Abstract
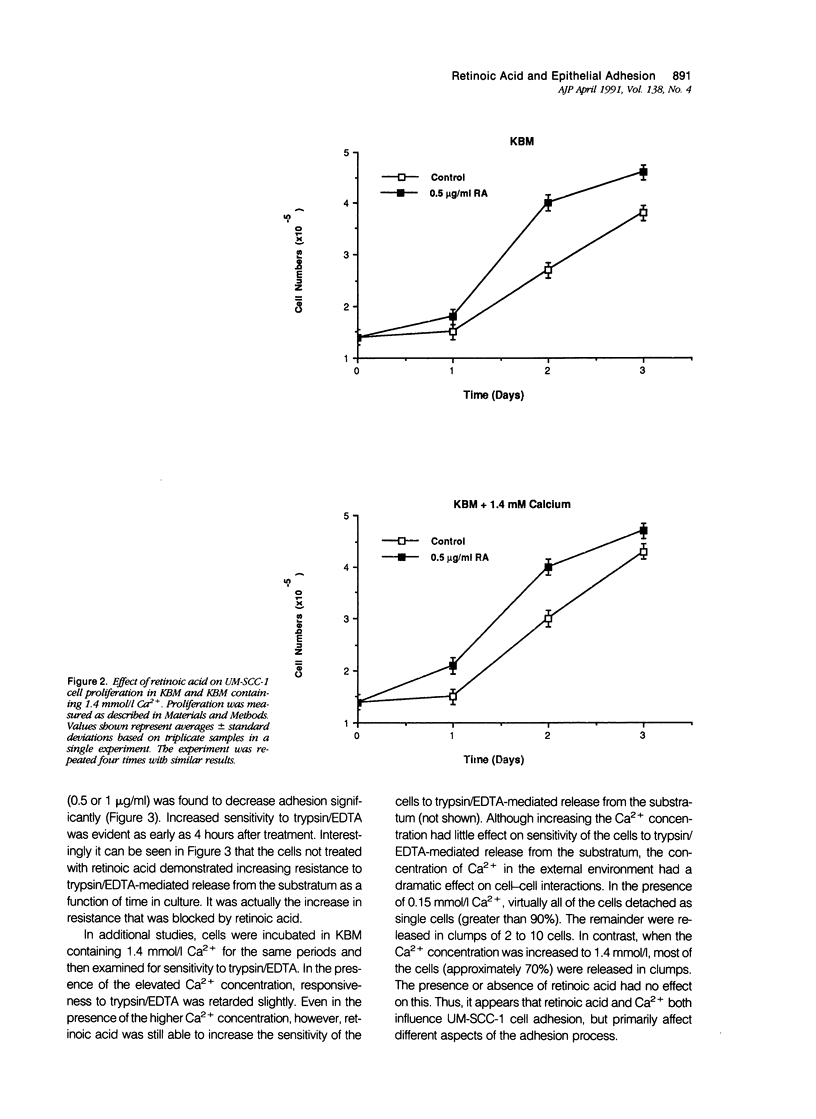
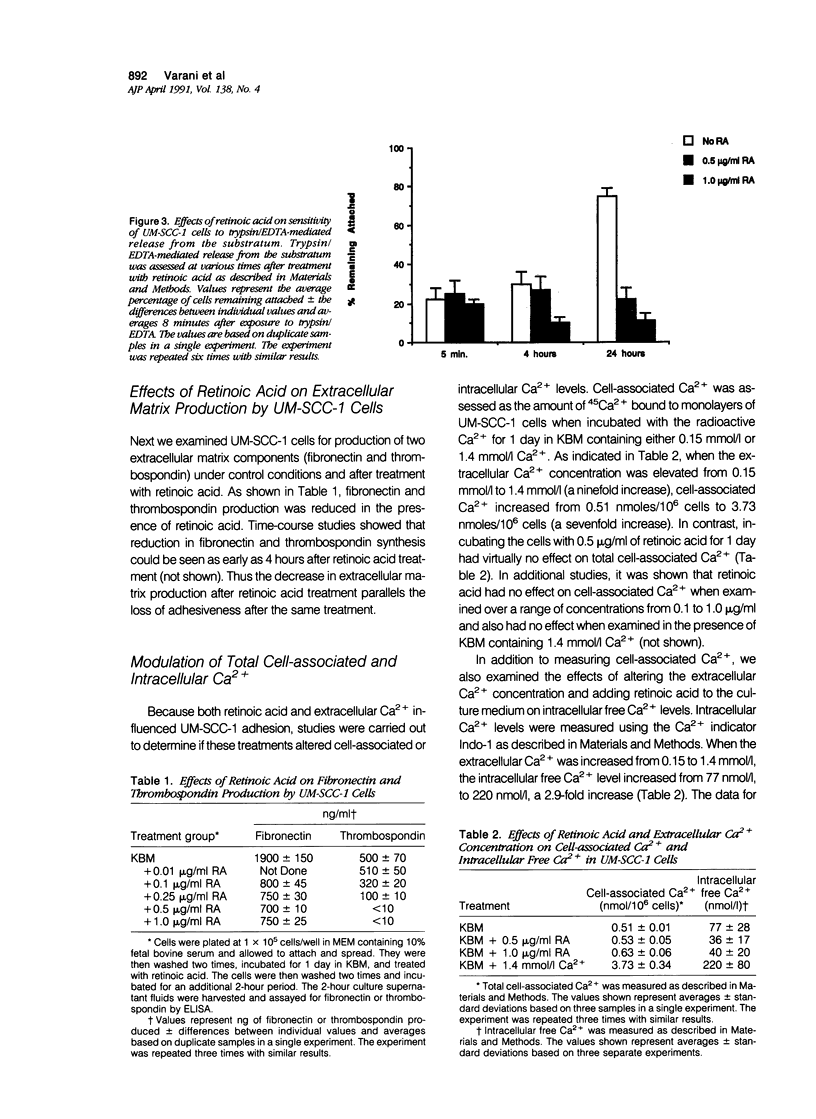
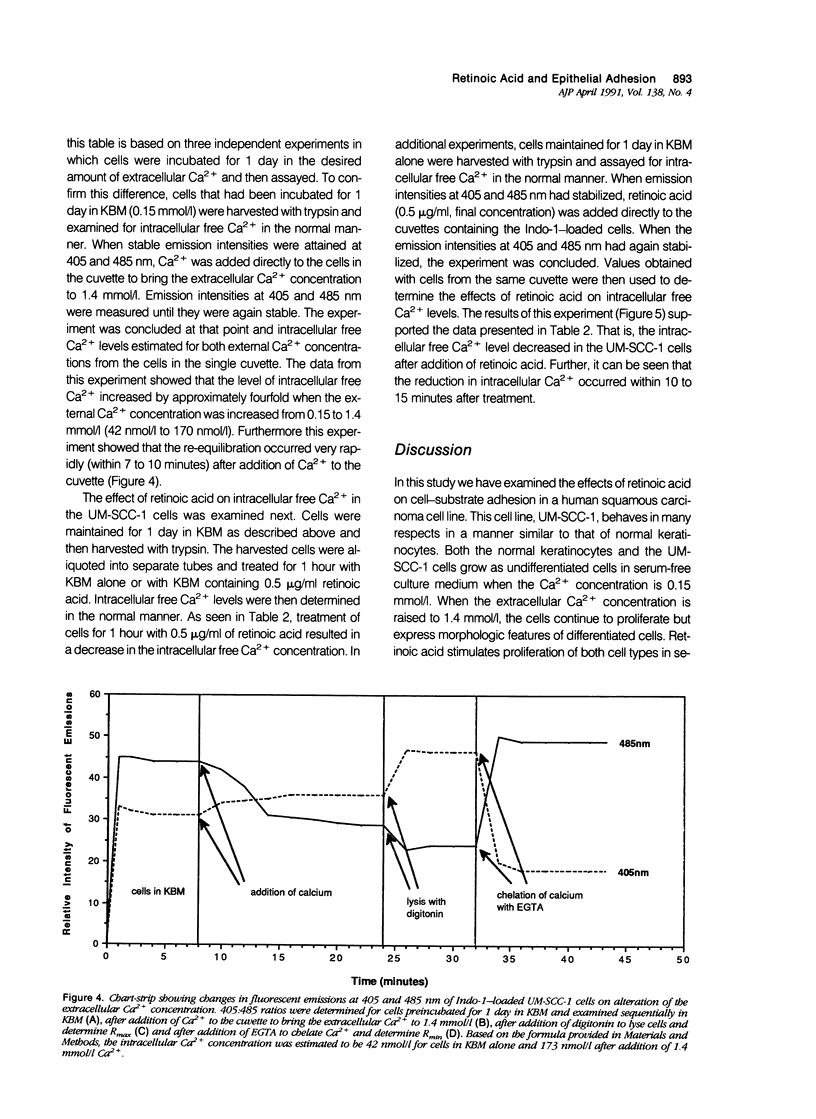
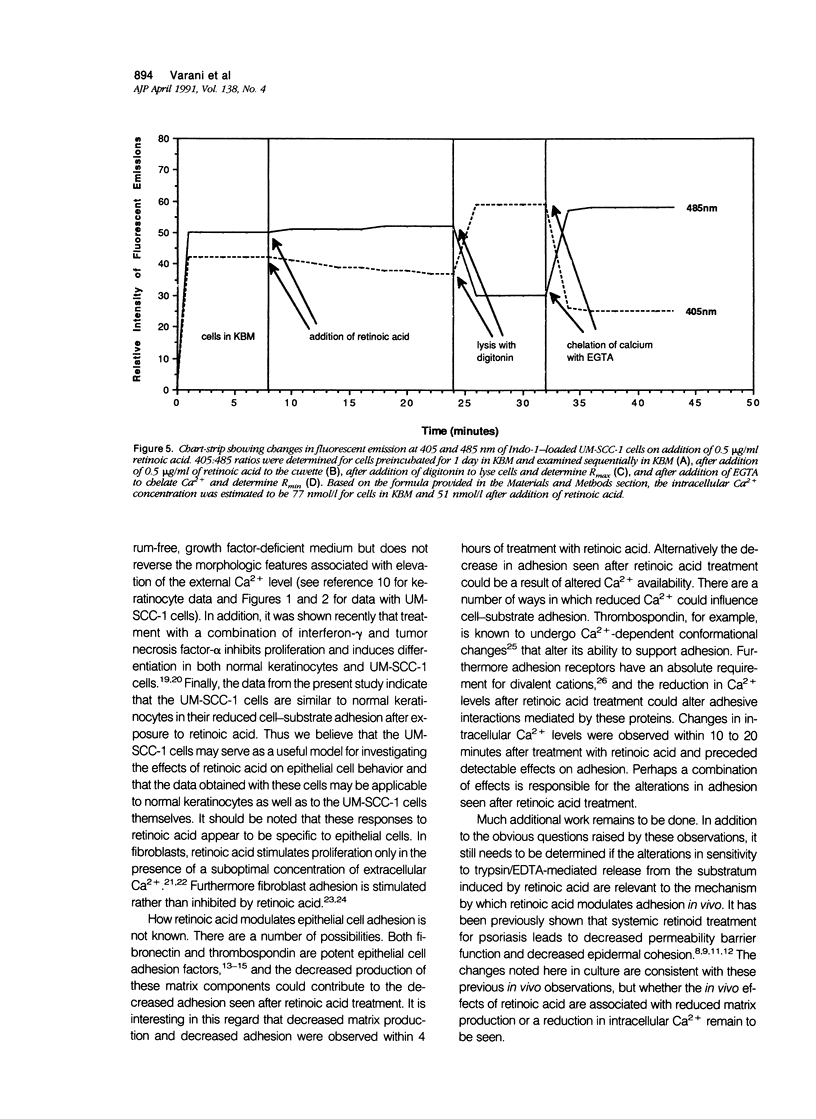
Human squamous epithelial cells maintained in growth factor-deficient medium were examined for sensitivity to all-trans retinoic acid (retinoic acid). Under conditions of low external Ca2+ (0.15 mmol/l [millimolar]), or high external Ca2+ (1.4 mmol/l), retinoic acid stimulated proliferation. Concomitantly, cell-substrate adhesion was decreased. Enzyme-linked immunosorbent assays were used to assess production of two extracellular matrix components, ie, fibronectin and thrombospondin. In the presence of retinoic acid, production of both was decreased. Because both fibronectin and thrombospondin serve as epithelial cell adhesion factors, the decreased production of these moieties could contribute to reduced adhesion. Using 45Ca2+ to measure total cell-associated Ca2+ and the Ca2(+)-sensitive dye Indo-1 to measure intracellular free Ca2+, it was found that concentrations of retinoic acid that altered cell-substrate adhesion in the squamous epithelial cells had no effect on total, cell-associated Ca2+, but reduced intracellular free Ca2+ by 50% to 60%. Because Ca2+ is a regulator of adhesion, the ability of retinoic acid to modulate Ca2+ levels in the squamous epithelial cells may explain, in part, how retinoic acid influences their adhesiveness.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamo S., Sasak W., Dion L. D., De Luca L. M. Studies on the mechanism of retinoid-induced adhesion of spontaneously transformed mouse fibroblasts. Acta Vitaminol Enzymol. 1983;5(1):3–10. [PubMed] [Google Scholar]
