Abstract
Immunohistochemistry of human atherosclerotic arteries demonstrates expression of the intercellular adhesion molecule-1 (ICAM-1) on endothelial cells, macrophages, and smooth muscle cells of the plaques. Normal arterial endothelial cells and intimal smooth muscle outside plaques give weaker or negative reactions; these differ from the strong endothelial expression in small vessels. Quantitative color-image analysis of the endothelial layer shows increased expression of ICAM-1 in all subtypes of atherosclerotic lesions, except fibrous plaques. Endothelial expression of ICAM-1 may be involved in the recruitment of monocytes to the lesion, as suggested by its role in the entry of leukocytes, including monocytes, into foci of inflammation. Collaboration with other mechanisms, particularly chemoattractant factors, may be important for this effect. ICAM-1 enhanced monocyte recruitment is a potential mechanism for the growth of an atherosclerotic plaque.
Full text
PDF


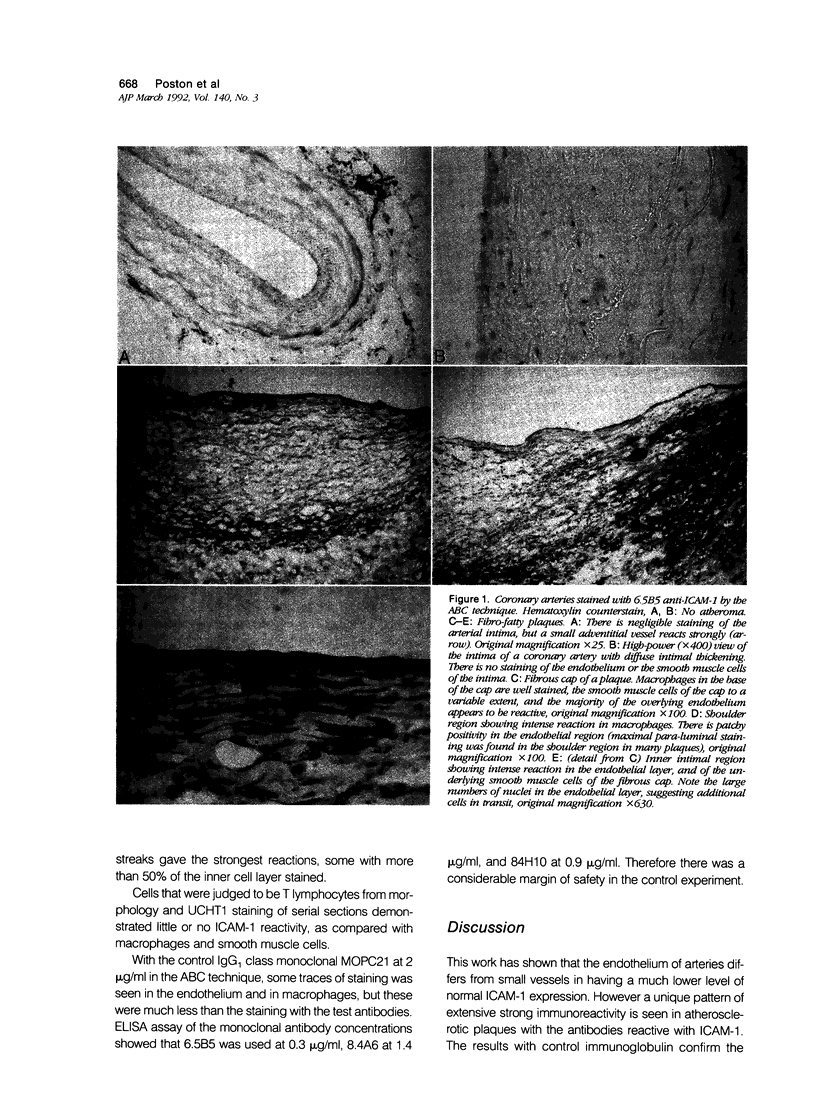
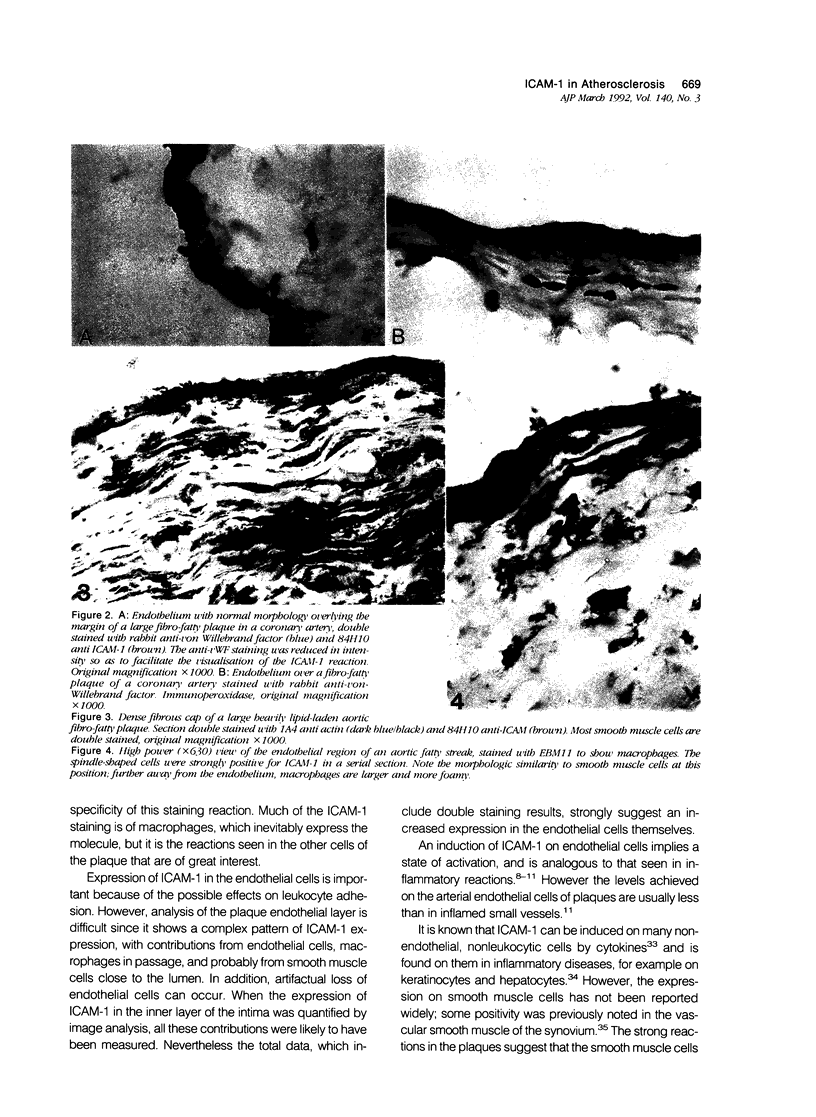
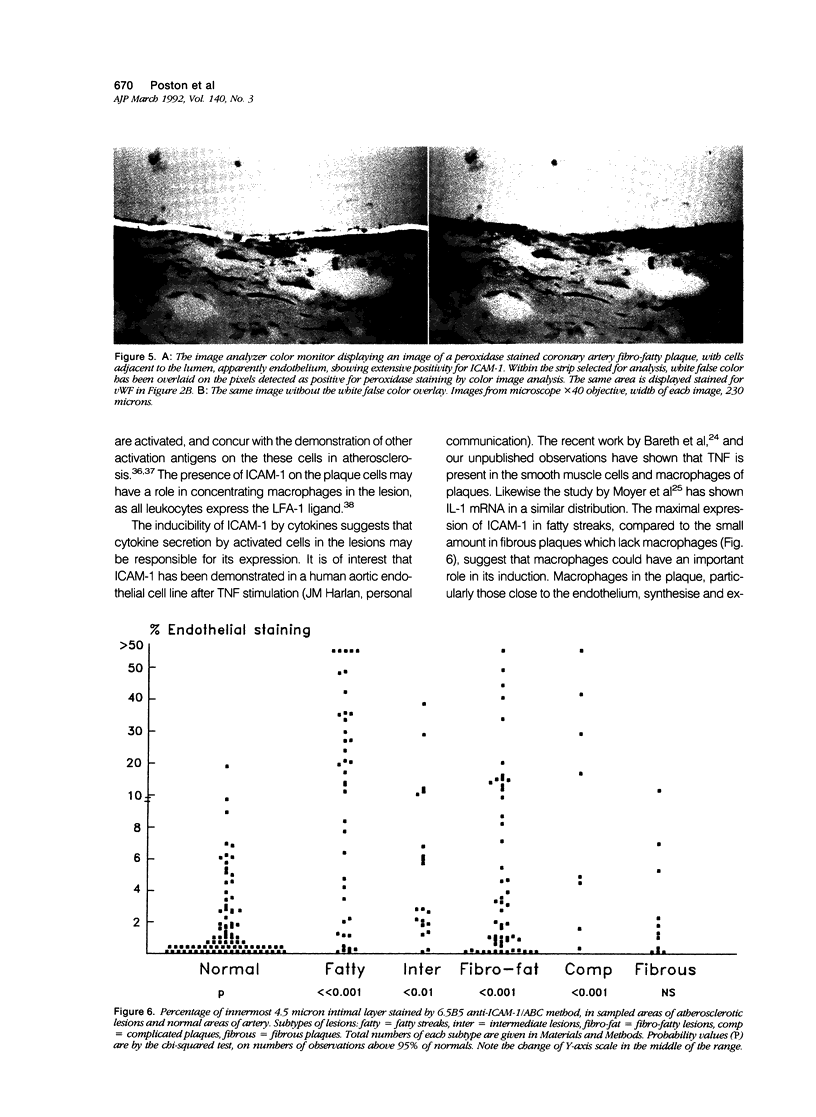



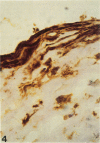
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barath P., Fishbein M. C., Cao J., Berenson J., Helfant R. H., Forrester J. S. Tumor necrosis factor gene expression in human vascular intimal smooth muscle cells detected by in situ hybridization. Am J Pathol. 1990 Sep;137(3):503–509. [PMC free article] [PubMed] [Google Scholar]
- Bayne E. K., Rupp E. A., Limjuco G., Chin J., Schmidt J. A. Immunocytochemical detection of interleukin 1 within stimulated human monocytes. J Exp Med. 1986 May 1;163(5):1267–1280. doi: 10.1084/jem.163.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekhuizen H., Corsèl-van Tilburg A. J., van Furth R. Characterization of monocyte adherence to human macrovascular and microvascular endothelial cells. J Immunol. 1990 Jul 15;145(2):510–518. [PubMed] [Google Scholar]
