Abstract
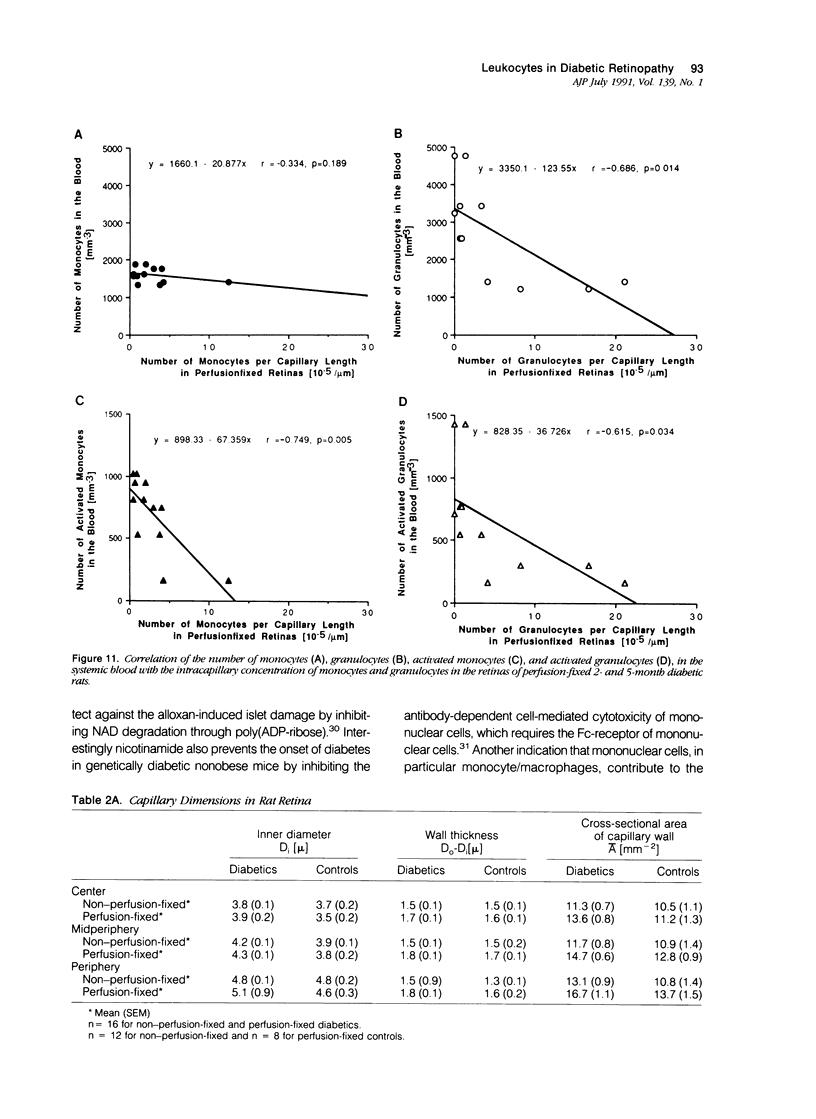
Capillary occlusions are characteristic features of the early diabetic retinopathy and are presumed to initiate neovascularization. Activated leukocytes can cause microvascular occlusions and cell damage by release of cytotoxic products. To explore the role of leukocytes in capillary occlusion, nonperfusion, and neovascularization of diabetic retinopathy, a rat model was used, in which a diabetic state was induced by alloxan. Retina flat preparations were differentially stained for monocytes and granulocytes. Capillary occlusion, nonperfusion, and neovascularization were assessed microscopically in the center, midperiphery, and periphery of the retina. In contrast to control retinas, 2- to 9-month diabetic rats showed many capillary occlusions by leukocytes, especially monocytes, endothelial cell damage, extravascular macrophage accumulation, and tissue damage. The percentage of activated monocytes and granulocytes in the circulating blood of diabetic rats was greatly increased, and areas of capillary 'loss' and neovascularization in the retina coincided with sites of extravascular leukocytes. The authors' results suggest a potential role of monocytes and macrophages in the pathogenesis of diabetic retinopathy.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton A., Born G. V. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972 Apr;222(2):447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagge U., Amundson B., Lauritzen C. White blood cell deformability and plugging of skeletal muscle capillaries in hemorrhagic shock. Acta Physiol Scand. 1980 Feb;108(2):159–163. doi: 10.1111/j.1748-1716.1980.tb06513.x. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Knighton D. R., Hunt T. K., Werb Z. Isolation of a nonmitogenic angiogenesis factor from wound fluid. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7773–7777. doi: 10.1073/pnas.79.24.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar R. S., Kahn C. R., Koren H. S. Insulin inhibition of antibody-dependent cytoxicity and insulin receptors in macrophages. Nature. 1977 Feb 17;265(5595):632–635. doi: 10.1038/265632a0. [DOI] [PubMed] [Google Scholar]
- Barroso-Aranda J., Schmid-Schönbein G. W., Zweifach B. W., Engler R. L. Granulocytes and no-reflow phenomenon in irreversible hemorrhagic shock. Circ Res. 1988 Aug;63(2):437–447. doi: 10.1161/01.res.63.2.437. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt A., Braide M., Myrhage R., Bagge U. Vital microscopic studies on the capillary distribution of leukocytes in the rat cremaster muscle. Int J Microcirc Clin Exp. 1987 Aug;6(3):273–286. [PubMed] [Google Scholar]