- Bryce G. F., Bogdan N. J., Brown C. C. Retinoic acids promote the repair of the dermal damage and the effacement of wrinkles in the UVB-irradiated hairless mouse. J Invest Dermatol. 1988 Aug;91(2):175–180. doi: 10.1111/1523-1747.ep12464445. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Folkvord J. M., Wertz R. L. Fibronectin, as well as other extracellular matrix proteins, mediate human keratinocyte adherence. J Invest Dermatol. 1985 May;84(5):378–383. doi: 10.1111/1523-1747.ep12265466. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Brown B. E. The mammalian cutaneous permeability barrier: defective barrier function is essential fatty acid deficiency correlates with abnormal intercellular lipid deposition. Lab Invest. 1978 Dec;39(6):574–583. [PubMed] [Google Scholar]
- Elias P. M., Fritsch P. O., Lampe M., Williams M. L., Brown B. E., Nemanic M., Grayson S. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981 Jun;44(6):531–540. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kato S., De Luca L. M. Retinoic acid modulates attachment of mouse fibroblasts to laminin substrates. Exp Cell Res. 1987 Dec;173(2):450–462. doi: 10.1016/0014-4827(87)90285-0. [DOI] [PubMed] [Google Scholar]
- Kimmel K. A., Carey T. E. Altered expression in squamous carcinoma cells of an orientation restricted epithelial antigen detected by monoclonal antibody A9. Cancer Res. 1986 Jul;46(7):3614–3623. [PubMed] [Google Scholar]
- Kligman A. M., Grove G. L., Hirose R., Leyden J. J. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Duo C. H., Kligman A. M. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12(2):139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- Kligman L. H. Effects of all-trans-retinoic acid on the dermis of hairless mice. