Abstract
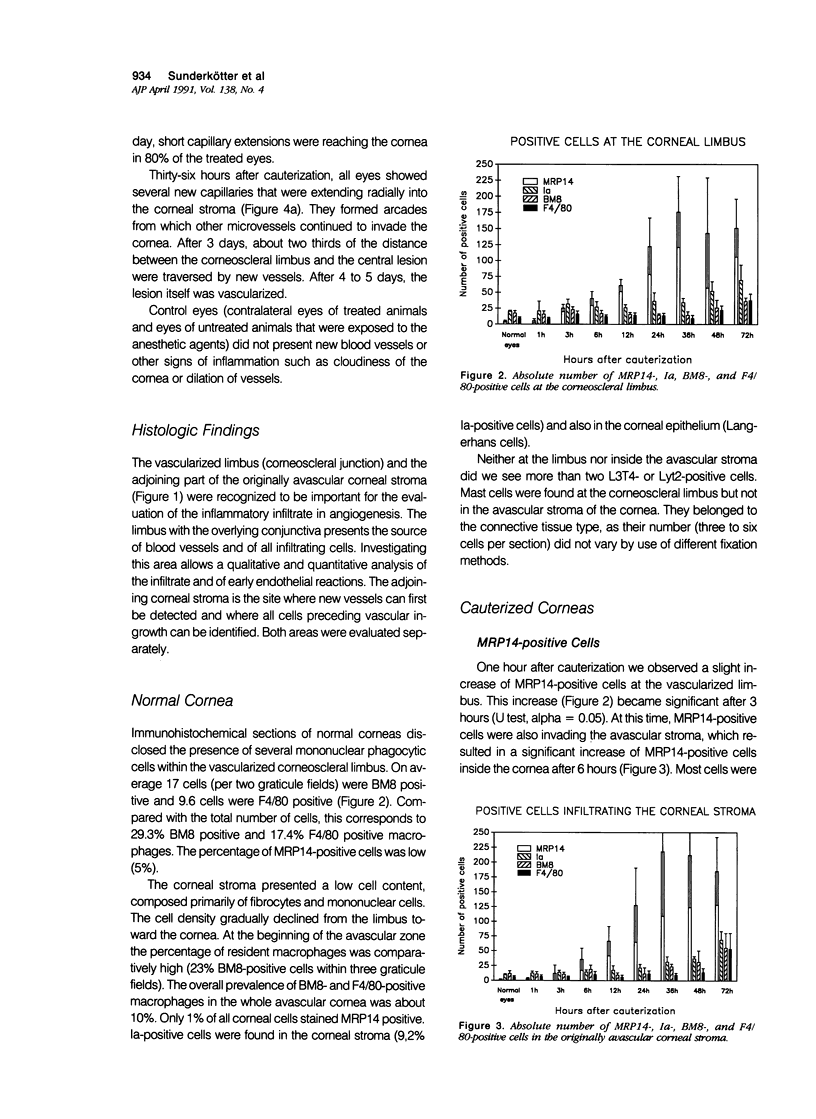
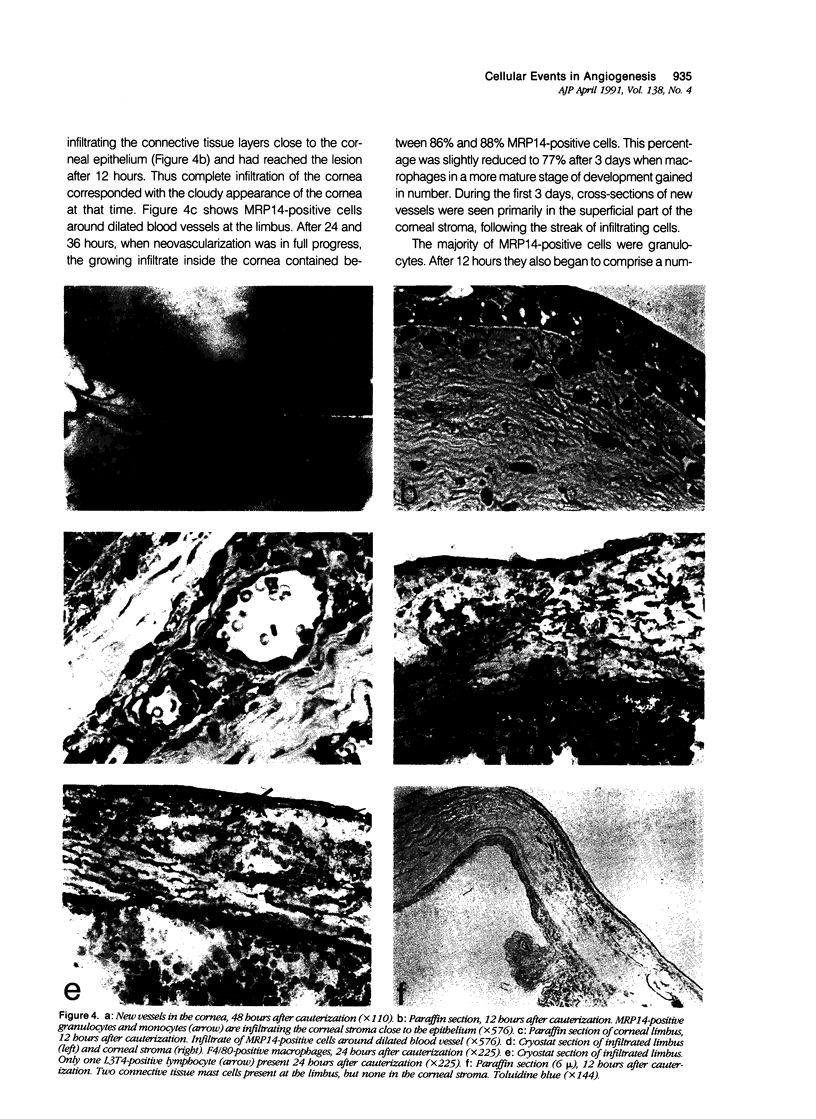
The aim of this study was to establish an angiogenesis model in the mouse and to define immunohistochemically the cellular events that precede angiogenesis. After chemical cauterization of the murine cornea, neovascularization was observed within 36 hours. The cellular infiltrate was analyzed by using antibodies on cryostat and paraffin sections and by histochemical staining for mast cells. It was found that neither T lymphocytes nor mast cells nor macrophages in a more mature stage of development were part of the infiltrate that preceded the ingrowth of new blood vessels. Instead, the infiltrating cells appearing from 3 hours on were granulocytes and inflammatory monocytes, as detected by an antibody against the calcium-binding protein MRP14. The authors conclude that the induction of angiogenesis during nonspecific inflammation is associated with the early influx of myelomonocytic cells, but not with the infiltration of mature macrophages, T lymphocytes, or mast cells. This study shows that immunohistochemical analysis of cauterized murine corneas presents a useful tool for further studies on cells and cell products involved in the angiogenic process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessandri G., Raju K., Gullino P. M. Mobilization of capillary endothelium in vitro induced by effectors of angiogenesis in vivo. Cancer Res. 1983 Apr;43(4):1790–1797. [PubMed] [Google Scholar]
- Auerbach R., Sidky Y. A. Nature of the stimulus leading to lymphocyte-induced angiogenesis. J Immunol. 1979 Aug;123(2):751–754. [PubMed] [Google Scholar]
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Barnhill R. L., Wolf J. E., Jr Angiogenesis and the skin. J Am Acad Dermatol. 1987 Jun;16(6):1226–1242. doi: 10.1016/s0190-9622(87)70161-3. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Dvorak A. M., Dvorak H. F. Leaky vessels, fibrin deposition, and fibrosis: a sequence of events common to solid tumors and to many other types of disease. Am Rev Respir Dis. 1989 Oct;140(4):1104–1107. doi: 10.1164/ajrccm/140.4.1104. [DOI] [PubMed] [Google Scholar]
- Cheng Y. F., Kramer R. H. Human microvascular endothelial cells express integrin-related complexes that mediate adhesion to the extracellular matrix. J Cell Physiol. 1989 May;139(2):275–286. doi: 10.1002/jcp.1041390209. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. II. Studies on the effect of leukocytic elimination on corneal vascularization. Am J Pathol. 1975 Dec;81(3):531–544. [PMC free article] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. III. Studies related to the vasoproliferative capability of polymorphonuclear leukocytes and lymphocytes. Am J Pathol. 1976 Jan;82(1):157–170. [PMC free article] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. Am J Pathol. 1975 Jun;79(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Furcht L. T. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986 Nov;55(5):505–509. [PubMed] [Google Scholar]