- Chien S., Schmalzer E. A., Lee M. M., Impelluso T., Skalak R. Role of white blood cells in filtration of blood cell suspensions. Biorheology. 1983;20(1):11–27. doi: 10.3233/bir-1983-20102. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Aikin B. S. Polymorphonuclear leukocytes in immunologic reactions. The destruction of vascular basement membrane in vivo and in vitro. J Exp Med. 1966 Oct 1;124(4):733–752. doi: 10.1084/jem.124.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Vaz J., Faria de Abreu J. R., Campos A. J. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975 Nov;59(11):649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITZEL J., SARGEANT L., HADLEY W. B. The relationship of abnormal vascular responses to retinopathy and nephropathy in diabetics. AMA Arch Intern Med. 1958 May;101(5):912–920. doi: 10.1001/archinte.1958.00260170068009. [DOI] [PubMed] [Google Scholar]
- Davis M. D. Diabetic retinopathy: a clinical overview. Diabetes Metab Rev. 1988 Jun;4(4):291–322. doi: 10.1002/dmr.5610040402. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Meyer R. K. Development of retinal vasculature in rats. Am J Ophthalmol. 1965 Oct;60(4):628–641. doi: 10.1016/0002-9394(65)92251-8. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Ernst E., Matrai A. Leukozytenrheologie bei Diabetes mellitus. Fortschr Med. 1987 Oct 20;105(30):590–592. [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fussganger R. D., Kahn C. R., Roth J., De Meyts P. Binding and degradation of insulin by human peripheral granulocytes. Demonstration of specific receptors with high affinity. J Biol Chem. 1976 May 10;251(9):2761–2769. [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler J. R., Morel D. W., Lewis L. J., Chisolm G. M. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983 May-Jun;3(3):215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- Janoff A., Schaefer S., Scherer J., Bean M. A. Mediators of inflammation in leukocyte lysosomes. II. Mechanism of action of lysosomal cationic protein upon vascular permeability in the rat. J Exp Med. 1965 Nov 1;122(5):841–851. doi: 10.1084/jem.122.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Zeligs J. D. Vascular injury and lysis of basement membrane in vitro by neutral protease of human leukocytes. Science. 1968 Aug 16;161(3842):702–704. doi: 10.1126/science.161.3842.702. [DOI] [PubMed] [Google Scholar]
- Kador P. F. The role of aldose reductase in the development of diabetic complications. Med Res Rev. 1988 Jul-Sep;8(3):325–352. doi: 10.1002/med.2610080302. [DOI] [PubMed] [Google Scholar]
