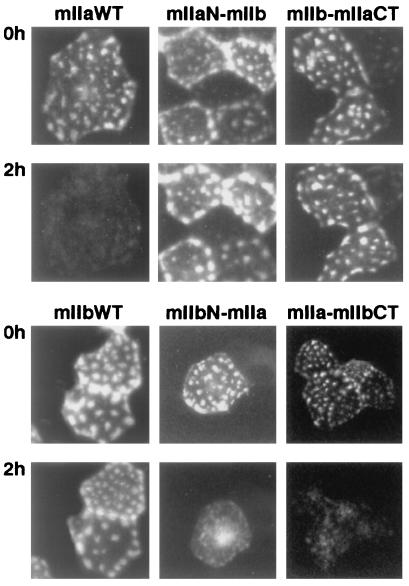
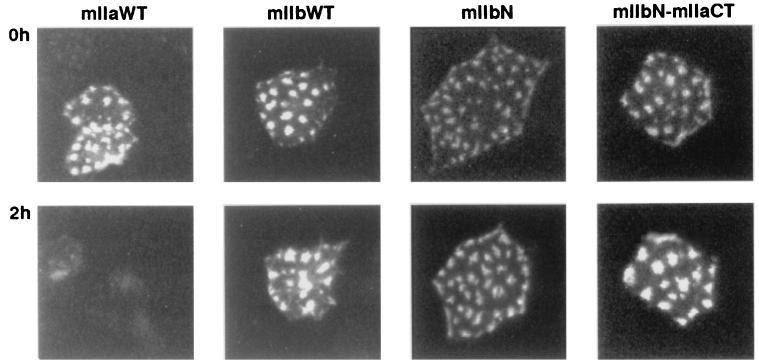
We generated several chimeras in which either the N terminus (mIIaN-mIIb, mIIbN-mIIa) or the C terminus (mIIa-mIIbCT, mIIb-mIIaCT) of mIIa and mIIb was interchanged (Figs. 1 and 2), and we studied their apical expression after 1–34 PTH treatment. Whereas mIIa was internalized and degraded after 2 h of incubation with 10−8 M 1–34 PTH, neither the mIIaN-mIIb nor the mIIb-mIIaCT chimera was affected after 2 h (Fig. 4) or 4 h of PTH treatment (not shown). However, the mIIa-mIIbCT and mIIbN-mIIa chimeras were still totally removed from the apical membrane after 2 h of treatment with PTH 1–34 (Fig. 4). These experiments indicated that the N-terminal tail, the putative first intracellular loop, and the associated transmembrane domains as well as the C-terminal tail are not involved in the PTH-induced internalization and/or degradation of type IIa NaPi cotransporter. Therefore, the residual part (amino acids 216 to 558), suggested to contain at least two putative intracellular loops (26), must contain the domain(s) involved in the observed PTH effect.
Figure 4.
PTH regulation of chimeric NaPi cotransporters. OK cells were transfected with wt mIIa or mIIb cotransporters or with the chimeras mIIaN-mIIb, IIbN-IIa, IIa-IIbCT, and IIb-IIaCT (see Fig. 1) and processed for PTH experiment (see Materials and Methods). Cells were incubated for 2 h with 10−8 M 1–34 PTH, and the patterns of green fluorescent cells before (Upper) and after (Lower) the treatment were compared. Down-regulation of mIIa, mIIbN-mIIa, and mIIa-mIIbCT by 1–34 PTH is indicated by the disappearance of their specific “patches.” Results are representative of 4 to10 independent experiments.
K503R504 Residues Are Crucial for Type IIa PTH-Induced Down-Regulation.
The above-mentioned intracellular loops are highly conserved between IIa and IIb cotransporters with only a few different amino acids (20). In one loop (K369-W397), only an uncharged amino acid (N372) present in type IIa is replaced by a charged amino acid (G388) in type IIb; in the last loop (W486-W511), two charged amino acids (K503R504) present in type IIa are replaced by uncharged residues (N520I521) in type IIb (Fig. 5). The specific role of these amino acids in PTH-dependent internalization was analyzed by site-directed mutagenesis.
Figure 5.
