Abstract
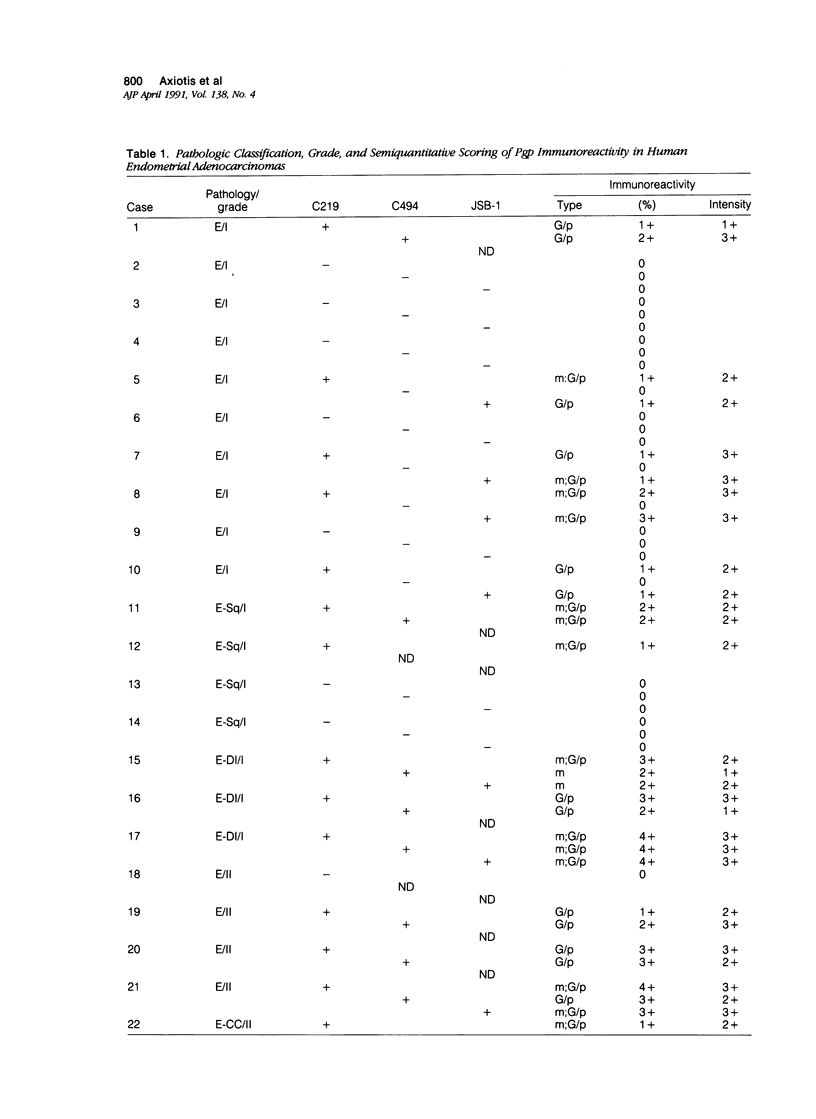
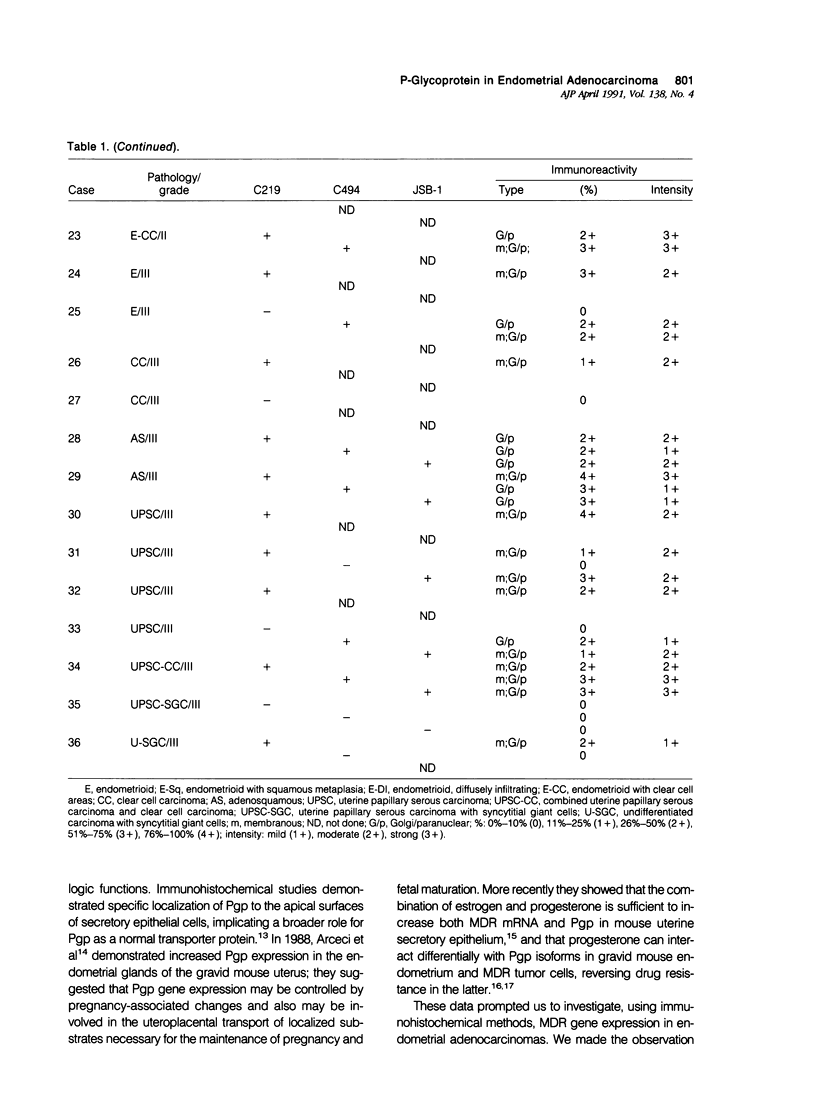
P-glycoprotein (Pgp) has emerged as the central mediator in classic multidrug resistance in model systems in vitro. High levels of Pgp also have been detected in many normal human tissues and tumors; and its role in clinical drug resistance is currently under investigation. Recently significant levels of Pgp were localized to gravid and secretory endometrium; and it was demonstrated that the combination of estrogen and progesterone is sufficient to induce high levels of both Pgp mRNA and Pgp in uterine secretory epithelium. These findings suggest that increased Pgp expression also may be present in hormone-responsive malignancies such as endometrial adenocarcinoma. To determine whether Pgp is expressed in endometrial adenocarcinoma, 36 endometrial adenocarcinomas (grade I [n = 17]; grade II [n = 6]; grade III [n = 13]) were investigated retrospectively by the avidin-biotin-complex immunohistochemical procedure using three murine monoclonal antibodies (MAb) MAb C219, MAb C494, and MAb JSB-1, which recognize spatially distinct cytoplasmic epitopes of Pgp. Seventy-two percent of the tumors showed positive immunostaining with at least one MAb; 67% showed immunostaining with MAb C219, 50% with MAb C494, and 62% with MAb JSB-1. Forty-six percent of tumors were immunoreactive to two and 29% to all three antibodies. Membranous and Golgi/paranuclear type staining patterns were observed. Overall the intensity of immunostaining varied from one sample to another for a given tumor type, and considerable heterogeneity of expression was commonly seen within a given tumor. Strong to moderate immunoreactivity was seen in diffusely infiltrating, adenosquamous, and serous papillary carcinomas. In general, immunoreactivity to MAb C494 was weaker than MAb C219 or MAb JSB-1. Adenomatous and non-neoplastic endometrium adjacent to the tumors displayed strong membranous immunostaining with MAb JSB-1. Endometrial capillaries showed weak-to-moderate immunostaining to all three antibodies. It is concluded that Pgp is commonly expressed in endometrial adenocarcinoma and may be a significant factor responsible for their drug-resistant nature subject to modulation by progesterone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Baas F., Raponi R., Horwitz S. B., Housman D., Croop J. M. Multidrug resistance gene expression is controlled by steroid hormones in the secretory epithelium of the uterus. Mol Reprod Dev. 1990 Feb;25(2):101–109. doi: 10.1002/mrd.1080250202. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Thorner P. S., Haddad G., Ling V. Immunohistochemical detection of P-glycoprotein: prognostic correlation in soft tissue sarcoma of childhood. J Clin Oncol. 1990 Apr;8(4):689–704. doi: 10.1200/JCO.1990.8.4.689. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Casals D., Rittman-Grauer L., Biedler J. L., Melamed M. R., Bertino J. R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989 Jan;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J. M., Raymond M., Haber D., Devault A., Arceci R. J., Gros P., Housman D. E. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989 Mar;9(3):1346–1350. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars K. L., Ling V. P-glycoprotein and multidrug resistance in cancer chemotherapy. Semin Oncol. 1989 Apr;16(2):156–165. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Shen D. W., Mickley L. A., Pastan I., Gottesman M. M. Intrinsic drug resistance in human kidney cancer is associated with expression of a human multidrug-resistance gene. J Clin Oncol. 1987 Dec;5(12):1922–1927. doi: 10.1200/JCO.1987.5.12.1922. [DOI] [PubMed] [Google Scholar]
- Georges E., Bradley G., Gariepy J., Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Jan;87(1):152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Greenberger L. M., Croop J. M., Horwitz S. B., Arceci R. J. P-glycoproteins encoded by mdr 1b in murine gravid uterus and multidrug resistant tumor cell lines are differentially glycosylated. FEBS Lett. 1989 Nov 6;257(2):419–421. doi: 10.1016/0014-5793(89)81586-8. [DOI] [PubMed] [Google Scholar]
- Grogan T., Dalton W., Rybski J., Spier C., Meltzer P., Richter L., Gleason M., Pindur J., Cline A., Scheper R. Optimization of immunocytochemical P-glycoprotein assessment in multidrug-resistant plasma cell myeloma using three antibodies. Lab Invest. 1990 Dec;63(6):815–824. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Inoue M., Sasagawa T., Saito J., Shimizu H., Ueda G., Tanizawa O., Nakayama M. Expression of blood group antigens A, B, H, Lewis-a, and Lewis-b in fetal, normal, and malignant tissues of the uterine endometrium. Cancer. 1987 Dec 15;60(12):2985–2993. doi: 10.1002/1097-0142(19871215)60:12<2985::aid-cncr2820601222>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kane S. E., Gottesman M. M. Multidrug resistance in the laboratory and clinic. Cancer Cells. 1989 Sep;1(1):33–36. [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Mittal K. R., Barwick K. W. Diffusely infiltrating adenocarcinoma of the endometrium. A subtype with poor prognosis. Am J Surg Pathol. 1988 Oct;12(10):754–758. doi: 10.1097/00000478-198810000-00003. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Cowan K. H. Multidrug resistance. J Natl Cancer Inst. 1988 Mar 2;80(1):14–20. doi: 10.1093/jnci/80.1.14. [DOI] [PubMed] [Google Scholar]