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):779-85, 884-7. doi: 10.1016/s0190-9622(86)70234-x. [DOI] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J., Riser B. L., Mitra R. S., Dixit V. M., Varani J. Inhibitory effect of gamma interferon on cultured human keratinocyte thrombospondin production, distribution, and biologic activities. J Invest Dermatol. 1988 Sep;91(3):213–218. doi: 10.1111/1523-1747.ep12465005. [DOI] [PubMed] [Google Scholar]
- Orfanos C. E., Runne U. Tissue changes in psoriatic plaques after oral administration of retinoid. Dermatologica. 1978;157 (Suppl 1):19–25. doi: 10.1159/000250880. [DOI] [PubMed] [Google Scholar]
- Schuger L., Dixit V. M., Carey T. E., Varani J. Modulation of squamous carcinoma cell growth, morphology, adhesiveness and extracellular matrix production by interferon-gamma and tumor necrosis factor-alpha. Pathobiology. 1990;58(5):279–286. doi: 10.1159/000163597. [DOI] [PubMed] [Google Scholar]
- Skoglund G., Patarroyo M., Forsbeck K., Nilsson K., Ingelman-Sundberg M. Evidence for separate control by phorbol esters of CD18-dependent adhesion and translocation of protein kinase C in U-937 cells. Cancer Res. 1988 Jun 1;48(11):3168–3172. [PubMed] [Google Scholar]
- Varani J., Dixit V. M., Fligiel S. E., McKeever P. E., Carey T. E. Thrombospondin-induced attachment and spreading of human squamous carcinoma cells. Exp Cell Res. 1986 Dec;167(2):376–390. doi: 10.1016/0014-4827(86)90178-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Lovett E. J., 3rd, McCoy J. P., Jr, Shibata S., Maddox D. E., Goldstein I. J., Wicha M. Differential expression of a lamininlike substance by high- and low-metastatic tumor cells. Am J Pathol. 