Abstract
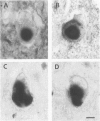
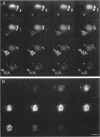
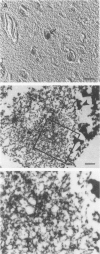
A subset of demented elderly patients exhibit large numbers of cortical intraneuronal inclusions similar to the neurofilament (NF)-rich Lewy bodies (LB) found in pigmented subcortical neurons of patients with Parkinson's disease (PD). Because these cortical inclusions may contribute to the emergence of cognitive impairments in afflicted individuals, the authors mapped the distribution of NF epitopes in these so-called cortical LBs. This was done using ethanol-fixed tissues and a large library of monoclonal antibodies (MAbs) with well-characterized binding specificities to various regions of each NF triplet protein. Cortical LBs were examined by light, confocal, and electron microscopy, and they were compared with the subcortical LBs of PD and LBs in the peripheral nervous system (PNS). Monoclonal antibodies specific for the rod regions of each of the three NF subunits, or for phosphate-dependent and independent antigenic sites in the tail region of the high- (NF-H) and middle- (NF-M) molecular weight (Mr) NF subunits as well as other MAbs to the extreme COOH terminus of NF-L and NF-M or the head region of NF-M labeled a variable number of cortical LBs. Remarkably one of these anti-NF MAbs, RMO32, which recognized a phosphorylated epitope in the tail region of NF-M, immunolabeled nearly all cortical LBs, whereas each of the other anti-NF MAbs never labeled more than 10% of ubiquitin- or RMO32-positive cortical LBs. Further LBs in the PNS resembled those in the central nervous system (CNS) in their immunologic properties, and LBs in both sites were dominated by filamentous aggregates at the ultrastructural level. These findings suggest that NF proteins are profoundly altered during their incorporation into cortical and PNS LBs. Further the authors here identified immunologic and ultrastructural properties common to cortical LBs, PNS LBs, and classic substantia nigra LBs in PD. The accumulation of filamentous, perikaryal inclusions rich in NF proteins at diverse sites in the CNS and PNS of patients with a variety of neurodegenerative disorders suggests a widespread disruption of NF metabolism or transport.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Lee V. M., Otvos L., Jr, Greenberg B. D., Lowery D. E., Sharma S. K., Schmidt M. L., Trojanowski J. Q. Defined neurofilament, tau, and beta-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaques. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2249–2253. doi: 10.1073/pnas.87.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancher C., Lassmann H., Budka H., Jellinger K., Grundke-Iqbal I., Iqbal K., Wiche G., Seitelberger F., Wisniewski H. M. An antigenic profile of Lewy bodies: immunocytochemical indication for protein phosphorylation and ubiquitination. J Neuropathol Exp Neurol. 1989 Jan;48(1):81–93. doi: 10.1097/00005072-198901000-00007. [DOI] [PubMed] [Google Scholar]
- Burkhardt C. R., Filley C. M., Kleinschmidt-DeMasters B. K., de la Monte S., Norenberg M. D., Schneck S. A. Diffuse Lewy body disease and progressive dementia. Neurology. 1988 Oct;38(10):1520–1528. doi: 10.1212/wnl.38.10.1520. [DOI] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Dickson D. W., Crystal H., Mattiace L. A., Kress Y., Schwagerl A., Ksiezak-Reding H., Davies P., Yen S. H. Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques. Acta Neuropathol. 1989;78(6):572–584. doi: 10.1007/BF00691284. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Davies P., Mayeux R., Crystal H., Horoupian D. S., Thompson A., Goldman J. E. Diffuse Lewy body disease. Neuropathological and biochemical studies of six patients. Acta Neuropathol. 1987;75(1):8–15. doi: 10.1007/BF00686786. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Wertkin A., Kress Y., Ksiezak-Reding H., Yen S. H. Ubiquitin immunoreactive structures in normal human brains. Distribution and developmental aspects. Lab Invest. 1990 Jul;63(1):87–99. [PubMed] [Google Scholar]