Sequence alignment of the last intracellular loop of type IIa and type IIb NaPi cotransporters. From each type II NaPi cotransporters, two species are shown: mouse and human. Conserved amino acids are shown in black and nonconserved in white. The two charged amino acids KR present in type IIa are replaced by uncharged residues NI in type IIb. Potential protein kinase C phosphorylation sites (consensus, S/TXK/R) are also indicated.
Mutation of the residue G388 to N in mIIb as well as in the mIIb-mIIaCT chimera did not alter the cotransporter insensitivity after 2 h (Fig. 6) or after 4 h PTH treatment (data not shown). These data eliminated a role of this residue (N) in the PTH-induced down-regulation. In view of the data to be described below, we have not performed the mutation of N383 to G in the mIIa as well as in the corresponding mIIa-mIIbCT chimera, but we assume that such mutations would not have affected the PTH sensitivity of these EGFP constructs.
Figure 6.
mIIbN and mIIbN-mIIaCT are not internalized by PTH. OK cells were transfected with wt mIIa or mIIb cotransporters or with the mutants mIIbN and mIIbN-mIIaCT and processed for the PTH experiment (see Materials and Methods). Cells were incubated for 2 h with 10−8 M 1–34 PTH, and the patterns of green fluorescent cells before (Upper) and after (Lower) treatment were compared. Down-regulation of mIIa by 1–34 PTH is indicated by the disappearance of its specific “patches.” Results are representative of three independent experiments.
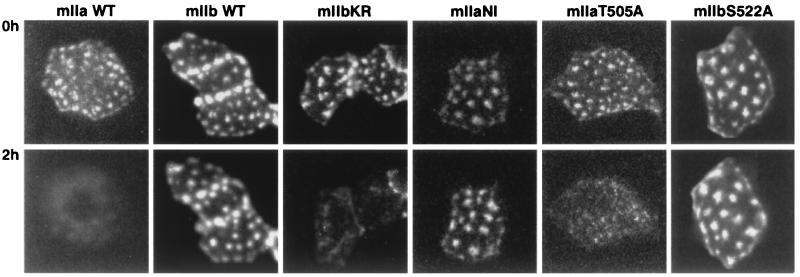
Two other mutants were generated, mIIaNI and mIIbKR, in which the specific residues in the last intracellular loop of type IIa (K503R504) and type IIb (N520I521) were interchanged. As shown in Fig. 7, the mIIbKR mutant behaved similarly to mIIa and was internalized and degraded after 2 h of incubation with 1–34 PTH. On the contrary, mIIaNI behaved like mIIb and was not sensitive to PTH treatment after 2 h (Fig. 7) or 4 h (data not shown). These data indicated that residues K503R504 play an essential role in the PTH-induced down-regulation of type IIa NaPi cotransporter.
Figure 7.
PTH regulation of mutated NaPi cotransporters. OK cells were transfected with wt mIIa or mIIb cotransporters or with the mutants mIIbKR, mIIaNI, mIIaT505A, and mIIbS522A and processed for the PTH experiment (see Materials and Methods). Cells were incubated for 2 h with 10−8 M 1–34 PTH, and the patterns of green fluorescent cells before (Upper) and after (lower) the treatment were compared. Down-regulation of mIIa and mIIbKR by 1–34 PTH is indicated by the disappearance of their specific “patches.” Results are representative of 4 to10 independent experiments.
The K503R504 residues of the type IIa NaPi cotransporter are located just upstream of a consensus site for protein kinase C phosphorylation (S/TXK/R, where X is any amino acid; Fig. 5). This site is present in both type IIa and type IIb (T505AK for IIa and S522AK for IIb). In the type IIa transporter, such nearby phosphorylation/dephosphorylation could lead to an activation/inactivation of the KR motif. On the other hand, the charged amino acids (KR) in IIa could modify an interaction of the kinase with this phosphorylation site. A specific phosphorylation of the type IIa protein could again modify its accessibility to the endocytotic machinery, e.g., also involving interacting proteins, similar to the kinase-mediated regulation of NHE-3 (44–47). To address the potential role of this phosphorylation site in PTH-dependent internalization of type IIa cotransporter, two mutants were generated in which either the T505 on mIIa or the S522 on mIIb were replaced by A (mIIaT505A and mIIbS522A). As shown in Fig. 7, none of these mutations had an effect on the PTH response. In agreement with this observation, removal of all protein kinase C sites on the rat IIa was unable to prevent the protein kinase C-dependent effect when tested in the Xenopus laevis oocytes system (48, 49).