- Kaminski P. M., Proctor K. G. Attenuation of no-reflow phenomenon, neutrophil activation, and reperfusion injury in intestinal microcirculation by topical adenosine. Circ Res. 1989 Aug;65(2):426–435. doi: 10.1161/01.res.65.2.426. [DOI] [PubMed] [Google Scholar]
- Kohner E. M., Oakley N. W. Diabetic retinopathy. Metabolism. 1975 Sep;24(9):1085–1102. doi: 10.1016/0026-0495(75)90102-x. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Lian J., Burleigh M. C. Role of granulocyte collagenase in collagen degradation. Am J Pathol. 1972 Sep;68(3):565–578. [PMC free article] [PubMed] [Google Scholar]
- Ley K., Pries A. R., Gaehtgens P. Preferential distribution of leukocytes in rat mesentery microvessel networks. Pflugers Arch. 1988 Jul;412(1-2):93–100. doi: 10.1007/BF00583736. [DOI] [PubMed] [Google Scholar]
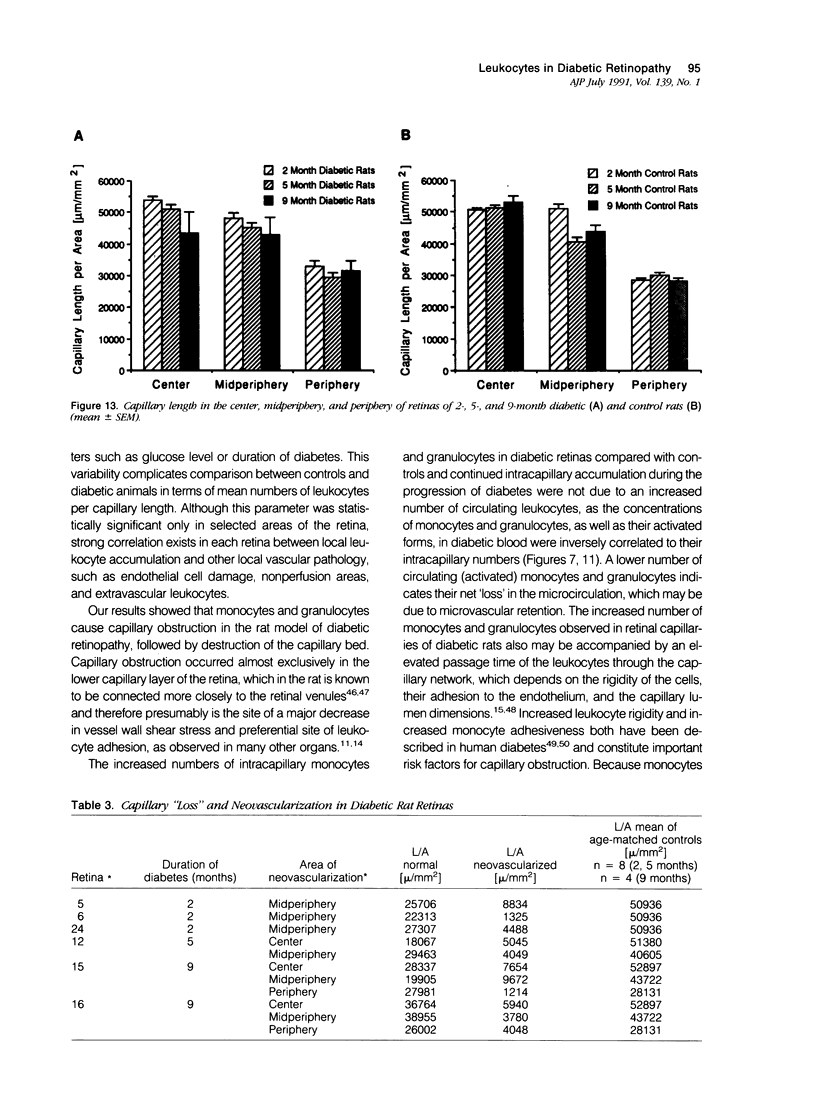
- Little H. L. Alterations in blood elements in the pathogenesis of diabetic retinopathy. Ophthalmology. 1981 Jul;88(7):647–654. doi: 10.1016/s0161-6420(81)34971-9. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J. Alloxan toxicity to the pancreatic B-cell. A new hypothesis. Biochem Pharmacol. 1982 Nov 15;31(22):3527–3534. doi: 10.1016/0006-2952(82)90571-8. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Sener A., Pipeleers D. G. Determinants of the selective toxicity of alloxan to the pancreatic B cell. Proc Natl Acad Sci U S A. 1982 Feb;79(3):927–930. doi: 10.1073/pnas.79.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Mayrovitz H. N., Wiedman M. P., Tuma R. F. Factors influencing leukocyte adherence in microvessels. Thromb Haemost. 1977 Dec 15;38(4):823–830. [PubMed] [Google Scholar]
- Muraoka K., Shimizu K. Intraretinal neovascularization in diabetic retinopathy. Ophthalmology. 1984 Dec;91(12):1440–1446. doi: 10.1016/s0161-6420(84)34125-2. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Yamada K., Hanafusa T., Fujino-Kurihara H., Miyagawa J., Miyazaki A., Saitoh R., Minami Y., Kono N., Nonaka K. Elevated antibody-dependent cell-mediated cytotoxicity and its inhibition by nicotinamide in the diabetic NOD mouse. Immunol Lett. 1986 Mar;12(2-3):91–94. doi: 10.1016/0165-2478(86)90088-x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H. Regulation of proinsulin synthesis in pancreatic islets and a new aspect to insulin-dependent diabetes. Mol Cell Biochem. 1981 Jun 9;37(1):43–61. doi: 10.1007/BF02355886. [DOI] [PubMed] [Google Scholar]
- Ooi B. S., MacCarthy E. P., Hsu A., Ooi Y. M. Human mononuclear cell modulation of endothelial cell proliferation. J Lab Clin Med. 1983 Sep;102(3):428–433. [PubMed] [Google Scholar]