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Breviario F., Aglietta M., Sanavio F., Bosia A., Dejana E. Studies on the mechanism of interleukin 1 stimulation of platelet activating factor synthesis in human endothelial cells in culture. Biochim Biophys Acta. 1987 Jan 19;927(1):43–54. doi: 10.1016/0167-4889(87)90064-4. [DOI] [PubMed] [Google Scholar]
- Bylock A. L., Gerrity R. G. Visualization of monocyte recruitment into atherosclerotic arteries using fluorescent labelling. Atherosclerosis. 1988 May;71(1):17–25. doi: 10.1016/0021-9150(88)90298-5. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Chensue S. W., Remick D. G., Shmyr-Forsch C., Beals T. F., Kunkel S. L. Immunohistochemical demonstration of cytoplasmic and membrane-associated tumor necrosis factor in murine macrophages. Am J Pathol. 1988 Dec;133(3):564–572. [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- De Jong A. S., Van Kessel-van Vark M., Raap A. K. Sensitivity of various visualization methods for peroxidase and alkaline phosphatase activity in immunoenzyme histochemistry. Histochem J. 1985 Oct;17(10):1119–1130. doi: 10.1007/BF01002537. [DOI] [PubMed] [Google Scholar]
- Denholm E. M., Wolber F. M., Phan S. H. Secretion of monocyte chemotactic activity by alveolar macrophages. Am J Pathol. 1989 Sep;135(3):571–580. [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Doukas J., Pober J. S. Lymphocyte-mediated activation of cultured endothelial cells (EC). CD4+ T cells inhibit EC class II MHC expression despite secreting IFN-gamma and increasing EC class I MHC and intercellular adhesion molecule-1 expression. J Immunol. 1990 Aug 15;145(4):1088–1098. [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Figdor C. G., van Kooyk Y., Keizer G. D. On the mode of action of LFA-1. Immunol Today. 1990 Aug;11(8):277–280. doi: 10.1016/0167-5699(90)90112-m. [DOI] [PubMed] [Google Scholar]
- Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb 22;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Graber N., Gopal T. V., Wilson D., Beall L. D., Polte T., Newman W. T cells bind to cytokine-activated endothelial cells via a novel, inducible sialoglycoprotein and endothelial leukocyte adhesion molecule-1. J Immunol. 1990 Aug 1;145(3):819–830. [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Seifert P. S., Stemme S. Immune mechanisms in atherosclerosis. Arteriosclerosis. 1989 Sep-Oct;9(5):567–578. doi: 10.1161/01.atv.9.5.567. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Burrows J. C., Haines G. K., Carlos T. M., Harlan J. M., Leibovich S. J. Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest. 1991 Mar;64(3):313–320. [PubMed] [Google Scholar]
- Lauener R. P., Geha R. S., Vercelli D. Engagement of the monocyte surface antigen CD14 induces lymphocyte function-associated antigen-1/intercellular adhesion molecule-1-dependent homotypic adhesion. J Immunol. 1990 Sep 1;145(5):1390–1394. [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Maddox P. H., Jenkins D. 3-Aminopropyltriethoxysilane (APES): a new advance in section adhesion. J Clin Pathol. 1987 Oct;40(10):1256–1257. doi: 10.1136/jcp.40.10.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Beckstead J. H., Moore K. L., Marshall-Carlson L., Bainton D. F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989 Jul;84(1):92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer C. F., Sajuthi D., Tulli H., Williams J. K. Synthesis of IL-1 alpha and IL-1 beta by arterial cells in atherosclerosis. Am J Pathol. 1991 Apr;138(4):951–960. [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Norris P., Poston R. N., Thomas D. S., Thornhill M., Hawk J., Haskard D. O. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991 May;96(5):763–770. doi: 10.1111/1523-1747.ep12471720. [DOI] [PubMed] [Google Scholar]
- Pallesen G., Knudsen L. M. Leucocyte antigens in human post mortem tissues: their preservation and loss as demonstrated by monoclonal antibody immunohistological staining. Histopathology. 1985 Aug;9(8):791–804. doi: 10.1111/j.1365-2559.1985.tb02867.x. [DOI] [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Poston R. N., Davies D. F. Immunity and inflammation in the pathogenesis of atherosclerosis. A review. Atherosclerosis. 1974 May-Jun;19(3):353–367. doi: 10.1016/s0021-9150(74)80001-8. [DOI] [PubMed] [Google Scholar]