In different cellular systems and for different membrane proteins, endosomal and lysosomal targeting is mediated via specific signals as di-leucine and tyrosine motifs (50). Type II NaPi cotransporters contain also such motifs; however, removal of all di-leucine or tyrosine motifs present in rat type IIa did not prevent the PTH internalization of this cotransporter (14), suggesting alternative mediating signals (motifs). The identified K503R504 (or RR for NaPi-4; ref. 51) could represent a novel internalization motif, at least for the PTH-induced regulation of the type IIa NaPi cotransporter in the OK cell system.
PTH-induced regulation of the type IIa may involve one or multiple regulatory proteins interacting with the cotransporter. In this case, K503R504 could mediate in part this interaction. Similar to the regulation of the epithelial Na/H exchanger, such interaction might be required for kinase-mediated effects (44–47, 52) and/or for directing the transporter to the endocytotic machinery. Thus, proteins interacting with the last intracellular loop of IIa containing the KR need to be identified. Such interactions might then be controlled by PTH-induced phosphorylation reactions also at the level of the interacting proteins. Using a two-hybrid screen, we have recently found that the C terminus of type IIa interacts with several proteins via PDZ domains (53). On the basis of the present data, there is, however, no evidence for an involvement of the C terminus in the PTH-induced down-regulation.
In conclusion, we have shown that type IIa and type IIb NaPi cotransporters differ in their sensitivity to PTH. In type IIa, the PTH-induced endocytosis involves two charged amino acids K503R504 located in the putative last intracellular loop of the cotransporter. Sequences in the cytoplasmic N- and C-terminal as well as in other intracellular loops are not involved in the PTH effect.
Acknowledgments
This work was supported by a Swiss National Science Foundation grant (to H.M.), the Hartmann Müller-Stiftung (Zürich, Switzerland), the Olga Mayenflsch-Stiftung, the Schwerzerische Bank-Gesellschaft (Zürich; Bu70417–1), and Novartis-Foundation.
Abbreviations
- OK
opossum kidney
- wt
wild type
- EGFP
enhanced green fluorescent protein
- PTH
parathyroid hormone
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220394197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220394197
References
- 1.Magagnin S, Werner A, Markovich D, Sorribas V, Stange G, Biber J, Murer H. Proc Natl Acad Sci USA. 1993;90:5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murer H, Biber J. Curr Opin Nephrol Hypertens. 1994;3:504–510. doi: 10.1097/00041552-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Beck L, Karaplis A C, Amizuka N, Hewson A S, Ozawa H, Tenenhouse H S. Proc Natl Acad Sci USA. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murer H, Hernando N, Forster I, Biber J. Physiol Rev. 2000;80:1373–1409. doi: 10.1152/physrev.2000.80.4.1373. [DOI] [PubMed] [Google Scholar]
- 5.Berndt T J, Knox F G. In: The Kidney, physiology and pathophysiology. Seldin D W, Giebisch G, editors. New York: Raven Press; 1992. pp. 2511–2532. [Google Scholar]
- 6.Evers C, Murer H, Kinne R. Biochem J. 1978;172:49–56. doi: 10.1042/bj1720049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keusch I, Traebert M, Lotscher M, Kaissling B, Murer H, Biber J. Kidney Int. 1998;54:1224–1232. doi: 10.1046/j.1523-1755.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- 8.Murer H, Forster I, Hernando N, Lambert G, Traebert M, Biber J. Am J Physiol. 1999;277:F676–F684. doi: 10.1152/ajprenal.1999.277.5.F676. [DOI] [PubMed] [Google Scholar]
