Abstract
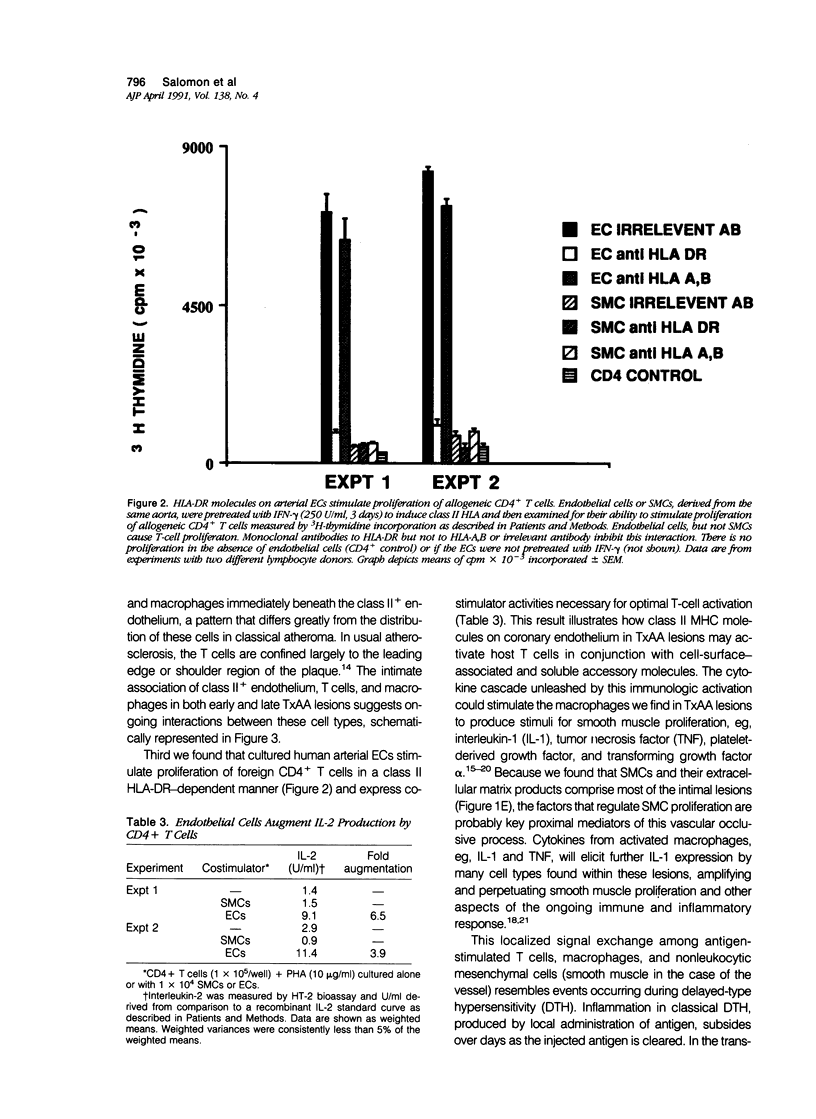
Occlusive disease of coronary arteries of engrafted hearts is the major obstacle to long-term survival of human cardiac allografts. The pathogenesis of this process remains uncertain. The identity and localization of cells found in transplantation-associated arteriosclerosis lesions from human cardiac allografts were evaluated, and their expression of class II major histocompatibility complex (human leukocyte antigen-DR [HLA-DR]), surface molecules required for recognition of foreign cells by CD4+ T lymphocytes, was noted. Expanded intimas of transplanted coronary arteries contain T lymphocytes (both CD4+ and CD8+ in approximately equal number) and HLA-DR+ macrophages, both localized primarily in a ring immediately below the luminal endothelium, a distribution strikingly different from that in typical atherosclerosis. Coronary arterial endothelium from six of six transplanted hearts studied bore high levels of HLA-DR. Normal human arteries or usual atherosclerotic lesions have few if any HLA-DR+ endothelial cells. The significance of these findings was tested by evaluating the ability of HLA-DR+ arterial cells to interact with allogeneic T cells in vitro. Endothelial cells (but not smooth muscle cells) cultured from human arteries stimulated foreign CD4+ T cells to proliferate and augmented their secretion of interleukin-2. These findings suggest that ongoing stimulation of recipient T lymphocytes by HLA-DR+ endothelium of donor coronary arteries contributes to a sustained regional immune response. Consequent local release of cytokines may regulate smooth muscle cell proliferation and matrix accumulation within the coronary arteries of allografted hearts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billingham M. E. Cardiac transplant atherosclerosis. Transplant Proc. 1987 Aug;19(4 Suppl 5):19–25. [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Doherty P. C., Allan J. E., Lynch F., Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990 Feb;11(2):55–59. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- Doukas J., Pober J. S. Lymphocyte-mediated activation of cultured endothelial cells (EC). CD4+ T cells inhibit EC class II MHC expression despite secreting IFN-gamma and increasing EC class I MHC and intercellular adhesion molecule-1 expression. J Immunol. 1990 Aug 15;145(4):1088–1098. [PubMed] [Google Scholar]