- Hirsch S., Austyn J. M., Gordon S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med. 1981 Sep 1;154(3):713–725. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. K., Knighton D. R., Thakral K. K., Goodson W. H., 3rd, Andrews W. S. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984 Jul;96(1):48–54. [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989 Sep 8;58(5):803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Junghans B. M., Collin H. B. The limbal vascular response to corneal injury. An autoradiographic study. Cornea. 1989;8(2):141–149. [PubMed] [Google Scholar]
- Kaminski M., Auerbach R. Angiogenesis induction by CD-4 positive lymphocytes. Proc Soc Exp Biol Med. 1988 Sep;188(4):440–443. doi: 10.3181/00379727-188-42757. [DOI] [PubMed] [Google Scholar]
- Kessler D. A., Langer R. S., Pless N. A., Folkman J. Mast cells and tumor angiogenesis. Int J Cancer. 1976 Nov 15;18(5):703–709. doi: 10.1002/ijc.2910180520. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Induction of neovascularization by activated human monocytes. J Leukoc Biol. 1986 Feb;39(2):233–238. doi: 10.1002/jlb.39.2.233. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Stimulation of neovascularization by human rheumatoid synovial tissue macrophages. Arthritis Rheum. 1986 Apr;29(4):471–479. doi: 10.1002/art.1780290403. [DOI] [PubMed] [Google Scholar]
- Lagasse E., Clerc R. G. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol Cell Biol. 1988 Jun;8(6):2402–2410. doi: 10.1128/mcb.8.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malorny U., Michels E., Sorg C. A monoclonal antibody against an antigen present on mouse macrophages and absent from monocytes. Cell Tissue Res. 1986;243(2):421–428. doi: 10.1007/BF00251059. [DOI] [PubMed] [Google Scholar]
- McCracken J. S., Burger P. C., Klintworth G. K. Morphologic observations on experimental corneal vascularization in the rat. Lab Invest. 1979 Dec;41(6):519–530. [PubMed] [Google Scholar]
- Motro B., Itin A., Sachs L., Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3092–3096. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukkaruppan V., Auerbach R. Angiogenesis in the mouse cornea. Science. 1979 Sep 28;205(4413):1416–1418. doi: 10.1126/science.472760. [DOI] [PubMed] [Google Scholar]
- Norrby K., Jakobsson A., Sörbo J. Mast-cell secretion and angiogenesis, a quantitative study in rats and mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(4):251–256. doi: 10.1007/BF02899089. [DOI] [PubMed] [Google Scholar]