1983 Apr;111(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Mitra R. S., Gibbs D., Phan S. H., Dixit V. M., Mitra R., Jr, Wang T., Siebert K. J., Nickoloff B. J., Voorhees J. J. All-trans retinoic acid stimulates growth and extracellular matrix production in growth-inhibited cultured human skin fibroblasts. J Invest Dermatol. 1990 May;94(5):717–723. doi: 10.1111/1523-1747.ep12876294. [DOI] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Dixit V. M., Mitra R. S., Voorhees J. J. All-trans retinoic acid stimulates growth of adult human keratinocytes cultured in growth factor-deficient medium, inhibits production of thrombospondin and fibronectin, and reduces adhesion. J Invest Dermatol. 1989 Oct;93(4):449–454. doi: 10.1111/1523-1747.ep12284020. [DOI] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Riser B. L., Mitra R. S., O'Rourke K., Dixit V. M. Thrombospondin-induced adhesion of human keratinocytes. J Clin Invest. 1988 May;81(5):1537–1544. doi: 10.1172/JCI113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Shayevitz J., Perry D., Mitra R. S., Nickoloff B. J., Voorhees J. J. Retinoic acid stimulation of human dermal fibroblast proliferation is dependent on suboptimal extracellular Ca2+ concentration. Am J Pathol. 1990 Jun;136(6):1275–1281. [PMC free article] [PubMed] [Google Scholar]
- Weiss J. S., Ellis C. N., Headington J. T., Tincoff T., Hamilton T. A., Voorhees J. J. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988 Jan 22;259(4):527–532. [PubMed] [Google Scholar]
- Williams M. L., Elias P. M. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981 Oct;117(10):611–619. [PubMed] [Google Scholar]