- Eggertson D. E., Sima A. A. Dementia with cerebral Lewy bodies. A mesocortical dopaminergic defect? Arch Neurol. 1986 May;43(5):524–527. doi: 10.1001/archneur.1986.00520050094034. [DOI] [PubMed] [Google Scholar]
- Galloway P. G., Bergeron C., Perry G. The presence of tau distinguishes Lewy bodies of diffuse Lewy body disease from those of idiopathic Parkinson disease. Neurosci Lett. 1989 May 22;100(1-3):6–10. doi: 10.1016/0304-3940(89)90651-4. [DOI] [PubMed] [Google Scholar]
- Gibb W. R., Luthert P. J., Janota I., Lantos P. L. Cortical Lewy body dementia: clinical features and classification. J Neurol Neurosurg Psychiatry. 1989 Feb;52(2):185–192. doi: 10.1136/jnnp.52.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Yen S. H., Chiu F. C., Peress N. S. Lewy bodies of Parkinson's disease contain neurofilament antigens. Science. 1983 Sep 9;221(4615):1082–1084. doi: 10.1126/science.6308771. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin pathway for the degradation of intracellular proteins. Prog Nucleic Acid Res Mol Biol. 1986;33:19-56, 301. doi: 10.1016/s0079-6603(08)60019-7. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Ikeda S., Yoshimura T., Kato H., Namba M. Idiopathic Parkinsonism with Lewy-type inclusions in cerebral cortex. A case report. Acta Neuropathol. 1978 Feb 20;41(2):165–168. doi: 10.1007/BF00689769. [DOI] [PubMed] [Google Scholar]
- Itoh T., Momma Y., Ogasawara N. An electron microscopic study on atypical presenile dementia with numerous Lewy bodies in the cerebral cortex. Folia Psychiatr Neurol Jpn. 1982;36(1):99–106. doi: 10.1111/j.1440-1819.1982.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Knopman D. S., Mastri A. R., Frey W. H., 2nd, Sung J. H., Rustan T. Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology. 1990 Feb;40(2):251–256. doi: 10.1212/wnl.40.2.251. [DOI] [PubMed] [Google Scholar]
- Kosaka K. Lewy bodies in cerebral cortex, report of three cases. Acta Neuropathol. 1978 May 24;42(2):127–134. doi: 10.1007/BF00690978. [DOI] [PubMed] [Google Scholar]
- Kosaka K., Yoshimura M., Ikeda K., Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree--a new disease? Clin Neuropathol. 1984 Sep-Oct;3(5):185–192. [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Binder L. I., Yen S. H. Alzheimer disease proteins (A68) share epitopes with tau but show distinct biochemical properties. J Neurosci Res. 1990 Mar;25(3):420–430. doi: 10.1002/jnr.490250320. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Chien C. H., Lee V. M., Yen S. H. Mapping of the Alz 50 epitope in microtubule-associated proteins tau. J Neurosci Res. 1990 Mar;25(3):412–419. doi: 10.1002/jnr.490250319. [DOI] [PubMed] [Google Scholar]
- Kuljis R. O., Martín-Vasallo P., Peress N. S. Lewy bodies in tyrosine hydroxylase-synthesizing neurons of the human cerebral cortex. Neurosci Lett. 1989 Nov 20;106(1-2):49–54. doi: 10.1016/0304-3940(89)90200-0. [DOI] [PubMed] [Google Scholar]
- Kuzuhara S., Mori H., Izumiyama N., Yoshimura M., Ihara Y. Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol. 1988;75(4):345–353. doi: 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W. Structural similarities and differences between neurofilament proteins from five different species as revealed using monoclonal antibodies. J Neurosci. 1986 Aug;6(8):2179–2186. doi: 10.1523/JNEUROSCI.06-08-02179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Schmidt M. L., Trojanowski J. Q. Alzheimer disease tangles share immunological similarities with multiphosphorylation repeats in the two large neurofilament proteins. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7384–7388. doi: 10.1073/pnas.85.19.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox G., Lowe J., Morrell K., Landon M., Mayer R. J. Anti-ubiquitin immunocytochemistry is more sensitive than conventional techniques in the detection of diffuse Lewy body disease. J Neurol Neurosurg Psychiatry. 1989 Jan;52(1):67–71. doi: 10.1136/jnnp.52.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S., Saitoh T., Quijada S., Cole G. M., Terry R. D. Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tangles, Pick bodies and Lewy bodies. J Neuropathol Exp Neurol. 1988 Jul;47(4):393–405. doi: 10.1097/00005072-198807000-00001. [DOI] [PubMed] [Google Scholar]