- 9.Lotscher M, Scarpetta Y, Levi M, Halaihel N, Wang H, Zajicek H K, Biber J, Murer H, Kaissling B. J Clin Invest. 1999;104:483–494. doi: 10.1172/JCI3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfister M F, Lederer E, Forgo J, Ziegler U, Lotscher M, Quabius E S, Biber J, Murer H. J Biol Chem. 1997;272:20125–20130. doi: 10.1074/jbc.272.32.20125. [DOI] [PubMed] [Google Scholar]
- 11.Pfister M F, Ruf I, Stange G, Ziegler U, Lederer E, Biber J, Murer H. Proc Natl Acad Sci USA. 1998;95:1909–1914. doi: 10.1073/pnas.95.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lederer E D, Sohi S S, Mathiesen J M, Klein J B. Am J Physiol. 1998;275:F270–F277. doi: 10.1152/ajprenal.1998.275.2.F270. [DOI] [PubMed] [Google Scholar]
- 13.Jankowski M, Biber J, Murer H. Pflugers Arch Eur J Physiol. 1999;438:689–693. doi: 10.1007/s004249900093. [DOI] [PubMed] [Google Scholar]
- 14.Hernando N, Forgo J, Biber J, Murer H. J Am Soc Nephrol. 2000;11:1961–1968. doi: 10.1681/ASN.V11111961. [DOI] [PubMed] [Google Scholar]
- 15.Malmstrom K, Stange G, Murer H. Biochem J. 1988;251:207–213. doi: 10.1042/bj2510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole J A, Eber S L, Poelling R E, Thorne P K, Forte L R. Am J Physiol. 1987;253:E221–E227. doi: 10.1152/ajpendo.1987.253.2.E221. [DOI] [PubMed] [Google Scholar]
- 17.Cole J A, Forte L R, Eber S, Thorne P K, Poelling R E. Endocrinology. 1988;122:2981–2989. doi: 10.1210/endo-122-6-2981. [DOI] [PubMed] [Google Scholar]
- 18.Pfister M F, Forgo J, Ziegler U, Biber J, Murer H. Am J Physiol. 1999;276:F720–F725. doi: 10.1152/ajprenal.1999.276.5.F720. [DOI] [PubMed] [Google Scholar]
- 19.Traebert M, Völkl H, Biber J, Murer H, Kaissling B. Am J Physiol. 2000;278:F792. doi: 10.1152/ajprenal.2000.278.5.F792. [DOI] [PubMed] [Google Scholar]
- 20.Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J. Proc Natl Acad Sci USA. 1998;95:14564–14569. doi: 10.1073/pnas.95.24.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Custer M, Lotscher M, Biber J, Murer H, Kaissling B. Am J Physiol. 1994;266:F767–F774. doi: 10.1152/ajprenal.1994.266.5.F767. [DOI] [PubMed] [Google Scholar]
- 22.Traebert M, Hattenhauer O, Murer H, Kaissling B, Biber J. Am J Physiol. 1999;277:L868–L873. doi: 10.1152/ajplung.1999.277.5.L868. [DOI] [PubMed] [Google Scholar]
- 23.De la Horra C, Hernando N, Lambert G, Forster I, Biber J, Murer H. J Biol Chem. 2000;275:6284–6287. doi: 10.1074/jbc.275.9.6284. [DOI] [PubMed] [Google Scholar]
- 24.De la Horra, C., Hernando, N., Biber, J. & Murer, H., submitted for publication.
- 25.Hattenhauer O, Traebert M, Murer H, Biber J. Am J Physiol. 1999;277:G756–G762. doi: 10.1152/ajpgi.1999.277.4.G756. [DOI] [PubMed] [Google Scholar]
- 26.Lambert G, Traebert M, Hernando N, Biber J, Murer H. Pflugers Arch Eur J Physiol. 1999;753:7–14. doi: 10.1007/s004240050869. [DOI] [PubMed] [Google Scholar]
- 27.Lambert G, Forster I C, Stange G, Biber J, Murer H. J Gen Physiol. 1999;114:637–652. doi: 10.1085/jgp.114.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reshkin S J, Wuarin F, Biber J, Murer H. J Biol Chem. 1990;265:15261–15266. [PubMed] [Google Scholar]
- 29.Reshkin S J, Forgo J, Murer H. Pflugers Arch Eur J Physiol. 1990;416:554–560. doi: 10.1007/BF00382689. [DOI] [PubMed] [Google Scholar]
- 30.Caverzasio J, Rizzoli R, Bonjour J P. J Biol Chem. 1986;261:3233–3237. [PubMed] [Google Scholar]