- Gao S. Z., Alderman E. L., Schroeder J. S., Silverman J. F., Hunt S. A. Accelerated coronary vascular disease in the heart transplant patient: coronary arteriographic findings. J Am Coll Cardiol. 1988 Aug;12(2):334–340. doi: 10.1016/0735-1097(88)90402-0. [DOI] [PubMed] [Google Scholar]
- Grattan M. T., Moreno-Cabral C. E., Starnes V. A., Oyer P. E., Stinson E. B., Shumway N. E. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989 Jun 23;261(24):3561–3566. [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
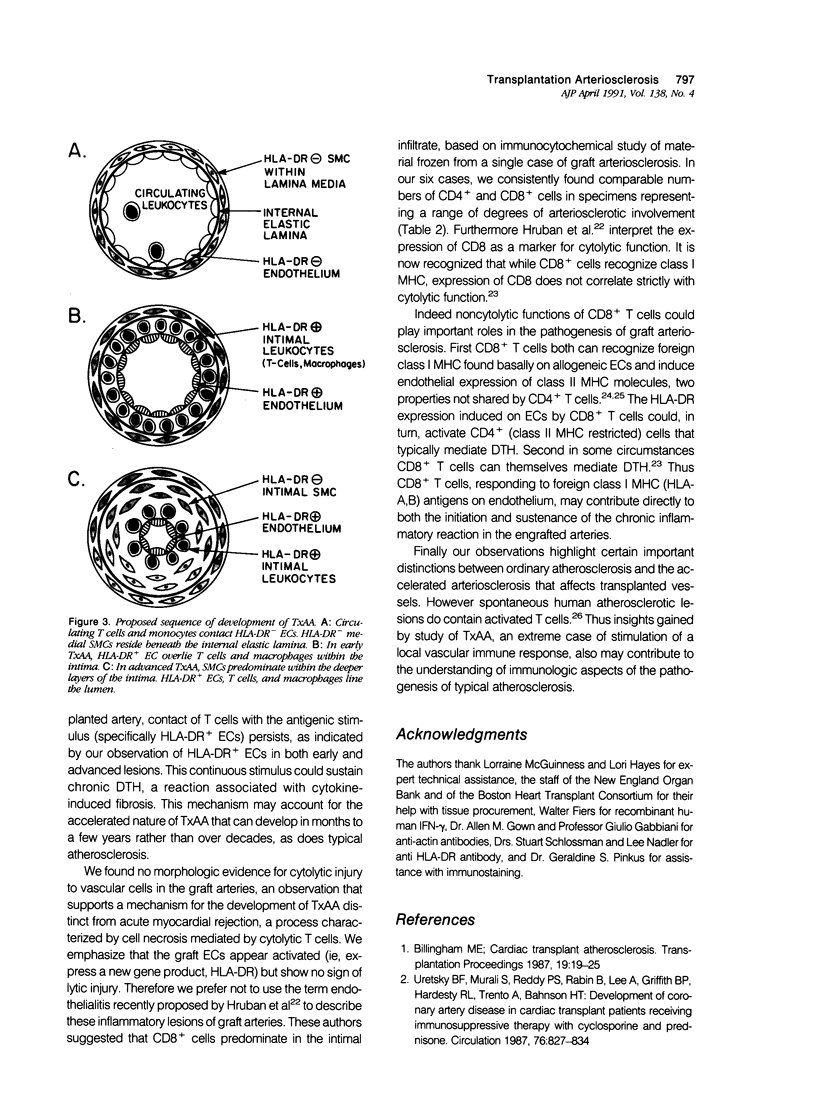
- Hruban R. H., Beschorner W. E., Baumgartner W. A., Augustine S. M., Ren H., Reitz B. A., Hutchins G. M. Accelerated arteriosclerosis in heart transplant recipients is associated with a T-lymphocyte-mediated endothelialitis. Am J Pathol. 1990 Oct;137(4):871–882. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Savage C. O., Pober J. S. Endothelial cells augment T cell interleukin 2 production by a contact-dependent mechanism involving CD2/LFA-3 interaction. J Exp Med. 1990 May 1;171(5):1453–1467. doi: 10.1084/jem.171.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Kosek J. C., Bieber C., Lower R. R. Heart graft arteriosclerosis. Transplant Proc. 1971 Mar;3(1):512–514. [PubMed] [Google Scholar]
- Libby P., Salomon R. N., Payne D. D., Schoen F. J., Pober J. S. Functions of vascular wall cells related to development of transplantation-associated coronary arteriosclerosis. Transplant Proc. 1989 Aug;21(4):3677–3684. [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Pardi R., Bender J. R., Engleman E. G. Lymphocyte subsets differentially induce class II human leukocyte antigens on allogeneic microvascular endothelial cells. J Immunol. 1987 Oct 15;139(8):2585–2592. [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Libby P., Reiss C. S. Inducible expression of class II major histocompatibility complex antigens and the immunogenicity of vascular endothelium. Transplantation. 1986 Feb;41(2):141–146. doi: 10.1097/00007890-198602000-00001. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai M., Kohno Y., Namba M., Umemiya T., Niwa K., Nakajima H., Mikata A. Class II major histocompatibility antigen expression on coronary arterial endothelium in a patient with Kawasaki disease. Hum Pathol. 1990 Feb;21(2):231–234. doi: 10.1016/0046-8177(90)90135-r. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Uretsky B. F., Murali S., Reddy P. S., Rabin B., Lee A., Griffith B. P., Hardesty R. L., Trento A., Bahnson H. T. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987 Oct;76(4):827–834. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]