- Odink K., Cerletti N., Brüggen J., Clerc R. G., Tarcsay L., Zwadlo G., Gerhards G., Schlegel R., Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987 Nov 5;330(6143):80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- Ohuchi T., Kuriyama S., Yoshimura N., Honda Y., Hiraoka M., Abe M. Suppression of human retinal pigment epithelial cell proliferation by hyperthermia. Arch Ophthalmol. 1990 Jun;108(6):873–875. doi: 10.1001/archopht.1990.01070080117047. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Regulation of endothelial cell growth, architecture, and matrix synthesis by TGF-beta. Am Rev Respir Dis. 1989 Oct;140(4):1126–1128. doi: 10.1164/ajrccm/140.4.1126. [DOI] [PubMed] [Google Scholar]
- SCHOEFL G. I. STUDIES ON INFLAMMATION. III. GROWING CAPILLARIES: THEIR STRUCTURE AND PERMEABILITY. Virchows Arch Pathol Anat Physiol Klin Med. 1963 Nov 8;337:97–141. [PubMed] [Google Scholar]
- Sidky Y. A., Auerbach R. Lymphocyte-induced angiogenesis: a quantitative and sensitive assay of the graft-vs.-host reaction. J Exp Med. 1975 May 1;141(5):1084–1100. doi: 10.1084/jem.141.5.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. S., Basu P. K. Mast cells in corneal immune reaction. Can J Ophthalmol. 1970 Apr;5(2):175–183. [PubMed] [Google Scholar]
- Starkey J. R., Crowle P. K., Taubenberger S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988 Jul 15;42(1):48–52. doi: 10.1002/ijc.2910420110. [DOI] [PubMed] [Google Scholar]
- Strobel S., Miller H. R., Ferguson A. Human intestinal mucosal mast cells: evaluation of fixation and staining techniques. J Clin Pathol. 1981 Aug;34(8):851–858. doi: 10.1136/jcp.34.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkötter C., Roth J., Sorg C. Immunohistochemical detection of bFGF and TNF-alpha in the course of inflammatory angiogenesis in the mouse cornea. Am J Pathol. 1990 Sep;137(3):511–515. [PMC free article] [PubMed] [Google Scholar]
- Thompson W. D., Campbell R., Evans T. Fibrin degradation and angiogenesis: quantitative analysis of the angiogenic response in the chick chorioallantoic membrane. J Pathol. 1985 Jan;145(1):27–37. doi: 10.1002/path.1711450103. [DOI] [PubMed] [Google Scholar]
- Weil G. J., Chused T. M. Eosinophil autofluorescence and its use in isolation and analysis of human eosinophils using flow microfluorometry. Blood. 1981 Jun;57(6):1099–1104. [PubMed] [Google Scholar]
- West D. C., Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res. 1989 Jul;183(1):179–196. doi: 10.1016/0014-4827(89)90428-x. [DOI] [PubMed] [Google Scholar]
- Wilson D. J. Mast cells are present during angiogenesis in the chick extraembryonic vascular system. Experientia. 1985 Feb 15;41(2):269–271. doi: 10.1007/BF02002631. [DOI] [PubMed] [Google Scholar]
- Zwadlo G., Brüggen J., Gerhards G., Schlegel R., Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988 Jun;72(3):510–515. [PMC free article] [PubMed] [Google Scholar]