- Ng W. F., Sarangi F., Zastawny R. L., Veinot-Drebot L., Ling V. Identification of members of the P-glycoprotein multigene family. Mol Cell Biol. 1989 Mar;9(3):1224–1232. doi: 10.1128/mcb.9.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooter K., Sonneveld P., Janssen A., Oostrum R., Boersma T., Herweijer H., Valerio D., Hagemeijer A., Baas F. Expression of the mdr3 gene in prolymphocytic leukemia: association with cyclosporin-A-induced increase in drug accumulation. Int J Cancer. 1990 Apr 15;45(4):626–631. doi: 10.1002/ijc.2910450409. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989 Feb;37(2):159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- Thigpen T. Systemic therapy with single agents for advanced or recurrent endometrial carcinoma. Cancer Treat Res. 1989;49:93–106. doi: 10.1007/978-1-4613-0867-6_7. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Kuszak J. R., Jakate S. M., Lebovitz M. D., Kluskens L. F., Coon J. S. ABO blood type predicts the cytolocalization of anti-P-glycoprotein monoclonal antibody reactivity in human colon and ureter. Hum Pathol. 1990 Sep;21(9):949–958. doi: 10.1016/0046-8177(90)90180-d. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Kuszak J. R., Kluskens L. F., Coon J. S. P-glycoproteins in pathology: the multidrug resistance gene family in humans. Hum Pathol. 1990 Jan;21(1):34–48. doi: 10.1016/0046-8177(90)90073-e. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Richert N. D., Cornwell M. M., Tsuruo T., Hamada H., Gottesman M. M., Pastan I. H. Immunocytochemical localization of P170 at the plasma membrane of multidrug-resistant human cells. J Histochem Cytochem. 1987 Dec;35(12):1451–1456. doi: 10.1177/35.12.2890686. [DOI] [PubMed] [Google Scholar]
- Yang C. P., Cohen D., Greenberger L. M., Hsu S. I., Horwitz S. B. Differential transport properties of two mdr gene products are distinguished by progesterone. J Biol Chem. 1990 Jun 25;265(18):10282–10288. [PubMed] [Google Scholar]
- Yang C. P., DePinho S. G., Greenberger L. M., Arceci R. J., Horwitz S. B. Progesterone interacts with P-glycoprotein in multidrug-resistant cells and in the endometrium of gravid uterus. J Biol Chem. 1989 Jan 15;264(2):782–788. [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]