- 31.Malmstrom K, Murer H. Am J Physiol. 1986;251:C23–C31. doi: 10.1152/ajpcell.1986.251.1.C23. [DOI] [PubMed] [Google Scholar]
- 32.Reshkin S J, Forgo J, Murer H. J Membr Biol. 1991;124:227–237. doi: 10.1007/BF01994356. [DOI] [PubMed] [Google Scholar]
- 33.Reshkin S J, Murer H. Am J Physiol. 1992;262:F572–F577. doi: 10.1152/ajprenal.1992.262.4.F572. [DOI] [PubMed] [Google Scholar]
- 34.Hernando N, Sheikh S, Karim-Jimenez Z, Galliker H, Forgo J, Biber J, Murer H. Am J Physiol. 2000;278:F361–F368. doi: 10.1152/ajprenal.2000.278.3.F361. [DOI] [PubMed] [Google Scholar]
- 35.Karim-Jimenez Z, Hernando N, Biber J, Murer H. Proc Natl Acad Sci USA. 2000;97:2916–2921. doi: 10.1073/pnas.97.6.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakabayashi S, Fafournoux P, Sardet C, Pouyssegur J. Proc Natl Acad Sci USA. 1992;89:2424–2428. doi: 10.1073/pnas.89.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine S A, Nath S K, Yun C H, Yip J W, Montrose M, Donowitz M, Tse C M. J Biol Chem. 1995;270:13716–13725. doi: 10.1074/jbc.270.23.13716. [DOI] [PubMed] [Google Scholar]
- 38.Cabado A G, Yu F H, Kapus A, Lukacs G, Grinstein S, Orlowski J. J Biol Chem. 1996;271:3590–3599. doi: 10.1074/jbc.271.7.3590. [DOI] [PubMed] [Google Scholar]
- 39.Nath S K, Kambadur R, Yun C H, Donowitz M, Tse C M. Am J Physiol. 1999;276:C873–C882. doi: 10.1152/ajpcell.1999.276.4.C873. [DOI] [PubMed] [Google Scholar]
- 40.Jung C Y. Exp Physiol. 1998;83:267–273. doi: 10.1113/expphysiol.1998.sp004112. [DOI] [PubMed] [Google Scholar]
- 41.Hall R A, Ostedgaard L S, Premont R T, Blitzer J T, Rahman N, Welsh M J, Lefkowitz R J. Proc Natl Acad Sci USA. 1998;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall R A, Premont R T, Chow C W, Blitzer J T, Pitcher J A, Claing A, Stoffel R H, Barak L S, Shenolikar S, Weinman E J, et al. Nature (London) 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 43.Naren A P, Nelson D J, Xie W, Jovov B, Pevsner J, Bennett M K, Benos D J, Quick M W, Kirk K L. Nature (London) 1997;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- 44.Weinman E J, Steplock D, Tate K, Hall R A, Spurney R F, Shenolikar S. J Clin Invest. 1998;101:2199–2206. doi: 10.1172/JCI204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamprecht G, Weinman E J, Yun C H. J Biol Chem. 1998;273:29972–29978. doi: 10.1074/jbc.273.45.29972. [DOI] [PubMed] [Google Scholar]
- 46.Zizak M, Lamprecht G, Steplock D, Tariq N, Shenolikar S, Donowitz M, Yun C H, Weinman E J. J Biol Chem. 1999;274:24753–24758. doi: 10.1074/jbc.274.35.24753. [DOI] [PubMed] [Google Scholar]
- 47.Minkoff C, Shenolikar S, Weinman E J. Curr Opin Nephrol Hypertens. 1999;8:603–608. doi: 10.1097/00041552-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Hayes G, Busch A E, Lang F, Biber J, Murer H. Pflugers Arch Eur J Physiol. 1995;430:819–824. doi: 10.1007/BF00386181. [DOI] [PubMed] [Google Scholar]
- 49.Forster I C, Traebert M, Jankowski M, Stange G, Biber J, Murer H. J Physiol. 1999;517:327–340. doi: 10.1111/j.1469-7793.1999.0327t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mellman I. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 51.Sorribas V, Markovich D, Hayes G, Stange G, Forgo J, Biber J, Murer H. J Biol Chem. 1994;269:6615–6621. [PubMed] [Google Scholar]
- 52.Moe O W. J Am Soc Nephrol. 1999;10:2412–2425. doi: 10.1681/ASN.V10112412. [DOI] [PubMed] [Google Scholar]
- 53.Gisler S, Stagljar I, Biber J, Murer H. J Am Soc Nephrol. 1999;10:609A. [Google Scholar]