- Oschilewski U., Kiesel U., Kolb H. Administration of silica prevents diabetes in BB-rats. Diabetes. 1985 Feb;34(2):197–199. doi: 10.2337/diab.34.2.197. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. H., Biggar W. D., L'Esperance P., Good R. A. N.B.T. test on monocytes of neutropenic patients. Lancet. 1972 May 13;1(7759):1064–1064. doi: 10.1016/s0140-6736(72)91236-6. [DOI] [PubMed] [Google Scholar]
- Park B. H., Fikrig S. M., Smithwick E. M. Infection and nitroblue-tetrazolium reduction by neutrophils. A diagnostic acid. Lancet. 1968 Sep 7;2(7567):532–534. doi: 10.1016/s0140-6736(68)92406-9. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- ROOT H. F., MIRSKY S., DITZEL J. Proliferative retinopathy in diabetes mellitus; review of eight hundred forty-seven cases. J Am Med Assoc. 1959 Feb 28;169(9):903–909. doi: 10.1001/jama.1959.03000260001001. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Modulation of macrophage Fc receptor expression in vitro by insulin and cyclic nucleotides. Nature. 1975 Oct 16;257(5527):597–599. doi: 10.1038/257597a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Ryan U. S. The endothelial surface and responses to injury. Fed Proc. 1986 Feb;45(2):101–108. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Hotta N., Sakamoto N., Matsuoka S., Ohishi N., Yagi K. Lipid peroxide level in plasma of diabetic patients. Biochem Med. 1979 Feb;21(1):104–107. doi: 10.1016/0006-2944(79)90061-9. [DOI] [PubMed] [Google Scholar]
- Schmid-Schoenbein G. W., Fung Y. C., Zweifach B. W. Vascular endothelium-leukocyte interaction; sticking shear force in venules. Circ Res. 1975 Jan;36(1):173–184. doi: 10.1161/01.res.36.1.173. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Leukocyte kinetics in the microcirculation. Biorheology. 1987;24(2):139–151. doi: 10.3233/bir-1987-24207. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Skalak R., Simon S. I., Engler R. L. The interaction between leukocytes and endothelium in vivo. Ann N Y Acad Sci. 1987;516:348–361. doi: 10.1111/j.1749-6632.1987.tb33055.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Skalak R., Usami S., Chien S. Cell distribution in capillary networks. Microvasc Res. 1980 Jan;19(1):18–44. doi: 10.1016/0026-2862(80)90082-5. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Usami S., Skalak R., Chien S. The interaction of leukocytes and erythrocytes in capillary and postcapillary vessels. Microvasc Res. 1980 Jan;19(1):45–70. doi: 10.1016/0026-2862(80)90083-7. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein H., Volger E. Red-cell aggregation and red-cell deformability in diabetes. Diabetes. 1976;25(2 Suppl):897–902. [PubMed] [Google Scholar]