- Mori H., Yoshimura M., Tomonaga M., Yamanouchi H. Progressive supranuclear palsy with Lewy bodies. Acta Neuropathol. 1986;71(3-4):344–346. doi: 10.1007/BF00688061. [DOI] [PubMed] [Google Scholar]
- Perry R. H., Irving D., Blessed G., Fairbairn A., Perry E. K. Senile dementia of Lewy body type. A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci. 1990 Feb;95(2):119–139. doi: 10.1016/0022-510x(90)90236-g. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Carden M. J., Lee V. M., Trojanowski J. Q. Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest. 1987 Mar;56(3):282–294. [PubMed] [Google Scholar]
- Schmidt M. L., Gur R. E., Gur R. C., Trojanowski J. Q. Intraneuronal and extracellular neurofibrillary tangles exhibit mutually exclusive cytoskeletal antigens. Ann Neurol. 1988 Feb;23(2):184–189. doi: 10.1002/ana.410230212. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Lee V. M., Hurtig H., Trojanowski J. Q. Properties of antigenic determinants that distinguish neurofibrillary tangles in progressive supranuclear palsy and Alzheimer's disease. Lab Invest. 1988 Oct;59(4):460–466. [PubMed] [Google Scholar]
- Schmidt M. L., Lee V. M., Trojanowski J. Q. Analysis of epitopes shared by Hirano bodies and neurofilament proteins in normal and Alzheimer's disease hippocampus. Lab Invest. 1989 Apr;60(4):513–522. [PubMed] [Google Scholar]
- Schmidt M. L., Lee V. M., Trojanowski J. Q. Relative abundance of tau and neurofilament epitopes in hippocampal neurofibrillary tangles. Am J Pathol. 1990 May;136(5):1069–1075. [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Chau V. Ubiquitin and microtubule-associated protein tau immunoreactivity each define distinct structures with differing distributions and solubility properties in Alzheimer brain. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2854–2858. doi: 10.1073/pnas.85.8.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Fischer S., Weber K. alpha-MSH and neurofilament M-protein share a continuous epitope but not extended sequences. An explanation for neurofibrillary staining with alpha-MSH antibodies. FEBS Lett. 1985 Feb 25;181(2):343–346. doi: 10.1016/0014-5793(85)80289-1. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Clark A. W., Sternberger N. A., Sternberger L. A. Lewy body dementia without Alzheimer changes. Can J Neurol Sci. 1986 Nov;13(4 Suppl):490–497. doi: 10.1017/s0317167100037185. [DOI] [PubMed] [Google Scholar]
- Stern R. A., Otvos L., Jr, Trojanowski J. Q., Lee V. M. Monoclonal antibodies to a synthetic peptide homologous with the first 28 amino acids of Alzheimer's disease beta-protein recognize amyloid and diverse glial and neuronal cell types in the central nervous system. Am J Pathol. 1989 May;134(5):973–978. [PMC free article] [PubMed] [Google Scholar]
- Tiller-Borcich J. K., Forno L. S. Parkinson's disease and dementia with neuronal inclusions in the cerebral cortex: Lewy bodies or Pick bodies. J Neuropathol Exp Neurol. 1988 Sep;47(5):526–535. doi: 10.1097/00005072-198809000-00004. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Kelsten M. L., Lee V. M. Phosphate-dependent and independent neurofilament protein epitopes are expressed throughout the cell cycle in human medulloblastoma (D283 MED) cells. Am J Pathol. 1989 Oct;135(4):747–758. [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of phosphate-independent MAP2 epitopes revealed with monoclonal antibodies in microwave-denatured human nervous system tissues. J Neurosci Methods. 1989 Aug;29(2):171–180. doi: 10.1016/0165-0270(89)90030-7. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989 Feb;37(2):209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Takahashi H., Takeda S., Ohama E., Ikuta F. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol. 1988;76(3):217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- den HARTOG JAGER W. A., BETHLEM J. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry. 1960 Nov;23:283–290. doi: 10.1136/jnnp.23.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]