- Prescott M. F., McBride C. K., Court M. Development of intimal lesions after leukocyte migration into the vascular wall. Am J Pathol. 1989 Nov;135(5):835–846. [PMC free article] [PubMed] [Google Scholar]
- Printseva O. J., Peclo M. M., Tjurmin A. V., Faerman A. I., Danilov S. M., Repin V. S., Smirnov V. N. A 90-kd surface antigen from a subpopulation of smooth muscle cells from human atherosclerotic lesions. Am J Pathol. 1989 Feb;134(2):305–313. [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Saegusa Y., Cavender D., Ziff M. Stimulation of mononuclear cell binding to human endothelial cell monolayers by thrombin. J Immunol. 1988 Dec 15;141(12):4140–4145. [PubMed] [Google Scholar]
- Schwartz C. J., Valente A. J., Sprague E. A., Kelley J. L., Suenram C. A., Rozek M. M. Atherosclerosis as an inflammatory process. The roles of the monocyte-macrophage. Ann N Y Acad Sci. 1985;454:115–120. doi: 10.1111/j.1749-6632.1985.tb11849.x. [DOI] [PubMed] [Google Scholar]
- Seifert P. S., Hansson G. K. Decay-accelerating factor is expressed on vascular smooth muscle cells in human atherosclerotic lesions. J Clin Invest. 1989 Aug;84(2):597–604. doi: 10.1172/JCI114204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert P. S., Hugo F., Tranum-Jensen J., Zâhringer U., Muhly M., Bhakdi S. Isolation and characterization of a complement-activating lipid extracted from human atherosclerotic lesions. J Exp Med. 1990 Aug 1;172(2):547–557. doi: 10.1084/jem.172.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Kim D. N. Biology of disease. Atherosclerosis as a hyperplastic and/or neoplastic process. Lab Invest. 1983 Mar;48(3):245–255. [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- Tsukada T., McNutt M. A., Ross R., Gown A. M. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987 May;127(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Mergenhagen S. E. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989 Aug;10(8):258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]