- Schröder S., Brab M., Schmid-Schönbein G. W., Reim M., Schmid-Schönbein H. Microvascular network topology of the human retinal vessels. Fortschr Ophthalmol. 1990;87(1):52–58. [PubMed] [Google Scholar]
- Schröder S., Schmid-Schönbein G. W., Schmid-Schönbein H., Brub M., Reim M. Methode zur Erfassung der Netzwerktopologie der menschlichen Retinagefässe. Klin Monbl Augenheilkd. 1990 Jul;197(1):33–39. doi: 10.1055/s-2008-1046240. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Bianco A. R., Handwerger B. S., Kahn C. R. Demonstration that monocytes rather than lymphocytes are the insulin-binding cells in preparations of humah peripheral blood mononuclear leukocytes: implications for studies of insulin-resistant states in man. Proc Natl Acad Sci U S A. 1975 Feb;72(2):474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiadi H., Wautier J. L., Courillon-Mallet A., Passa P., Caen J. Increased adhesion to fibronectin and Mo-1 expression by diabetic monocytes. J Immunol. 1987 May 15;138(10):3230–3234. [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Stefansson E., Landers M. B., 3rd, Wolbarsht M. L. Oxygenation and vasodilatation in relation to diabetic and other proliferative retinopathies. Ophthalmic Surg. 1983 Mar;14(3):209–226. [PubMed] [Google Scholar]
- Uchigata Y., Yamamoto H., Nagai H., Okamoto H. Effect of poly(ADP-ribose) synthetase inhibitor administration to rats before and after injection of alloxan and streptozotocin on islet proinsulin synthesis. Diabetes. 1983 Apr;32(4):316–318. doi: 10.2337/diab.32.4.316. [DOI] [PubMed] [Google Scholar]
- WISE G. N. Retinal neovascularization. Trans Am Ophthalmol Soc. 1956;54:729–826. [PMC free article] [PubMed] [Google Scholar]
- Wall R. T., Harker L. A., Quadracci L. J., Striker G. E. Factors influencing endothelial cell proliferation in vitro. J Cell Physiol. 1978 Aug;96(2):203–213. doi: 10.1002/jcp.1040960209. [DOI] [PubMed] [Google Scholar]
- Werb Z. How the macrophage regulates its extracellular environment. Am J Anat. 1983 Mar;166(3):237–256. doi: 10.1002/aja.1001660302. [DOI] [PubMed] [Google Scholar]
- Wierusz-Wysocka B., Wysocki H., Siekierka H., Wykretowicz A., Szczepanik A., Klimas R. Evidence of polymorphonuclear neutrophils (PMN) activation in patients with insulin-dependent diabetes mellitus. J Leukoc Biol. 1987 Nov;42(5):519–523. doi: 10.1002/jlb.42.5.519. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yanoff M. Ocular pathology of diabetes mellitus. Am J Ophthalmol. 1969 Jan;67(1):21–38. doi: 10.1016/0002-9394(69)90004-x. [DOI] [PubMed] [Google Scholar]